Abstract
The application of magnetic oxides in spintronics has recently attracted much attention. The epitaxial growth of magnetic oxide on Si could be the first step of new functional spintronics devices with semiconductors. However, epitaxial spinel ferrite films are generally grown on oxide substrates, not on semiconductors. To combine oxide spintronics and semiconductor technology, we fabricated Fe3O4 films through epitaxial growth on a Si(111) substrate by inserting a γ-Al2O3 buffer layer. Both of γ-Al2O3 and Fe3O4 layer grew epitaxially on Si and the films exhibited the magnetic and electronic properties as same as bulk. Furthermore, we also found the buffer layer dependence of crystal structure of Fe3O4 by X-ray diffraction and high-resolution transmission electron microscope. The Fe3O4 films on an amorphous-Al2O3 buffer layer grown at room temperature grew uniaxially in the (111) orientation and had a textured structure in the plane. When Fe3O4 was deposited on Si(111) directly, the poly-crystal Fe3O4 films were obtained due to SiOx on Si substrate. The epitaxial Fe3O4 layer on Si substrates enable us the integration of highly functional spintoronic devices with Si technology.
Similar content being viewed by others
Introduction
In the field of spintronics, spin injection and transport phenomena have attracted much attention owing to the possibility of producing novel functional devices1,2,3. In particular, the combination of spintronics and semiconductors is a promising technology for the development of the next stage of spintronic devices, e.g., spin-FET or logic devices4, 5. The spin injection technique, in which the spin-polarized currents are injected from ferromagnetic metals into conventional semiconductor materials2, 3, 6, has been intensely investigated for the preparation of spintronic devices. As a result, researchers have succeeded in nonlocal detection7 or the observation of the Hanle effect1, which demonstrates the spin state in the semiconductor; thus, the behavior of the spin current in the semiconductor can be determined8. Recently, graphene has also been the subject of spin injection because the spin diffusion length in such light elements is expected to be long owing to small spin–orbit interaction9, 10.
The source of the spin current plays an important role in obtaining high-efficiency spin injection. Magnetic oxides are one of the most promising spin source candidates. However, ferromagnetic metals have been used so far because of convenience during fabrication. Magnetic oxides possess unique properties11,12,13,14; Fe3O4 or (LaSr)MnO3 have a half-metallic state, which provides highly spin polarized current15, and NiFe2O4 or CoFe2O4 are magnetic insulators, which means that they could work as a spin filter tunnel barrier16,17,18. γ-Fe2O3 is another candidate as the spin filter barrier. It is the spinel type ferrimagnetic insulator that is obtained by over oxidation of Fe3O4 19. Recently, NiCo2O4 with spinel structure was discovered to exhibit large magnetoresistance effects20. Therefore, the combination of magnetic oxides and semiconductors enables us to produce new functional devices. Some research groups fabricated the magnetic oxide on oxide semiconductor, Nb:SrTiO3, and investigated the transport characteristics including spin transport of the junctions19, 21. However, epitaxial growth of magnetic oxide on Si, which is the most important semiconductor, has not been established because the surface of Si is easily oxidized by the oxygen atmosphere during the evaporation of the magnetic oxides22.
In this study, we grew Fe3O4 epitaxially on a Si(111) substrate by the insertion of an ultrathin γ-Al2O3 buffer layer. Fe3O4 is the ferrimagnetic conducting oxide with spinel crystal structure. At 120K, Fe3O4 shows phase transition called Verwey transition, at which the electric resistivity increases drastically and the crystal symmetry decreases from face-centered cubic to monoclinic23,24,25. Fe3O4 is expected to be half-metallic theoretically, meaning to have a spin polarization of 100%15, and a spin polarization of more than 80% was observed experimentally using a spin-resolved photoemission spectroscopy26. An ultrathin γ-Al2O3 layer was inserted to prevent surface oxidation of Si during the Fe3O4 growth. γ-Al2O3 is an aluminum oxide with the same spinel structure as Fe3O4 and the lattice constant of γ-Al2O3 is 7.91 Å, which is three halves of that of Si with lattice mismatch of −2.9%27. From the viewpoint of the crystal structure, Fe3O4 and γ-Al2O3 seems to grow on Si epitaxially.
γ-Al2O3 (111) epitaxial growth on Si(111) was reported by two research groups recently. Jung et al. formed a γ-Al2O3 (111) layer by annealing an Al layer on protective Si oxide, which was carefully oxidized to be reduced by the Al layer28. Merckling et al. fabricated γ-Al2O3 (111) by the deposition of an Al2O3 source under ultra-high vacuum29. In the former method, it is difficult to optimize the oxidation of the Si layer and the thickness of Al film. In contrast, the latter method is simple if an ultra-high vacuum system is accessible.
In this study, the epitaxial γ-Al2O3 buffer layers were prepared using an ultra-high vacuum system and the Fe3O4 layer was fabricated by reactive molecular beam epitaxy. We investigated the crystal structure, magnetic and electric properties of the Fe3O4 layer on Si(111) with an epitaxial γ-Al2O3 buffer layer, an amorphous-Al2O3 buffer layer, and without a buffer layer. We succeeded in the fabrication of high quality Fe3O4 films on Si(111) substrates. The buffer layer had a significant effect on the crystal structure of the Fe3O4 layers.
Results and Discussion
Epitaxial growth
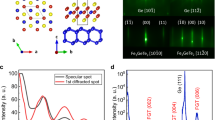
The γ-Al2O3 and Fe3O4 layers were grown by molecular beam epitaxy method. The structures of the samples were (a) Si(111)/γ-Al2O3 2.4 nm/Fe3O4 50 nm/amorphous-Al2O3 2.0 nm, (b) Si(111)/amorphous-Al2O3 2.4 nm/Fe3O4 50 nm/amorphous-Al2O3 2.0 nm and (c) Si(111)/Fe3O4 50 nm/amorphous-Al2O3 2.0 nm, as shown in Fig. 1 (hereafter referred to as (a) EPI, (b) AMO and (c) W/O), respectively. After treatment of the Si substrate, we confirmed that the in-situ reflection high energy electron diffraction (RHEED) pattern of the Si substrate had a (7 × 7) streak pattern (Supplementary Fig. S1). This means that the surface of Si was clean and flat. Figure 2(a) and (b) show the RHEED pattern of γ-Al2O3 and Fe3O4 in EPI. The direction of the incident electron beam was [11-2]. The RHEED patterns of γ-Al2O3 and Fe3O4 were clear streak patterns indicating that γ-Al2O3 and Fe3O4 grew epitaxially. Therefore, the γ-Al2O3 film was considered to play a role of a buffer layer for epitaxial growth of Fe3O4. The surface roughness of γ-Al2O3 and Fe3O4 were estimated to be very small in value by atomic force microscope (AFM) (shown in Supplementary Fig. S2).
Figure 2(c) and (d) show the RHEED pattern of amorphous-Al2O3 and Fe3O4 in AMO. The amorphous-Al2O3 layer was deposited at room temperature. After the deposition of Al2O3, as shown in Fig. 2(c), the Si (7 × 7) streak pattern turned into a halo pattern, which indicated that the Al2O3 layer was amorphous. Figure 2(d) shows the RHEED pattern of Fe3O4 on the amorphous-Al2O3. A ring and streak pattern was observed, which implied the presence of a polycrystalline surface. Thus, the epitaxial γ-Al2O3 played a crucial role in the formation of epitaxial Fe3O4 on the Si substrate.
Figure 2(e) and (f) show the RHEED pattern of the Si substrate and Fe3O4 in W/O. The surface of the Si substrate exhibited a diffused streak pattern owing to the introduction of oxygen gas, which oxidized the Si surface. In Fig. 2(f), the RHEED pattern of Fe3O4 on SiOx shows a halo pattern, which indicated that spinel-type Fe3O4 was not formed.
X-ray diffraction
To confirm the crystallization, the θ–2θ X-ray diffraction (XRD) measurements were carried out on three samples, as shown in Fig. 3(a). The diffraction pattern of Fe3O4 on an γ-Al2O3 buffer layer in EPI (red line) exhibited four peaks (18.3°, 37.2°, 57.2°, 79.4°), which were in agreement with the diffraction patterns of Fe3O4 (111), (222), (333) and (444) planes. This indicated that the Fe3O4 film was (111)-oriented without other orientations or phases. The lattice constant measured by XRD was estimated to be 8.39 Å. The lattice constant of the in-plane direction was estimated to be 8.23 Å (Supplementary Fig. S3), which is smaller than the bulk lattice parameter. Therefore, the Fe3O4 was considered to be compressed in-plane.
To investigate the in-plane epitaxial relationship, we conducted ϕ-scan measurements of Si(311) and Fe3O4 (4-40), as shown in Fig. 3(b). The six peaks of Fe3O4 (4-40) appeared at 60° intervals, indicating the presence of two 180° rotated domains in the Fe3O4 layer. The epitaxial relationships were [11-2]Fe3O4(111) and [-1-12]Fe3O4(111) parallel to [11-2]Si(111), as exhibited in Fig. 3(c). In addition, the peaks of the Fe3O4 film were broader than that of the Si substrate. There was a lattice mismatch of 5.7% at γ-Al2O3/Fe3O4.
The θ–2θ XRD diffraction pattern of Fe3O4 in AMO (blue line) exhibited four peaks, which was identical with the diffraction pattern of Fe3O4 in EPI. Therefore, the Fe3O4 in AMO was also (111)-oriented. However, the RHEED pattern in Fig. 1(d) implied the presence of a polycrystalline structure. Furthermore, the Fe3O4(4-40) diffraction peak was not observed in the ϕ-scan measurement. Therefore, we concluded that the Fe3O4 had a textured structure and the growth direction was (111).
The θ–2θ XRD diffraction pattern of Fe3O4 in W/O (green line) exhibited small peaks related to Fe3O4(311), (400), (422) and unknown peaks. In a previous study30, the XRD of Fe3O4 on SiO2 indicated that the Fe3O4 layer was polycrystalline and contained other phases.
To investigate the crystallinity of the Fe3O4 layer in detail, we carried out X-ray reciprocal space mapping around the symmetric (222) diffraction for Fe3O4 in EPI and AMO (Fig. 3(d)). The symmetrical scan showed that the Fe3O4(222) spot on amorphous-Al2O3 was larger than the Fe3O4 spot on γ-Al2O3, which means that the Fe3O4 in AMO had an angle distribution in the growth directions. Although the reason for the (111) oriented Fe3O4 growth on amorphous-Al2O3/Si(111) was unclear, two possibilities exist that could explain this growth. The first is a reduction in the total anisotropy energy related to the surface energy and interface energy31. The difference between AMO and W/O could be attributed to the difference of the surface and interface energy of amo-Al2O3 and amo-SiO. The second possibility is that the amo-Al2O3 maintains a crystal structure of Si locally because the amo-Al2O3 layer was very thin. Fe3O4 could utilize such a microcrystal-like region as a growth nucleus.
Transmission electron microscope observation
We conducted cross-sectional transition electron microscopy (TEM) analysis to confirm the crystallinity and compositions of the materials. Figure 4 shows the cross-section TEM images in which the electron beams were incident along the Si [1-10] zone axis. In Fig. 4(a), the TEM image shows that the Fe atoms of Fe3O4 were orderly aligned; thus, the Fe3O4 film was epitaxial. The electron diffraction (ED) of Fe3O4 in EPI shown in Fig. 4(b) was in good agreement with the simulation of spinel structure. The left side in Fig. 4(a) shows the epitaxial relationship on [11-2]Fe3O4(111)/[11-2]Si(111), whereas the center of image shows the epitaxial relationship on [-1-12]Fe3O4(111)/[11-2]Si(111), which were consistent with the results of the ϕ-scan measurements in the XRD. In addition, the spacing of the (111) planes were estimated at 4.87 Å from the high angle annular dark-field scanning (HAADF) image (Supplementary Fig. S4(c)), which were almost the same as the out-of-plane lattice constant (4.84 Å) determined by XRD in Fig. 3(a) and that of bulk Fe3O4 (4.85 Å). In contrast, the TEM image of Fe3O4 in AMO shown in Fig. 4(c) demonstrated that the structure was polycrystalline and grain boundaries were clearly observed. The ED image in Fig. 4(d) consisted of the diffraction from the grains with some crystal orientations. In the low magnification TEM image (supplementary Fig. S4(b)), some grains with a size of 15–30 nm appeared.
With respect to the buffer layer, the thickness of γ-Al2O3 was estimated from the HRTEM image (Fig. 4(a)) to be approximately 1 nm, which was thinner than the nominal value measured by the crystal oscillator in the chamber. The reason for this difference in thickness was unclear; however, it could be due to the fluctuation of the crystal oscillator or re-evaporation of Al2O3 because the γ-Al2O3 was grown at a high temperature (900 °C). We could see the amorphous layer under the γ-Al2O3 layer, which was determined to be a SiOx layer by HAADF and Energy dispersive X-ray spectroscopy (EDS) mapping images (Fig. 5). The SiOx layer was considered to form during the growth of Fe3O4 because the Fe3O4 was grown in 4 × 10−4 Pa O2 gas. It was reported that Si was oxidized through the γ-Al2O3 layer of less than 2.0 nm by introducing oxygen (>10−3 Pa)32. To confirm that, we fabricated a γ-Al2O3 (7.5 nm) film on Si(111), and carried out XRD and TEM observations (supplementary Fig. S5(a) and (b)). The γ-Al2O3 grew epitaxially on Si and we found no amorphous layer at the Si(111)/γ-Al2O3(7.5 nm) interface.
Magnetic characteristics
The magnetic character of Fe3O4 is one of its fundamental properties. The magnetization curves at room temperature for the Fe3O4 films on γ-Al2O3 layer are shown in Fig. 6(a). The directions of the magnetic field were in-plane [11-2], in-plane [1-10] and out-of-plane [111]. The hysteresis curve along [11-2] was the same as that along [1-10] and the Fe3O4 film had in-plane magnetization. The saturation magnetization (Ms) was 480 emu/cm3 for all magnetic field directions. The remanent magnetization (Mr), the coercive field (Hc), and the remanent ratio (Mr/Ms) in the in-plane field were 280 emu/cm3 500 Oe, and 0.48, respectively, and those for the out-of-plane field were 47 emu/cm3, 225 Oe, and 0.08, respectively. The hysteresis loops for Fe3O4 in EPI, AMO, and W/O are illustrated in Fig. 6(b). Fe3O4 in EPI had the largest Hc and Ms among the three samples. The Ms of Fe3O4 in EPI was the same as the value of bulk Fe3O4. Although the reason for small magnetization for AMO and W/O has not been clear so far, the antiphase boundary or disordered structure at grain boundary could be responsible for it33, 34.
Transport characteristics
Figure 7 shows that the dependence of the resistance on temperature for the Fe3O4 film in EPI. As is well-known, Fe3O4 is an electric conductor at room temperature and the resistivity increases exponentially with decreasing temperature. The resistivity of the film at 300 K was 2.5 × 10−4 Ωcm, which is lower than the bulk value (5 × 10−3 Ωcm)35. The dlogR/dT plots (inset) show a valley at approximately 120 K. This anomaly corresponds to a Verwey transition36, which is a famous phase transition in Fe3O4. The Verwey transition has been reported to sharply change the resistivity by approximately one digit37; however, the transition is easily disappeared by impurities or structure defects34, 38. As the Fe3O4 in EPI possessed magnetic and electric characteristics that were comparable to bulk Fe3O4, the Fe3O4 on γ-Al2O3 buffer layer was very good quality.
Conclusions
We fabricated an epitaxial Fe3O4 film on a Si substrate by inserting an γ-Al2O3 buffer layer. From the XRD measurement and TEM observation, the γ-Al2O3 buffer layer contributed to the growth of epitaxial Fe3O4(111) on Si(111). In contrast, the Fe3O4 film on an amo-Al2O3 buffer layer had an (111)-orientation with a textured structure. The Fe3O4 on γ-Al2O3 had magnetic properties corresponding to the bulk Fe3O4, furthermore the resistivity exhibited a Verwey transition at 120 K. The results indicate that the heterostructure of Si substrate/γ-Al2O3/Fe3O4 could be used as a part of magnetic tunnel junctions or spin injection devices and will allow us to integrate spintronic devices including Fe3O4 electrode, e.g., spin-FET or magnetic tunnel junctions, on Si.
Methods
Preparation of the samples
Before deposition, the Si substrate was cleaned by a standard Radio Corporation of America clean39 and hydrofluoric (HF) acid solution and annealed at 900 °C under a vacuum of < 10−6 Pa[29]. The γ-Al2O3 buffer layer was formed by evaporating the Al2O3 source material at 900 °C and annealing at 900 °C for 30 minutes. In previous reports, γ-Al2O3 was grown at >850 °C and under a vacuum of <10−6 Pa40. The growth conditions we used for γ-Al2O3 were in the range of the report. In Si(111)/amo-Al2O3/Fe3O4, the amo-Al2O3 was grown at room temperature under a vacuum of <3 × 10−6 Pa. Then, the Fe3O4 film was formed by reactive deposition at 300 °C under a O2 atmosphere of 4.0 × 10−4 Pa41. All the samples were fabricated under the same growth conditions to investigate the dependence of the quality of Fe3O4 films on the buffer layer.
Measurements
The epitaxial growth and crystal structure were confirmed by RHEED, XRD (Rigaku SmartLab (9 kW)), and TEM (FEI Titan3 G2 60-300). Cross-sectional samples for TEM were prepared by using conventional mechanical polishing and dimpling techniques42. The magnetic properties of Fe3O4 were measured by vibrating sample magnetometer (VSM) and the electrical properties were measured by direct current (DC) measurements.
References
Jedema, F. J., Heersche, H. B., Filip, A. T., Baselmans, J. J. A. & van Wees, B. J. Electrical detection of spin precession in a metallic mesoscopic spin valvex. Nature 416, 713–716 (2002).
van’t Erve, O. M. J. et al. Electrical injection and detection of spin-polarized carriers in silicon in a lateral transport geometry. Appl. Phys. Lett. 91, 212109 (2007).
Dash, S. J., Sharma, S., Patel, R. S., de Jong, M. P. & Jansen, R. Electrical creation of spin polarization in silicon at room temperature. Nature 462, 491–494 (2009).
Datta, S. & Das, B. Electronic analog of the electro-optic modulator. Appl. Phys. Lett. 56, 665 (1990).
Sugahara, S. & Tanaka, M. A spin metal–oxide–semiconductor field-effect transistor using half-metallic-ferromagnet contacts for the source and drain. Appl. Phys. Lett. 84, 2307 (2004).
Sasaki, T. et al. Electrical Spin Injection into Silicon Using MgO Tunnel Barrier. Appl. Phys. Exp. 2, 053003 (2009).
Johnson, M. & Silsbee, R. H. Interfacial charge-spin coupling: Injection and detection of spin magnetization in metals. Phys. Rev. Lett. 55, 1790 (1985).
Sasaki, T. et al. Spin Transport in Nondegenerate Si with a Spin MOSFET Structure at Room Temperature. Phys. Rev. Applied 2, 034005 (2014).
Ohishi, M. et al. Spin Injection into a Graphene Thin Film at Room Temperature. Jpn. J. Appl. Phys. 46, L605–L607 (2007).
Shiraishi, M. et al. Robustness of Spin Polarization in Graphene-Based Spin Valves. Adv. Funct. Mater. 19, 3711 (2009).
Bibes, M. & Barthélémy, A. Oxide spintronics. IEEE Trans. Electron Devices 54, 1003 (2007).
Yanagihara, H., Utsumi, Y., Niizeki, T., Inoue, J. & Kita, E. Perpendicular magnetic anisotropy in epitaxially strained cobalt-ferrite (001) thin films. J. Appl. Phys. 115, 17A719 (2014).
Takaobushi, J., Kanki, T., Kawai, T. & Tanaka, H. Preparation of ferroelectric field effect transistor based on sustainable strongly correlated (Fe,Zn)3O4 oxide semiconductor and their electrical transport properties. Appl. Phys. Lett. 98, 102506 (2011).
Niizeki, T. et al. Observation of longitudinal spin-Seebeck effect in cobalt-ferrite epitaxial thin films. AIP Advances 5, 053603 (2015).
Yanase, A. & Shiratori, K. Band Structure in the High Temperature Phase of Fe3O4. J. Phys. Soc. Jpn. 53, 312 (1984).
Ramos, A. V. et al. Room temperature spin filtering in epitaxial cobalt-ferrite tunnel barriers. Appl. Phys. Lett. 91, 122107 (2007).
Santos, T. S. & Moodera, J. S. Observation of spin filtering with a ferromagnetic EuO tunnel barrier. Phys. Rev. B 69, 241203 (2004).
Takahashi, Y. K. et al. High spin-filter efficiency in a Co ferrite fabricated by a thermal oxidation. Appl. Phys. Lett. 96, 072512 (2010).
Li, P. et al. Ultrathin Epitaxial Ferromagnetic γ-Fe2O3 Layer as High Efficiency Spin Filtering Materials for Spintronics Device Based on Semiconductors. Adv. Funct. Mater. 26, 5679–5689 (2016).
Li, P. et al. Spin Filtering in Epitaxial Spinel Films with Nanoscale Phase Separation. ACS Nano 11, 5011–5019 (2017).
Ziese, M., Köhler, U., Bollero, A., Höhne, R. & Esquinazi, P. Schottky barrier and spin polarization at the Fe3O4-Nb:SrTiO3 interface. Phys. Rev. B 71, 180406R (2005).
Watanabe, H., Fujita, K. & Ichikawa, M. Thermal decomposition of ultrathin oxide layers on Si(111) surfaces mediated by surface Si transport. Appl. Phys. Lett. 70, 1095 (1997).
Senn, M. S., Wright, J. P. & Attfield, J. P. Charge order and three-site distortions in the Verwey structure of magnetite. Nature 481, 173–176 (2012).
Mi, W., Guo, Z., Wang, Q., Yang, Y. & Bai, H. Charge ordering in reactive sputtered (100) and (111) oriented epitaxial Fe3O4 films. Scripta Materialia 68, 972–975 (2013).
Liu, X., Yin, L. & Mi, W. Biaxial strain effect induced electronic structure alternation and trimeron recombination in Fe3O4. Sci. Rep. 7, 43403 (2017).
Dedkov, Y. S., Rüdiger, U. & Güntherodt, G. Evidence for the half-metallic ferromagnetic state of Fe3O4 by spin-resolved photoelectron spectroscopy. Phys. Rev. B 65, 064417 (2002).
Merckling, C. et al. Growth of crystalline γ-Al2O3 on Si by molecular beam epitaxy: Influence of the substrate orientation. J. Appl. Phys. 102, 024101 (2007).
Jung, Y. C., Miura, H. & Ishida, M. Formation of Very Thin Epitaxial Al2O3 Pre-layer with Very Smooth Surface on Si (111) Using a Protective Oxide Layer. Jpn. J. Appl. Phys. 38, 2333 (1999).
Merckling, C. et al. Epitaxial growth and relaxation of γ-Al2O3 on silicon. Thin Solid Films 515, 6479–6483 (2007).
Park, C. et al. Interfacial composition and microstructure of Fe3O4 magnetic tunnel junctions. IEEE Trans. Magn. 39, 2806 (2003).
Shirsath, S. E., Liu, X., Yasukawa, Y., Li, S. & Morisako, A. Switching of magnetic easy-axis using crystal orientation for large perpendicular coercivity in CoFe2O4 thin film. Sci. Rep. 6, 30074 (2016).
Bachelet, R. et al. Functional spinel oxide heterostructures on silicon. Cryst. Eng. Comm. 16, 10741–10745 (2014).
Margulies, D. T. et al. Origin of the Anomalous Magnetic Behavior in Single Crystal Fe3O4 Films. Phys. Rev. Lett. 79, 5162 (1997).
Mi, W. B., Shen, J. J., Jiang, E. Y. & Bai, H. L. Microstructure, magnetic and magneto-transport properties of polycrystalline Fe3O4 films. Acta Materialia 55, 1919–1926 (2007).
Eerenstein, W., Palsra, T. T., Hibma, T. & Celotto, S. Origin of the increased resistivity in epitaxial Fe3O4 films. Phys. Rev. B 66, 201101(R) (2002).
Verwey, E. J. W. Electronic Conduction of Magnetite (Fe3O4) and its Transition Point at Low Temperatures. Nature 144, 327–328 (1939).
Kado, T., Saito, H. & Ando, K. Room-temperature magnetoresistance in magnetic tunnel junctions with Fe3O4 electrode. J. Appl. Phys. 101, 09J511 (2007).
Tsuda, N. et al. Electronic Conduction in Oxide (Springer, Berlin-Heidelberg, 2000)
Kern, W. & Puotinen, D. Cleaning solutions based on hydrogen peroxide for use in silicon semiconductor technology. RCA Review 31, 187–206 (1970).
Wu, S. Y. et al. High-quality thin single-crystal γ-Al2O3 films grown on Si(111). Appl. Phys. Lett. 87, 091908 (2005).
Nagahama, T. et al. Magnetic properties of epitaxial Fe3O4 films with various crystal orientations and tunnel magnetoresistance effect at room temperature. Appl. Phys. Lett. 105, 102410 (2014).
Lari, L., Lea, S., Feeser, C., Wessels, B. W. & Lazarov, V. K. Ferromagnetic InMnSb multi-phase films study by aberration-corrected (scanning) transmission electron microscopy. J. Appl. Phys. 111, 07C311 (2012).
Acknowledgements
We would like to express our gratitude to Prof. Yamamoto’s group, Prof. Tokeshi’s group for their cooperation in the microfabrication. We especially thank Prof. Sakaguchi for his advice and helpful suggestions. This work was partly supported by JSPS KAKENHI Grant Numbers 15H05702, 23686006, the Collaborative Research Program of Institute for Chemical Research, Kyoto University (grant 2016-57), and the OPEN FACILITY, Hokkaido University Sousei Hall and Nanotechnology Collaborative Research in Hokkaido University. TH and AH are supported by EU-FP7 HARFIR project (NMP3-SL-2013-604398).
Author information
Authors and Affiliations
Contributions
N.T. and T.N. conceived and designed the experiments using the help of other authors. N.T., T.N., Y.Y., T.Y. and T.S. performed the sample preparation, XRD measurements, magnetic and electrical measurements. T.H., A.H., N.T. and T.N. performed TEM observations. All the authors contributed to analysing and interpreting the data, and to writing the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takahashi, N., Huminiuc, T., Yamamoto, Y. et al. Fabrication of Epitaxial Fe3O4 Film on a Si(111) Substrate. Sci Rep 7, 7009 (2017). https://doi.org/10.1038/s41598-017-07104-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07104-z
This article is cited by
-
Observation of superconductivity in structure-selected Ti2O3 thin films
NPG Asia Materials (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.