Abstract
A tubular nervous system is present in the deuterostome groups Chordata (cephalochordates, tunicates, vertebrates) and in the non-chordate Enteropneusta. However, the worm-shaped enteropneusts possess a less complex nervous system featuring only a short hollow neural tube, whereby homology to its chordate counterpart remains elusive. Since the majority of data on enteropneusts stem from the harrimaniid Saccoglossus kowalevskii, putative interspecific variations remain undetected resulting in an unreliable ground pattern that impedes homology assessments. In order to complement the missing data from another enteropneust family, we investigated expression of key neuronal patterning genes in the ptychoderid Balanoglossus misakiensis. The collar cord of B. misakiensis shows anterior Six3/6 and posterior Otx + Engrailed expression, in a region corresponding to the chordate brain. Neuronal Nk2.1/Nk2.2 expression is absent. Interestingly, we found median Dlx and lateral Pax6 expression domains, i.e., a condition that is reversed compared to chordates. Comparative analyses reveal that adult nervous system patterning is highly conserved among the enteropneust families Harrimaniidae, Spengelidae and Ptychoderidae. BmiDlx and BmiPax6 have no corresponding expression domains in the chordate brain, which may be indicative of independent acquisition of a tubular nervous system in Enteropneusta and Chordata.
Similar content being viewed by others
Introduction
The evolution of the nervous system in Bilateria and Deuterostomia in particular has been hotly debated for decades1,2,3,4,5,6. In this debate, enteropneust hemichordates (or acorn worms) have occupied a pivotal role. Enteropneusts and echinoderms are two groups of non-chordate deuterostomes, distantly related to vertebrates7. In contrast to echinoderms that feature a highly derived body organization (pentamery, oral-aboral axis), the worm-shaped enteropneusts have retained putative ancestral bilaterian features such as bilateral symmetry, nephridia, coelomic cavities and a biphasic life style8. Enteropneusts share some characteristics with chordates, such as gill slits and, at least partly, a tubular nervous system. For these reasons, enteropneusts are ideal candidates to unravel nervous system evolution in Deuterostomia. The majority of enteropneust species belong to one of the three main families Harrimaniidae (e.g. Saccoglossus kowalevskii), Spengelidae (e.g. Schizocardium californicum) and Ptychoderidae (e.g. Balanoglossus misakiensis, Ptychodera flava)9. Harrimaniid species develop directly into the juvenile worm, whereas spengelid and ptychoderid enteropneusts develop indirectly via a specific larval type, the tornaria. Morphologically, the nervous system of enteropneusts is a basiepidermal plexus with additional condensed regions10. These comprise the proboscis stem, proboscis nerve ring, a dorsal nerve cord along the collar and trunk region, and a ventral nerve cord in the trunk connected to the dorsal nerve cord by a prebranchial nerve ring (Fig. 1A’)10, 11. The dorsal nerve cord within the collar region, the ‘collar cord’, is a subepidermal tubular nerve cord that is often thought to be reminiscent of the chordate neural tube and, like the latter, forms by neurulation12, 13. The collar cord is subdivided into a dorsal sheath of different neuronal cell types surrounding a central neural canal and a ventral neuropil13, 14. Although these morphological features would support homology of the chordate neural tube and the collar cord of enteropneusts, it remains unclear as to which part of the chordate neural tube the collar cord might correspond. Moreover, the results from gene expression analyses are somewhat contradictory. The nervous system of many bilaterians is patterned similarly from anterior to posterior by a number of specific transcription factors (see ref. 3 for review). For instance, Six3/6, Otx and Engrailed regionalize parts of the brain in bilaterians, while Hox genes pattern the postcerebral nerve cord3. Anteroposterior patterning of these transcription factors has been studied in the enteropneust S. kowalevskii and is similar to that in chordates3, 15, 16, yet the expression domains in S. kowalevskii are circumferential in the entire ectoderm and not restricted to the neuroectoderm as in chordates15, 16. The spengelid S. californicum exhibits similar expression domains of these transcription factors17. To complicate things further, Miyamoto and Wada18 showed that genes specifying the chordate neural plate border (e.g., SoxE, and Bmp2/4) have corresponding expression domains in the neural plate of the collar cord of the enteropneust Balanoglossus simodensis 18. Concluding so far, no unequivocal homology statement can be made at present concerning the collar cord and the chordate neural tube.
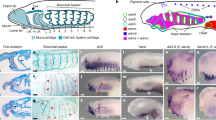
Establishment of the adult nervous system. Gene expression of BmiElav in the metamorphosing larva and juvenile of B. misakiensis. (A–E) Metamorphosing larva. (A’–D’) Juvenile. (A) Schematic illustration of BmiElav expression. BmiElav is expressed in the proboscis nerve ring, the developing dorsal nerve cord including the neural plate in the collar (B,D) and in the ventral nerve cord (C,D). Note that the expression is interrupted at the level of the telotroch. E Detail of the lateral trunk showing scattered neurons (arrowheads). (A’) Schematic illustration of BmiElav expression in juveniles. Note that the collar cord is neurulated. B’ Surface view from ventral, dorsal and lateral right (from top to bottom) showing strong expression in the proboscis nerve ring, proboscis plexus, and in the dorsal as well as ventral nerve cord. BmiElav expression is discontinuous in the middle of the dorsal nerve cord in the trunk region. (C’) Micrograph of cleared juvenile. (D’) Detail showing Elav+ cells in the subepidermal collar cord. cc = collar cord, dnc = dorsal nerve cord, np = neural plate, pn = peribranchial nerve ring, pr = proboscis nerve ring, pp = proboscis plexus, tt = telotroch, vnc = ventral nerve cord. (B) dorsal view. (C) ventral view. (D) view from lateral right.
Most of the gene expression data available for enteropneusts have been obtained from S. kowalevskii, a harrimaniid species with direct development. This data has been supplemented recently by a body patterning study of the spengelid S. californicum 17. However, a reliable ground pattern of neuronal patterning for Enteropneusta can only be reconstructed if data from members of many different enteropneust families are compared. Accordingly, a comparable developmental study of neuronal patterning in a ptychoderid enteropneust is of prime importance.
Here, we studied the expression domains of neuronal patterning genes in the indirectly developing ptychoderid Balanoglossus misakiensis, in order to provide the missing data. We focused on the expression patterns in the developing collar cord of anteroposterior (Six3/6, Otx, and Engrailed) as well as putative mediolateral patterning genes (Pax6, Dlx, Nk2.1, Nk2.2). The latter have been reported to form abutting domains of Nk and Pax genes in the annelid ventral nerve cord and in the vertebrate dorsal neural tube2, 19. In each of these progenitor domains specific neuronal cell types are formed (see Fig. 7 in ref. 19). For instance, serotonin-positive (+) neurons are exclusively restricted to the median Nk2.1 domain in the brain and to the median Nk2.2 in the spinal cord (see Figs 2 + 3 in ref. 2). Pax6 forms two bilaterally symmetric, intermediate progenitor domains and Dlx two lateral domains. Given the presence of a corresponding spatial organization of the vertebrate neural tube and the nerve cord in annelids, a similarly patterned nervous system has been proposed in the last common ancestor of Bilateria3. Therefore, we assess the presence of putative mediolateral patterning in the collar cord of B. misakiensis. The adult nervous system of B. misakiensis, including the collar cord, becomes morphologically distinct in early settled juveniles, indicating that neurogenic patterning of the collar cord starts in metamorphosing larvae20. In contrast, the larval nervous system (apical organ and neurite bundles of the ciliary bands) is independent of the adult nervous system and degrades during metamorphosis and settlement9, 18, 21. Therefore, we did not study the larval stages, but rather focus on the expression patterns in metamorphosing animals and early settled juveniles.
This study describes the first gene expression data for the ptychoderid B. misakiensis, and will enable the establishment of a reliable ground pattern for Enteropneusta. The 2nd objective of this study is then to compare the collar cord with the chordate neural tube, in order to elucidate the evolution of tubular nervous systems in Deuterostomia.
Results
Neuronal differentiation of the adult nervous system
In order to obtain an overview of the developing adult nervous system of Balanoglossus misakiensis, we first examined the expression of Elav, an RNA-binding protein that marks differentiating neurons22,23,24. BmiElav is expressed in the epidermis of the metamorphosing larva (Agassiz stage) of B. misakiensis as a stripe along the entire dorsal midline (except at the level of the telotroch) and extends circumferentially to the posterior base of the proboscis (Fig. 1A,B,D). In addition, BmiElav expression runs along the ventral midline of the trunk region with a gap in the region of the telotroch (Fig. 1A,C,D). BmiElav thus includes the region of the future dorsal and ventral nerve cords. Higher magnification of the perianal field reveals additional scattered BmiElav+ cells laterally outside the nerve cords (Fig. 1E).
In juvenile B. misakiensis, Elav+ cells are abundant in all condensed parts of the nervous system20, including the proboscis plexus at the base of the proboscis region and the proboscis nerve ring (Fig. 1A’B’). At the level of the collar region, BmiElav+ cells locate to the subepidermal collar cord (Fig. 1D’). BmiElav+ cells are also present in the prebranchial nerve ring, as well as in the dorsal and ventral nerve cords in the trunk region (Fig. 1B’,C’). The BmiElav signal is interrupted in the dorsal nerve cord at the former position of the telotroch.
Gene expression of anteroposterior patterning genes
We studied the expression of selected axial patterning genes to understand the interspecific variation of enteropneust collar cord development, and to compare with the expression pattern of the chordate neural tube.
The transcription factor BmiSix3/6 is strongly expressed throughout the entire ectoderm of the proboscis region and extends into the anterior rim of the collar ectoderm in metamorphosing larvae (Fig. 2A,B) and juvenile worms (Fig. 2C,D).
Anteroposterior patterning genes allocate the collar cord of B. misakiensis to the chordate brain region. Anterior is to the top left. (A–D) BmiSix3/6 is expressed throughout the ectoderm of the proboscis region and the anterior collar. (E–H) BmiOtx is expressed circumferentially in the posterior proboscis ectoderm and in the ectoderm of the collar region. (E) Dorsal view showing an additional domain in the pharyngeal endoderm (arrowheads). (G) BmiOtx is strongly expressed in the preoral ciliary organ (arrowhead). Section plane of inset indicated by dashed line. Inset shows Otx expression in the ciliary organ of the proboscis in a cross section of the posterior proboscis. (I–L) BmiEn is expressed in a narrow ring in the ectoderm of the posterior end of the collar region with an interruption on the dorsal side. co = collar. (A,C,E,G,I,K): dorsal views. (B,D,F,H,J,L): ventral views.
BmiOtx is expressed in the metamorphosing larva in the ventral area of the proboscis nerve ring (Fig. 2F) and in a distinct annular domain, encircling the anterior and middle collar region (Fig. 2E,F, Fig. S1B). There is an additional domain in the anterior pharyngeal region, which is the developing stomochord (Fig. 2E arrowheads). This is a non-neural endodermal domain where the skeletal horns of the cartilaginous proboscis skeleton will later form. In the juvenile enteropneust BmiOtx is expressed in the ventral and ventrolateral area of the proboscis nerve ring (Fig. 2G,H). The expression forms a U-shaped domain at the position where the sensory pre-oral ciliary organ develops (Fig. 2G inset). BmiOtx is also weakly expressed throughout the ectoderm of the collar region (Fig. 2F,H).
BmiEn(grailed) is expressed in a circumferential ring at the very posterior margin of the collar region in metamorphosing larvae (Fig. 2I,J and Fig. S1C). The signal is ectodermal and interrupted at the level of the dorsal midline. The juvenile enteropneust shows a similar expression pattern at the posterior margin of the collar region (Fig. 2K,L). The ring of BmiEn expression shows a gap on the dorsal side, as in the metamorphosing larva.
In summary, the collar cord, which is part of the enteropneust collar region (mesosome), borders anteriorly the expression domain of BmiSix3/6, lies within the BmiOtx-expression region, and is posteriorly delineated by a line of BmiEn expression.
Gene expression of mediolateral patterning genes
In metamorphosing larvae, BmiPax6 is strongly expressed in the proboscis nerve ring at the base of the proboscis and in an additional circular pattern in the ectoderm of the collar region (Fig. 3A,B). Between both circumferential domains, BmiPax6 is also expressed in two parallel, longitudinal domains of the collar (Fig. 3A, dashed area). This area of the neural plate will later neurulate to form the subepidermal collar cord13. In juveniles, BmiPax6 still shows a strong signal in the proboscis nerve ring. The circular domain in the posterior collar region becomes fainter in early juveniles (Fig. 3C inset) and is lost in older juveniles (Fig. 3C,D). No collar cord BmiPax6 expression domains are present in juveniles.
Expression domains of mediolateral patterning genes and serotonin-LIR in B. misakiensis. (A,B) BmiPax6 is expressed in the proboscis nerve ring and in a second circumferential domain in the collar ectoderm. Additionally, BmiPax6 forms paired longitudinal domains in the neural plate (dashed area) of the developing larva. In juveniles, the expression in the collar ectoderm fades (inset) and only the proboscis nerve ring shows strong signal of BmiPax6 (C,D). (E–H) BmiDlx is expressed as a median stripe in the collar and dorsal cord (arrowheads) with an interruption at the level of the telotroch (E). The strong staining in the protocoel is unspecific (see also Fig. S1). (I,J) Expression of BmiNk2.1 in the metamorphosing larva. BmiNk2.1 is strongly expressed in the stomochord (double arrowhead), in the ventrolateral ectoderm at the base of the proboscis (open arrowhead), in the posterior pharynx (black arrowhead), and weakly in the hindgut (white arrowhead). The degrading apical organ shows a faint signal (asterisk). (K–N) Expression of BmiNk2.2. (K) Surface view from ventral showing bilateral domains in the mid-pharynx region. (L) Lateral view from left. Inset shows a cross section of the collar region with the ventrolateral domain of BmiNk2.2 in the pharyngeal endoderm. In juveniles the expression domain of Nk2.2 is extended throughout the entire endoderm (M,N). Note that there is no ectodermal or neuronal expression domain of BmiNk2.2. (O–Q) Serotonin-LIR in the juvenile. (O) Overview. (P) Detail of the collar region as indicated in G. Partial Z-projection focussing on the collar cord (dashed area). (Q) Virtual cross section of the collar cord as indicated in H. Note that 5-HT + somata are absent from the collar cord (dashed area). Only two ventrolateral 5-HT + neurite bundles pass the ventral area of neurites. cc = collar cord, co = collar, ep = epidermis, g s = sill slit, lm = longitudinal muscles, nc = neural canal, pr = proboscis, sn = serotonin-LIR neuron, snb = serotonin-LIR neurite bundle, tr = trunk. (A,C,E,G,I,K,M): dorsal views, (B,J,L,N): lateral views left, (D,F,H): ventral views.
Expression of BmiDlx is present in the proboscis nerve ring and along the dorsal nerve cord with an interruption at the level of the telotroch in the metamorphosing larva (Fig. 3E,F). In juveniles of B. misakiensis, Dlx expression shows a faint signal in the ventral and ventrolateral portion of the proboscis nerve ring and in the dorsal nerve cord including the collar cord (Fig. 3G,H). Our data show that BmiDlx is expressed in the collar cord and in the dorsal nerve cord and forms a single median domain.
BmiNkx2.1 has four distinct expression domains in the metamorphosing larva (Fig. 3I,J). At this stage, the apical organ is degrading20 and BmiNkx2.1 is weakly expressed in this ectodermal region (Fig. 3I,J asterisk). BmiNkx2.1 shows a strong expression domain in the ventral ectoderm at the base of the proboscis region (Fig. 3J unfilled arrowhead). Further strong domains are within the developing endodermal stomochord (Fig. 3I, double arrowhead) and medially in the posterior pharyngeal endoderm (Fig. 3I,J black arrowhead). A fifth signal is present in the hindgut (Fig. 3J, white arrowhead).
The transcription factor BmiNkx2.2 is strongly expressed in the lateral and dorsal portions of the anterior pharyngeal endoderm in the metamorphosing larva (Fig. 3K, inset, L). In the juvenile worm the BmiNkx2.2 domain has extended posteriorly and is present throughout the endoderm, but absent from the hindgut (Fig. 3M,N). Thus, there is no expression domain of Nk2 genes in the collar cord or the trunk nerve cords in B. misakiensis.
We additionally checked the distribution of serotonin-LIR neuronal components within the collar cord, because these neurons are restricted to the Nkx2.1/2.2 domains in annelids and chordates. The serotonin-like immunoreactivity (LIR) nervous system of B. misakiensis has been previously described20, but the precise position of serotonin-LIR neurites within the collar cord has remained unknown. In the juvenile enteropneust serotonin-LIR neurons are present in the epidermis throughout all three body regions, with higher concentrations of somata in the proboscis and collar epidermis (Fig. 3O). Serotonin-LIR neurites form a basiepidermal nerve plexus in the proboscis and collar region. In the trunk region the serotonin-LIR neurites are condensed within the dorsal and ventral midline, in regions that constitute the nerve cords20. The neurulated collar cord is positioned between the dorsal mesenteries of the paired mesocoel and is composed of a dorsal sheath of cells and a ventral area of neurites (Fig. 3P,Q). The dorsal sheath of cells of the collar cord is devoid of serotonin-LIR somata (Fig. 3Q). Only two small ventrolateral serotonin-LIR neurite bundles pass through the whole neurite bundle of the collar cord. These serotonin-LIR lateral neurite bundles run adjacent to a pair of longitudinal muscle bundles, which run within the perihaemal diverticula that flank the collar cord ventrolaterally (Fig. 3Q).
Discussion
We investigated the expression domains of several genes involved in axial as well as mediolateral patterning of the nervous system of the indirect developing enteropneust Balanoglossus misakiensis. By using the pan-neuronal marker Elav for differentiating neurons22,23,24, we found that the condensed parts of the adult nervous system (proboscis plexus and ring, neural plate, ventral and dorsal nerve cords) are already prepatterned by Elav in metamorphosing larvae prior to settlement. In settled juveniles of B. misakiensis, neurulation results in a subepidermal tubular cord, as reported in other enteropneust species12, 13, 18.
Gene expression patterning of the collar cord in Enteropneusta
The transcription factors Six3/6, Otx and Engrailed play a conserved role in anteroposterior patterning and regionalization of the nervous system in chordates and in many other bilaterians3. Six3/6 patterns the anteromost region of the nervous system in numerous animals3, 25, 26. We found that in B. misakiensis the expression pattern of Six3/6 is likewise at the anteriormost region of the animal, while Otx and Engrailed form circular epidermal domains around the collar and the posterior margin of the collar region, respectively (Fig. 4B’). These expression domains are spatially similar to what has been described in the spengelid Schizocardium californicum 17 as well as the harrimaniid enteropneust Saccoglossus kowalevskii (Fig. 4C’15, 16,) Accordingly, we suggest a conserved role of neuronal and body region patterning for Six3/6, Otx and Engrailed in Enteropneusta that is independent from their mode of development (direct vs. indirect, Fig. 4B–C”). It is a plesiomorphic feature for Enteropneusta that has been inherited from a common bilaterian ancestor3, 15, 25.
Comparison of axial patterning genes in the neural plate of diverse deuterostomian taxa with focus on different developing modes in enteropneusts. Neuronal patterning in enteropneusts is highly conserved and independent of the mode of development. The ancestral condition of mediolateral patterning for Deuterostomia remains elusive. See text for discussion. (A) Expression domains of the hypothetical enteropneust ancestor. (B–B”) Selected developmental stages of B. misakiensis. (B) Metschnikoff larval stage. (B’) Metamorphosing Agassiz larval stage (this study). (B”) Juvenile worm. (C–C”) Selected developmental stages of S. kowalevskii. (C) Torpedo embryo stage. (C’) 1-gill slit hatchling (after data from refs 15, 16). (C”) Juvenile worm. (D) Expression domains in the neural plate of Branchiostoma floridae (after data from refs 34–36, 38, 39, 55). (E) Expression domains in the neural plate of the ascidian Ciona intestinalis (after data from refs 34, 40, 56–58). F Expression domains in the neural plate of the vertebrate Mus musculus. Scheme modified after2 (after data from refs 3, 16, 31, 37). (G) Expression domains of the hypothetical chordate ancestor. Mediolateral patterning of the postcerebral nerve cord is ambiguous. Note, all expression patterns are symmetrical, but are shown on one side only for clarity. b = brain, cv = cerebral vesicle, fb = forebrain, g = ganglion, hb = hindbrain, mb = midbrain, n = neck, nc = nerve cord, sc = spinal cord, sv = sensory vesicle.
Next, we examined the expression pattern of Dlx, Pax6 and Nk2.1/2.2. These transcription factors form mediolateral neurogenic domains in the neural tube of mouse, fruit fly and in the annelid Platynereis dumerilii 19, 27. Our analysis of B. misakiensis shows that BmiDlx is expressed in a narrow longitudinal stripe in the dorsal midline of the neural plate (Fig. 4B’). A similar pattern has been reported for Dlx in S. kowalevskii (Fig. 4C’16, 28,), S. californicum 17 and Balanoglossus simodensis 18, suggesting a conserved role of this transcription factor in neurogenesis in Enteropneusta. We then examined the expression pattern of BmiPax6 and found that it forms two lateral stripes along the neural plate of B. misakiensis (Fig. 4B’). We show that BmiPax6 is only expressed for a short period in the neural plate during metamorphosis and is entirely absent in early juveniles (2 d post-settlement) (Fig. 3C,D). This is the only report of a distinct expression pattern of Pax6 in the neural plate of an enteropneust species. In a comparable developmental stage of S. kowalevskii (1-gill-slit stage), Pax6 is expressed in corresponding circular domains (Fig. 4C’15, 16,), yet details from the neural plate are unknown. In B. simodensis and S. californicum, Pax6 expression was not detected in the neural plate17, 18. Thus, Pax6 expression in the collar cord might be a species-specific acquisition of B. misakiensis and not part of the enteropneust ground pattern (Fig. 4A).
Expression pattern analysis of the median progenitor markers Nk2.1 and Nk2.2 revealed that there are no expression domains for either of the BmiNk2 genes in the developing neural plate nor, later, in the collar cord in B. misakiensis. Instead, the main domains of Nk2.1 and Nk2.2 are detected in the pharyngeal endoderm (Fig. 3I–N). In the direct developer S. kowalevskii a similar endodermal expression of both genes has been reported previously15, 28 and in adult Ptychodera flava Nk2.1 also shows similar expression domains29, suggesting a more general role in endoderm specification of these genes in enteropneusts30. Moreover, serotonergic neurons in vertebrates are usually restricted to the progenitor domains of Nk2.1/2.2 31. Our data show that there is no median Nk2.2 domain in the collar cord in B. misakiensis. Concordantly, no serotonin-LIR somata are present in the collar cord of B. misakiensis. In fact, Nk2.2 does not co-localise with serotonin-LIR neurons in B. misakiensis Serotonin-LIR neurons comprise bipolar neurons throughout the epidermis of B. misakiensis, S. kowalevskii 20 as well as P. flava 14.
Concluding so far, with the exception of Pax6, the expression patterns of the genes investigated in this study are highly congruent among the enteropneusts S. kowalevskii, S. californicum, B. simodensis, P. flava as well as B. misakiensis, and thus similar functions appear most likely. The data further reveals that neuronal patterning of the adult nervous system in the different families of Enteropneusta (Harrimaniidae, Spengelidae and Ptychoderidae) is not affected by different developmental modes. This conclusion is also supported by morphogenetic data of the developing adult nervous system in enteropneusts20. On this basis, we propose that a similar collar cord patterning was present in the last common ancestor of Enteropneusta (Fig. 4A).
Comparative aspects of neural tube patterning among deuterostomes
Morphological similarities between the tubular collar cord and the chordate neural tube have not gone unnoticed, dating back more than 130 years32. Therefore, we compare the gene expression patterns of the transcription factors studied here among different deuterostomes, and discuss the evolutionary implications.
Chordata comprises three major taxa, Cephalochordata, Tunicata and Vertebrata, of which the latter two form the monophyletic Olfactores7, 33. All three groups share corresponding expression domains of the transcription factors Six3/6, Otx and Engrailed (Fig. 4D–F), which are restricted to the anterior portion of the neural plate, i.e., the future brain region3, 34. Thereby, coexpression of Otx and En mark the future midbrain-hindbrain boundary (MHB) in vertebrates and the posterior margin of the future sensory vesicle (brain) in the ascidian Ciona intestinalis. In contrast, the coexpressing domain of Otx and En in amphioxus is located in the midlevel of the brain region, whereas a second expression domain of Six3/6 is present at the posterior end of the cerebral vesicle (Fig. 4D)3, 35. Moreover, all three groups show a median/ventral Nk2.1 domain and expression domains of Pax6 and Dlx in the brain region15, 19, 31, 36,37,38,39,40. Thus, the chordate ancestor likely had a similar brain patterned by these transcription factors (Fig. 4G)3.
Mediolateral patterning of the postcerebral part of the neural tube by Pax6, Dlx and Nk2.1/2.2 differs considerably between chordates and needs further attention. The specific arrangement of lateral Dlx, mediolateral Pax6 and median Nk2 domains has been reported from the vertebrate spinal cord and hindbrain levels (posterior to MHB) as well as from the annelid and insect ventral nerve cord (postcerebral)2, 3. The median column of Nk2.2 is an exception as its domain projects anteriorly throughout the midbrain region and is replaced by Nk2.1 in the vertebrate forebrain (Fig. 4F). However, ascidians share only a mediolateral Pax6 domain with vertebrates, while Dlx and Nk2.2 expression is absent from the postcerebral neural tube (Fig. 4E)3, 40, 41. Ascidians belong to Tunicata, a taxon of rapidly evolving animals with reduced genome size that have lost about 25 genes involved in developmental patterning including Gbx, Wnt1 and Nk2.2 34, 42, 43. Thus, the aberrant and missing expression domains as compared to vertebrates could be explained by secondary gene losses in Tunicata. In comparison, amphioxus does not appear to be rapidly evolving. Cephalochordates have retained all of the putative ancestral bilaterian homeobox genes34, 43 and amphioxus exhibits one of the most ancestral genomes among chordates, in parallel with a less derived morphology43. However, Pax6 and Dlx expression are absent from the nerve cord in amphioxus, instead the median Nk2.1 domain extends throughout the posterior neural plate (Fig. 4D)39. It should be mentioned that a median Nk2.1/2.2 domain, a mediolateral Pax6 as well as a lateral Dlx expression domain are very well present in amphioxus, yet these expression domains are located in the posterior region of the cerebral vesicle (Fig. 4D) and not in the postcerebral nervous system as in vertebrates (Fig. 4D,F) and the protostomes Platynereis dumerilii and Drosophila melanogaster 2, 19. Taken together, mediolateral expression domains of Pax6, Dlx and Nk2 genes in the postcerebral nerve cord differ considerably among chordates, making it difficult to suggest a complete ancestral ground pattern for Chordata (Fig. 4G).
The reconstructed enteropneust ground pattern (Fig. 4A) allows for a comparison of tubular nervous systems among deuterostomes and will contribute to understanding their evolution. In this context, echinoderms are not considered, because they possess a highly derived body plan (pentamery, oral-aboral axis) and ancestrally a non-tubular nervous system44.
Comparison of the expression domains of Six3/6, Otx and Engrailed leads to the suggestion that the collar cord in enteropneusts might correspond to a region of the chordate brain rather than to the postcerebral neural tube (Fig. 4A,G). This is also supported by comparative Hox gene expression in S. kowalevskii 3, 15. In Enteropneusta the neural plate is patterned medially by Dlx (refs 15, 16 and 18, this study) (Fig. 4A), whereas Dlx expression is restricted to the very lateral area of the brain in amphioxus and ascidians (Fig. 4D,E) with respect to the spinal cord in vertebrates (Fig. 4F). Accordingly, there is no corresponding mediolateral patterning present in the enteropneust nervous system, and compared to chordates the expression domains of Dlx and Pax6 are flipped in B. misakiensis (Fig. 4B’,D–F). These incongruent expression patterns might be explained by the fact that dorsoventral expression of Bmp and Chordin, which are responsible for the placement of the mediolateral patterning domains, are reversed in enteropneusts relative to chordates28. In S. kowalevskii the tubular collar cord develops from the Bmp-expressing side, whereas the dorsal neural tube of chordates and the ventral nerve cord of protostomes form at the Chordin-expressing side28, 45. Concordantly, markers of midline cells in the chordate neural tube such as Sim and Netrin are expressed in the ventral ectoderm in enteropneusts while lateral markers of the chordate neural tube such as Dlx are expressed in the dorsal ectoderm (ref. 28, this study). Thus, following the dorsoventral (D-V) inversion hypothesis46, 47 (but see ref. 48), the dorsal side of chordates (neural tube) corresponds to the ventral side of enteropneusts, yet the collar cord is positioned dorsally. Accordingly, the enteropneust collar cord and the chordate neural tube do not represent corresponding parts of related organisms, making a homology hypothesis debatable.
Conclusion
A complex mediolateral patterning of the postcerebral nervous system by Pax6, Dlx and Nk2.1/Nk2.2 has been reported in vertebrates and the protostomes Platynereis dumerilii and Drosophila melanogaster 2, 3, 19. However, comparison of their expression domains between enteropneusts and chordates (amphioxus, ascidians and vertebrates) does not suggest that a similarly patterned postcerebral nervous system was present in the last deuterostomian ancestor (Fig. 4). Moreover, the tubular collar cord of Enteropneusta shows no expression domains of Dlx or Pax6 that correspond to the chordate brain. The “flipped” domains of Dlx and Pax6 in enteropneusts are likely the result of an inverted BMP/Chordin expression compared to chordates28. Previous electrophysiological and morphological data also proposed limited resemblances between the collar cord and neural tube10, 49. Altogether, it should be considered that the collar cord might represent an independent acquisition of Enteropneusta and that tubular nervous systems evolved convergently within Deuterostomia.
Materials and Methods
Balanoglossus misakiensis (Kuwano, 1902)
Adult B. misakiensis were collected at a depth of 1 to 2 m at Sunset beach, Aomori-Bay, Asamushi, Aomori, Japan, in June 2012 and June 2014. Specimens were transported to the Research Center for Marine Biology Tohoku University in Asamushi and were kept in aquaria with running filtered seawater at ambient water temperature (24–26 °C) as previously described20, 50. Spawning, in vitro fertilization, and fixations were performed as described earlier20.
Immunolabelling and confocal laser scanning microscopy
Juveniles of B. misakiensis (2-gill-slit juvenile = 3 days post settlement) were fixed with 4% paraformaldehyde (PFA) in phosphate buffer (PBS). Specimens were processed using standard protocols as previously described20.
RNA extraction, transcriptome analysis and gene cloning
More than 1,000 larvae from developmental stages of B. misakiensis ranging from early hatched tornaria to three day old juvenile worms were fixed in RNAlater (Sigma). Total RNA was extracted from a mix of developmental stages using RNeasy Mini Kit from Qiagen. Extracted RNA was sent to Eurofins (Germany) for Illumina HiSeq. 2000 sequencing using paired-end read module resulting in reads of 100 bp length. Obtained reads were assembled to contigs using Trinity v2.0.1 software under standard parameters and the transcriptome was analysed for sequences of interest with sequence search in Geneious 6.1 (Biomatters, New Zealand). Primers were generated with Primer3 software to obtain fragments of Elav, Six3/6, Pax6, Dlx, Otx, Engrailed, Nk2.1 and Nk2.2 (for primer sequences and accession numbers see supplemental material), synthesized by Microsynth AG (Balgach, Switzerland) in order to sub-clone into pGemT Easy vector (Promega).
Phylogenetic analysis
Full protein sequences were aligned using MUSCLE and regions with low-quality alignments for the Elav phylogenetic analysis were trimmed by TrimAl 1.2 rev 59 using the option “automated1” for the trimming51. For the reconstruction of the phylogenetic relationship between analysed homeobox proteins, the alignment was not trimmed. ProtTest 2.452 analysis retrieved LG (+G+F) and JTT (+I+G+F) as best-fitting models for the amino substitution rates for Elav (Fig. S2) and the homeobox protein analyses (Fig. S3), respectively. The maximum likelihood trees of protein sequences were then generated with PhyML 3.053 with default parameters and selecting the suitable amino acid substitution models previously identified. The tree topology search operations were set to the nearest neighbor interchange and subtree pruning and regrafting methods to retrieve the best solution for the estimation of the tree topology. The support values were calculated using 100 non-parametric bootstrap replicates.
Probe synthesis and in situ hybridization
Chromogenic in situ hybridizations were performed on whole-mounts with a pierced protocoel (see Controls for more information) following the protocol from Röttinger and Martindale54 with minor adjustments for B. misakiensis. Metamorphosing larvae (Agassiz stage) and juveniles (2-gill-slit stage) were treated with 10 ng/µl Proteinase K (Roth) for 4 min at room temperature. Colour development was stopped by three washes in PTw (phosphate buffered saline + 0.1% Tween20) and postfixed with 4% PFA for 1 hour. Animals were transferred into 100% EtOH over night for clearing and mounted in 80% glycerol.
Controls
Controls with sense probes were conducted in order to identify unspecific binding and probe trapping during in situ hybridization. The protocoel within the proboscis region turned out to be a perfect trap for any probe (Fig. S1A). Perforation of the proboscis using a thin tungsten needle helped to solve this problem (Fig. S1D,E). However, probe trapping could not always be eliminated, which is why a blue protocoel persists in the in situ hybridizations of Dlx (Fig. 3E–H), Otx and Engrailed in juveniles (Fig. 2G,H,K,L).
References
Holland, N. D. Early central nervous system evolution: An era of skin brains? Nat Rev Neurosci. 4, 1–11 (2003).
Arendt, D., Denes, A. S., Jekely, G. & Tessmar-Raible, K. The evolution of nervous system centralization. Phil. Trans. R. Soc. B 363, 1523–1528 (2008).
Holland, L. Z. et al. Evolution of bilaterian central nervous systems: a single origin? EvoDevo 4, 27 (2013).
Arendt, D., Tosches, M. A. & Marlow, H. From nerve net to nerve ring, nerve cord and brain – evolution of the nervous system. Nature Rev. Neurosci. 17, 61–72 (2015).
Hejnol, A. & Lowe, C. J. Embracing the comparative approach: how robust phylogenies and broader developmental sampling impacts the understanding of nervous system evolution. Phil. Trans. R. Soc. B 370, 20150045 (2015).
Lowe, C. J., Clarke, D. N., Medeiros, D. M., Rokhsar, D. S. & Gerhart, J. The deuterostome context of chordate origins. Nature 520, 456–465 (2015).
Cannon, J. T. et al. Xenacoelomorpha is the sister group to Nephrozoa. Nature 530, 89–93 (2016).
Dunn, C. W., Giribet, G., Edgecombe, G. D. & Hejnol, A. Animal Phylogeny and Its Evolutionary Implications. Annu. Rev. Ecol. Evol. Syst. 45, 371–95 (2014).
Kaul-Strehlow, S. & Röttinger, E. Hemichordata in: Evolutionary developmental biology of invertebrates Vol. 6 (ed. Andreas Wanninger). Springer Verlag, Berlin (2015).
Bullock, T. H. The anatomical organization of the nervous system of Enteropneusta. Q. J. Microsc. Sci. 86, 55–112 (1946).
Knight-Jones, E. W. On the nervous system of Saccoglossus cambrensis (Enteropneusta). Philos. Trans. R. Soc. Lond. B 236, 315–354 (1952).
Morgan, T. H. The development of Balanoglossus. J. Morphol. 9, 1–86 (1894).
Kaul, S. & Stach, T. Ontogeny of the collar cord: Neurulation in the hemichordate Saccoglossus kowalevskii. J. Morph. 271, 1240–1259 (2010).
Nomaksteinsky, M. et al. Centralization of the Deuterostome Nervous System Predates Chordates. Curr. Biol. 19, 1264–1269 (2009).
Lowe, C. J. et al. Anteroposterior patterning in hemichordates and the origin of the chordate nervous system. Cell 113, 853–865 (2003).
Pani, A. et al. Ancient deuterostome origins of vertebrate brain signalling centres. Nature 438, 289–295 (2012).
Gonzalez, P., Uhlinger, K. R. & Lowe, C. J. The Adult Body Plan of Indirect Developing Hemichordates Develops by Adding a Hox-Patterned Trunk to an Anterior Larval Territory. Curr. Biol. 27, 87–95 (2017).
Miyamoto, N. & Wada, H. Hemichordate neurulation and the origin of the neural tube. Nat. Commun. 4, 2713 (2013).
Denes, A. S. et al. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in Bilateria. Cell 129, 277–288 (2007).
Kaul-Strehlow, S., Urata, M., Minokawa, T., Stach, T. & Wanninger, A. Neurogenesis in directly and indirectly developing enteropneusts: of nets and cords. Org. Divers. Evol. 15, 405–422, doi:10.1007/s13127-015-0201-2 (2015).
Nielsen, C. & Hay-Schmidt, A. Development of the enteropneust Ptychodera flava: ciliary bands and nervous system. J. Morphol. 268, 551–570 (2007).
Soller, M. & White, K. Elav. Curr. Biol. 14, R53 (2004).
Berger, C., Renner, S., Lüer, K. & Technau, G. M. The commonly used marker ELAV is transiently expressed in neuroblasts and glial cells in the Drosophila embryonic CNS. Dev. Dyn. 236, 3562–3568 (2007).
Pascale, A., Amadio, M. & Quattrone, A. Defining a neuron: neuronal ELAV proteins. Cell Mol. Life Sci. 65, 128–140 (2008).
Steinmetz, P. R. et al. Six3 demarcates the anteriormost developing brain region in bilaterian animals. Evodevo 1, 14 (2010).
Marlow, H. et al. Larval body patterning and apical organs are conserved in animal evolution. BMC Biology 12, 7 (2014).
Holland, L.Z. The origin and evolution of chordate nervous systems. Phil. Trans. R. Soc. B 370, 20150048, http://dx.doi.org/10.1098/rstb.2015.0048 (2015).
Lowe, C. J. et al. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 4, e291 (2006).
Takacs, C. N., Moy, V. N. & Peterson, K. J. Testing putative hemichordate homologues of the chordate dorsal nervous system and endostyle: expression of NK2.1 (TTF-1) in the acorn worm Ptychodera flava (Hemichordata, Ptychoderidae). Evol. & Dev. 4, 405–417 (2002).
Okkema, P. G., Ha, E., Haun, C., Chen, W. & Fire, A. The Caenorhabditis elegans NK-2 homeobox gene ceh-22 activates pharyngeal muscle gene expression in combination with pha-1 and is required for normal pharyngeal development. Development 124, 3965–3973 (1997).
Shimamura, K., Hartigan, D. J., Martinez, S., Puelles, L. & Rubenstein, J. L. Longitudinal organization of the anterior neural plate and neural tube. Development 121, 3923–3933 (1995).
Bateson, W. The early stages of the development of Balanoglossus (sp. incert.). Q. J. Microsc. Sci. 24, 208–236, pls 18–21 (1884).
Delsuc, F., Brinkmann, H., Chourrout, D. & Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965–968 (2006).
Castro, L. F. C., Rasmussen, S. L. K., Holland, P. W. H., Holland, N. D. & Holland, L. Z. A Gbx homeobox gene in amphioxus: Insights into ancestry of the ANTP class evolution of the midbrain/hindbrain boundary. Dev. Biol. 295, 40–51 (2006).
Kozmik, Z. et al. Pax-Six-Eya-Dach network during amphioxus development: Conservation in vitro but context specificity in vivo. Dev. Biol. 306, 143–159 (2007).
Holland, N. D., Panganiban, G., Henyey, E. L. & Holland, L. Z. Sequence and developmental expression of AmphiDll, an amphioxus Distal-less gene transcribed in the ectoderm, epidermis and nervous system: insights into evolution of craniate forebrain and neural crest. Development 122, 2911–2920 (1996).
Mastick, G. S., Davis, N. M., Andrew, G. L. & Easter, S. S. Jr Pax-6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development 124, 1985–1997 (1997).
Glardon, S., Holland, L. Z., Gehring, W. J. & Holland, N. D. Isolation and developmental expression of the amphioxus Pax-6 gene (AmphiPax-6): insights into eye and photoreceptor evolution. Development 125, 2701–2710 (1998).
Venkatesh, T. V., Holland, N. D., Holland, L. Z., Su, M.-T. & Bodmer, R. Sequence and developmental expression of amphioxus AmphiNk2-1: insights into the evolutionary origin of the vertebrate thyroid gland and forebrain. Dev. Genes Evol. 209, 254–259 (1999).
Irvine, S. Q., Cangiano, M. C., Millette, B. J. & Gutter, E. S. Non-overlapping Expression Patterns of the Clustered Dll-A/B Genes in the Ascidian Ciona intestinalis. J. Exp. Zool. Part B 308, 428–441 (2007).
Edvardsen, R. B. et al. Remodelling of the homeobox gene complement in the tunicate Oikopleura dioica. Curr. Biol. 15, R12–R13 (2005).
Holland, L. Z. Chordate roots of the vertebrate nervous system: expanding the molecular toolkit. Nat. Rev. 10, 736–746 (2009).
Holland, N. D. From genomes to morphology: a view from amphioxus. Acta Zool. 91, 81–86 (2010).
Cavey, M. J. & Markel, K. Echinoidea in: Microscopic Anatomy of Invertebrates Vol. 14 (Eds. Harrison, F. W., Chia, F. S.). Wiley Liss, New York (1994).
De Robertis, E. M. Evo-Devo: Variations on Ancestral Themes. Cell 132, 185–195 (2008).
Geoffrey St-Hilaire, E. (1822) Considérations générales sur la vertèbre. Mémoires, Mus. d’His. Nat. 9, 89–119 (2008).
Arendt, D. & Nübler-Jung, K. Comparison of early nerve cord development in insects and vertebrates. Development 126, 2309–2325 (1999).
Gerhart, J. Inversion of the chordate body axis: Are there alternatives? Proc. Nat. Acad. Sci. 97, 4445–4448 (2000).
Cameron, C. B. & Mackie, G. O. Conduction-pathways in the nervous system of Saccoglossus sp. (Enteropneusta). Canad. J. Zool. 74, 15–19 (1996).
Urata, M. & Yamaguchi, M. The Development of the Enteropneust Hemichordate Balanoglossus misakiensis Kuwano. Zool. Sci. 21, 533–540 (2004).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Phylogenetics 25, 1972–1973 (2009).
Abascal, F., Zardoya, R. & Posada, D. ProtTest: selection of best-fit models of protein evolution. Phylogenetics 21, 2104–2105 (2005).
Guindon, S. et al. New Algorithms and Methods to Estimate Maximum-Liklihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Röttinger, E. & Martindale, M. Q. Ventralization of an indirect developing hemichordate by NiCl suggests a conserved mechanism of dorso-ventral (D/V) patterning in Ambulacraria (hemichordates and echinoderms). Dev. Biol. 354, 173–190 (2011).
Holland, L. Z., Venkatesh, T. V., Gorlin, A., Bodmer, R. & Holland, N. D. Characterization and developmental expression of AmphiNk2-2, an NK2 class homeobox gene from amphioxus (Phylum Chordata; Subphylum Cephalochordata). Dev. Genes Evol. 208, 100–105 (1998).
Moret, F. et al. The dopamine-synthesizing cells in the swimming larva of the tunicate Ciona intestinalis are located only in the hypothalamus-related domain of the sensory vesicle. Europ. J. Neurosci. 21, 3043–3055 (2005).
Hudson, C. & Lemaire, P. Induction of anterior neural fates in the ascidian Ciona intestinalis. Mech. Dev. 100, 189–203 (2001).
Mazet, F., Hutt, J. A., Millard, J. & Shimeld, S. M. Pax gene expression in the developing central nervous system of Ciona intestinalis. Gene Exp. Patt. 3, 743–745 (2003).
Acknowledgements
We thank Takuya Minokawa from the Research Center for Marine Biology Tohoku University in Asamushi, Japan for, providing laboratory space and equipment. This study was funded by a Lise-Meitner grant from the Austrian Science Fond (FWF) to SK-S (M 1485-B19). The collection trip of SK-S to Japan was financially supported by a stipend from the Research Center for Marine Biology, Tohoku University, Japan. Tim Wollesen (University Vienna) is thanked for his help in basic molecular biological methods. SK-S thanks Thomas Eder and Thomas Rattei (both University of Vienna) for their kind assistance with Illumina transcriptome assembly. SK-S kindly acknowledges the valuable support of Eric Röttinger (University of Nice) and Grigory Genikhovich (University of Vienna) that allowed the establishment of a reliable in situ protocol for B. misakiensis. Patrick Steinmetz (Sars Center, Bergen) is thanked for critical comments and suggestions on earlier versions of this manuscript.
Author information
Authors and Affiliations
Contributions
S.K.-S. and A.W. designed the study. M.U. and S.K.-S. collected and cultured material. S.K.-S. conducted I.H.C. and cLSM analyses. S.K.-S. extracted RNA, assembled the transcriptome, cloned all gene sequences, and performed in situ hybridizations. D.P. aligned sequences, conducted phylogenetic analyses and built orthology trees of the genes. S.K.-S. wrote the manuscript with input from A.W. All authors read, provided input, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Accession codes: BmiEngrailed: MF409250, BmiElav: MF409251, BmiSix3/6: MF409252, BmiDlx: MF409253, BmiPax6: MF409254, BmiOtx: MF409255, BmiNkx2.1: MF409256, BmiNkx2.2: MF409257.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaul-Strehlow, S., Urata, M., Praher, D. et al. Neuronal patterning of the tubular collar cord is highly conserved among enteropneusts but dissimilar to the chordate neural tube. Sci Rep 7, 7003 (2017). https://doi.org/10.1038/s41598-017-07052-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07052-8
This article is cited by
-
Molecular evidence of anteroposterior patterning in adult echinoderms
Nature (2023)
-
Comparisons of cell proliferation and cell death from tornaria larva to juvenile worm in the hemichordate Schizocardium californicum
EvoDevo (2022)
-
On the larva and the zooid of the pterobranch Rhabdopleura recondita Beli, Cameron and Piraino, 2018 (Hemichordata, Graptolithina)
Marine Biodiversity (2019)
-
Expression of NK cluster genes in the onychophoran Euperipatoides rowelli: implications for the evolution of NK family genes in nephrozoans
EvoDevo (2018)
-
Evolution of the bilaterian mouth and anus
Nature Ecology & Evolution (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.