Abstract
Major Depression is a prevalent mental disorder that is characterized by negative mood and reduced motivation, and frequently results in social withdrawal and memory-related deficits. Repeated stressors, such as adverse life events, increase the risk for development of the disorder. Consequently, individual variability in stress response greatly weighs on depression-vulnerability and -resilience. Here, we employed the social defeat-induced persistent stress (SDPS) paradigm to identify depression-prone individuals and to examine the temporal development of depression in the months following exposure to brief defeat stress. Male Wistar rats were socially defeated (5 defeat episodes) and single-housed for a prolonged period of time (~24 weeks). We assessed the emergence of a sustained depressive-like state by repeatedly evaluating social motivation (social approach avoidance) and spatial memory (object place recognition) in SDPS rats during the isolation period. Individual variability in the effects of SDPS yielded two extreme subpopulations: an SDPS-prone group that showed gradual affective and cognitive deterioration in terms of social approach and memory retention, and a SDPS-resilient group that did not develop this phenotype. Notably, in SDPS-prone individuals, the affective deficits preceded later cognitive impairments, providing a novel temporal profile of the development of pathology in this preclinical model of sustained depression.
Similar content being viewed by others
Introduction
Major Depressive Disorder (MDD) is considered one of the most debilitating psychiatric disorders, ascribed to a ~16% lifetime prevalence and ~60% probability for severe clinical manifestation1. According to the Diagnostic and Statistical Manual of mental disorders (DSM-5), MDD is characterized by a variety of psychological, somatic, and social deficits2. Amongst them, decreased mood and diminished interest for pleasurable activities (anhedonia) are at the core of the depressive state2, frequently accompanied by impaired cognitive function3.
Social withdrawal, defined as disengagement from social activities that leads to impoverished interpersonal relationships, is a common symptom in depression2. Depressed patients often display diminished motivation for social interaction, which augments their subjective feelings of loneliness, in turn intensifying their depressed mood4. It is thought that social withdrawal results from the lack of reinforcement normally achieved by maintaining healthy interpersonal relationships, reflecting anhedonia5, 6. In addition, social withdrawal might emerge as a result of affiliation problems7, 8, when depressed individuals experience negative feelings, such as social anxiety, inferiority and detachment, upon exposure to social settings. Integrating these two hypotheses, it is proposed that deficits in approach-avoidance behaviour eventually culminate in social withdrawal9. In agreement, depression severity is predictive of diminished approach towards stimuli of positive valence10, resulting in greater withdrawal magnitude.
Cognitive deficits in depression include alterations in executive function, attention and memory that significantly interfere with a patient’s daily activities11. Memory deficits, such as difficulty in recollection and declarative memory, heavily depend on aberrant function of the hippocampus12, 13, in which depression-induced structural and functional alterations are well-described14, 15. In support, depressed patients display impaired spatial memory performance in virtual reality navigation tasks16, 17, which is accompanied by functional deterioration of the hippocampus17. Currently, it is unclear whether this cognitive dysfunction renders individuals prone to depression or whether cognitive deficits appear following the first depressive episode18, 19. It is suggested that cognitive impairment is a core feature of depression that develops independently of depressed mood, as it lingers in patients remitting from mood-related depressive symptoms20, 21. On the other hand, affective vulnerability, i.e., inability to regulate the emotional response, is shown to disturb cognitive function in depressed patients22, indicating a level of interdependency between the two symptoms.
Exposure to repeated stress is considered a primary trigger of the depressive state23, 24 and factors that regulate the stress response, ranging from genetic predispositions to social influences, have been implicated in vulnerability (or resilience) to depression25. It was postulated that abnormal reactivity to stress, such as excessive and/or prolonged hypothalamic-pituitary-adrenal (HPA) axis activation, increases the probability of depression onset and its magnitude26. In contrast, the employment of resilience-inducing strategies, e.g., adaptive HPA axis habituation, is thought to protect an individual from depression in case of severe or persistent stress27. These coping mechanisms are strongly determined by individual predispositions, such as trait anxiety, and their development is intertwined with the frequency of stress experience28, 29. During adverse life-events, proactive coping is associated with resilience and adaptability, whereas passive coping is thought to contribute to the development of stress-induced depression30. Supporting this notion, in individuals who adopt avoidance-related coping strategies, high frequency of negative life events predicts greater severity of depressive symptoms31.
Given its importance, individual variability in stress response and its association with the development of susceptibility or resilience to depression have been extensively studied at the preclinical level27, 32. This allowed for detailed examination of sub-phenotypes of the disease and for elucidation of depression comorbidities33,34,35. Notably, rodent models employing acute social defeat stress have successfully outlined brain pathways36, 37 and molecular mechanisms38,39,40 underlying stress vulnerability. We recently adopted a rat paradigm that combines acute social defeat stress with prolonged social isolation, the Social Defeat-induced Persistent Stress (SDPS) model41. SDPS induces a sustained depressive-like state that persists long after (>2 months) exposure to social defeat stress and emulates behavioural and physiological hallmarks of the human disease, such as anhedonia42, social withdrawal43, cognitive dysfunction43, 44 and hippocampal pathology41, 45.
Here, we investigated whether the SDPS paradigm can be used to identify depression-prone and -resilient individuals, and thus, to facilitate the characterization of depressive-like symptoms that develop over time. Depression susceptibility was estimated based on approach-avoidance behaviour and short-term spatial memory retention, in terms of social withdrawal (affective function) and depression-induced memory deficits (cognitive function), respectively. Furthermore, we examined the temporal profile of these SDPS-triggered impairments acutely after short but severe stress exposure (social defeat) and in the following months, in the presence of a constant subthreshold stressor (social isolation).
Results
Individual variability in the effects of SDPS
As both affective and cognitive deficits determine the development and persistence of the depressive state21, 46, we used the performance of SDPS animals in the social approach avoidance (SAA) and object place recognition (OPR) tasks at 2 different time points (week 5 (w5) and 9 (w9) after defeat) to identify subpopulations of SDPS-prone and SDPS-resilient individuals in a large cohort of animals35, 39 (Fig. 1a). Based on this, we used data clustering for identification of two clearly divergent groups, in terms of affective and cognitive performance, as described below in detail. Individual data for both tests and all time points are presented in Supplementary Figure S1.
Cluster analysis of depressive-like behaviour over time: Overall model fit and predictor importance. (a) Experimental time-line of temporal profiling of affective and cognitive behaviour. Social approach-avoidance (SAA) and object place recognition (OPR) tasks took place in the weeks (w-1, w1, w5, w9, w24) before/after 5 daily defeat sessions (week 0). SDPS-vulnerability was estimated by a two-step cluster analysis of SAA and OPR performance as assessed at weeks 5 and 9 post-defeat (highlighted), using the Schwarz’s Bayesian criterion35. (b) Cluster quality for affective function (SAA) showed a good overall model fitting (0.60, orange open bar) when including individual data (n = 48) from the SAAw5 and SAAw9 tests. For comparison, poor (0.30) and fair (0.50) model fitting are depicted (grey shading). (c) From the two time points, performance at SAAw5 was the most important predictor of SDPS-proneness (predictor importance SAAw5, 1.00 vs. SAAw9, 0.57), indicating that SDPS-induced affective deficits that distinguish SDPS-prone from SDPS-resilient animals emerge at 1 month following the last defeat exposure. (d) Cluster quality for cognitive function (OPR) showed a good overall model fitting (0.60, orange open bar) when using individual data (n = 48) from the OPRw5 and OPRw9 tests. (e) From the two time points, performance at OPRw9 was the most important predictor of SDPS-proneness (predictor importance OPRw9, 1.00 vs. OPRw5, 0.06), indicating that cognitive deficits that distinguish SDPS-prone from SDPS-resilient animals develop later, namely at 2 months following the last defeat exposure.
SDPS effect on approach-avoidance behaviour
Based on clustering (Schwarz’s Bayesian criterion35) of approach-avoidance behaviour at two time-points after defeat (average model silhouette 0.60; Fig. 1b), the SAAw5 test was the most prominent predictor of SDPS-induced deficits in social motivation (predictor importance 1.00 vs. 0.57 for SAAw9, Fig. 1c), indicating establishment of affective vulnerability a month following defeat. Rats were clustered in two groups: 23 SDPS rats were identified as SDPSSAA-resilient (interaction index group mean: SAAw5, 0.91 ± 0.02; SAAw9, 0.92 ± 0.01), whereas the remaining 25 rats clustered in the SDPSSAA-prone group (interaction index group mean: SAAw5, 0.59 ± 0.03; SAAw9, 0.71 ± 0.03) (Fig. 2a). These two clusters showed distinct performance at the individual time points (SAAw5, U = 562.00, P < 0.001; SAAw9, U = 528.00, P < 0.001). Notably, SAA performance acutely following defeat was found to decrease the overall model fitting (Supplementary Fig. S2), suggesting that although immediate post-defeat SAA performance reflects the effects of acute defeat stress39, its relevance to predict the development of a long-lasting sustained depressed state is nominal.
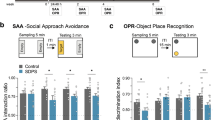
Cluster analysis: Individual variability in the effects of SDPS on the affective and cognitive domain. Two-step cluster analyses of social approach avoidance (SAA) and object place recognition (OPR) performance, each at weeks 5 and 9 post-defeat, revealed the emergence of two distinct SDPS groups, one displaying severely disrupted behaviour (SDPS-prone; Prn) and one exhibiting performance similar to controls (SDPS-resilient; Res). Individual performance at the SAAw5 (a,c) and OPRw9 (b,d) tests is depicted, as cluster analyses indicated these particular tests as the most important predictors of SDPS-vulnerability (cf. Fig. 1). (a,b) Individual performance at SAA (a) and OPR performance (b) on the total set of SDPS rats (n = 46–48), and the total set of control (Con) rats (n = 32) for comparison. (c,d), Individual performance of control (Con) rats (n = 16) and SDPS rats showing overlap in SAA and OPR after cluster analysis (see (a,b); n = 30; n = 15 per subgroup) at the SAA (c) and OPR (d) tasks. SDPS-resilient rats clustered together with all but two control rats at the SAA task (c), and all but three control rats at the OPR task (d), which are indicated by open circles. All panels: Group mean and standard deviation (vertical line with horizontal whiskers); red dashed line indicates interaction (a,c) or exploration (b,d) indices at chance level (0.50).
SDPS effect on short-term object place memory
Using task performance at two time points (average model silhouette 0.60; Fig. 1d), the OPRw9 test was the most prominent predictor of SDPS effects on cognitive function (predictor importance 1.00 vs. 0.06 for OPRw5, Fig. 1e), indicating the establishment of cognitive vulnerability at 2 months following defeat. Supporting this notion, in the two identified clusters a significant between-group difference was observed only at the OPRw9 test: OPRw5, F(1,44) = 2.29, P = 0.137; OPRw9, F(1,44) = 122.03, P < 0.001. In particular, 24 SDPS rats were identified as SDPSOPR-resilient (exploration index group mean: OPRw5, 0.61 ± 0.03; OPRw9, 0.70 ± 0.01), whereas the other 24 rats clustered in the SDPSOPR-prone group (exploration index group mean: OPRw5, 0.55 ± 0.03; OPRw9, 0.45 ± 0.02) (Fig. 2b).
Selection of SDPS-prone vs. -resilient subpopulation
Clustering based on the SAA and OPR tasks showed a substantial overlap, indicating that SDPS-induced depression proneness was reflected in deficits of both the affective and the cognitive domain. Particularly, 65% of the SDPSSAA-resilient group was part of the SDPSOPR-resilient group, and likewise, 64% of SDPSSAA-prone animals were clustered within the SDPSOPR-prone group. Rats with overlap in both domains were assigned to the SDPS-prone and SDPS-resilient populations, resulting in a total of 15 rats per subgroup (Fig. 2c,d). Re-analysis of cluster data, this time including the individual scores from controls (n = 16), validated the final population division, as SDPS-resilient animals clustered in general together with controls in both tasks (SAA, all but 2 controls; OPR, all but 3 controls) (Fig. 2c,d).
Temporal profile of SDPS-induced affective and cognitive deficits
Development of deficits in approach-avoidance behaviour
In order to clarify the temporal profile of depression-associated deficits in social behaviour, SAA performance of SDPS-prone, SDPS-resilient and control groups was plotted in a retrospective manner (Fig. 3). This revealed that before the start of the SDPS paradigm, no between-group differences in baseline approach behaviour were observed between the three groups (FSAA-bl(2,43) = 0.36, P = 0.700; Fig. 3a). Following social defeat, SAA performance (repeated measures ANOVA: SAAw1, SAAw5, SAAw9) showed no effect of time (FSAA(2,86) = 1.61, P = 0.207). A significant group by time interaction and group effect were observed (FSAAxGROUP(4,86) = 2.75, P = 0.033; FGROUP(2,43) = 28.06, P < 0.001), indicating that SDPS differentially affected SAA performance in each group during the weeks after defeat (Fig. 3a).
Development of affective and cognitive deficits in SDPS-prone and SDPS-resilient rats. (a) Approach-avoidance behaviour was examined in 5 subsequent SAA tests provided during a period of ~6 months (cf. Fig. 1a). Before defeat (baseline), no pre-existing differences were observed between groups. At SAAw5, only the SDPS-prone group failed to display preference for the social target. In the months following defeat, SDPS-prone rats showed reduced interaction index vs. controls and SDPS-resilient animals, indicating development of social withdrawal that persisted up to 6 months. (b) Left: Schematic representation of the object place recognition (OPR) task, with a 5-minute sampling phase, a 15-minute retention interval and the 5-minute testing phase, in which one of the two identical objects was displaced. Right: Short-term spatial memory was assessed in 3 OPR tests given during a period of 2 months (cf. Fig. 1a). Prior to defeat no between-group differences were seen. Already at 1 month from defeat, SDPS-prone animals showed inability to retain spatial information for a displaced object, which was exaggerated at the 2-month test. Both control and SDPS-resilient groups displayed intact memory retention. Repeated measures ANOVA main time (t), group (g) and time x group interaction (t x g) effects and pairwise comparisons are indicated. Dotted line represents chance levels (0.50) of interaction (SAA, a) or exploration (OPR, b). #No preference for the social target (SAA) or the displaced object (OPR); *P < 0.050; **P < 0.010; ***P < 0.001.
In particular, already acutely after social defeat (FSAAw1(2,43) = 3.87, P = 0.029), SDPS-prone animals showed reduced approach behaviour when compared with controls (P = 0.009). A trend for differential performance between SDPS-prone and SDPS-resilient rats (P = 0.065) was observed, as the latter group performed similar to controls (P = 0.430).
Analysis of SAAw5 test (H = 24.45, P < 0.001) confirmed the establishment of social avoidance in SDPS-prone rats (P < 0.001 vs. control and SDPS-resilient). The resilient group escaped the effects of SDPS and performed similar to controls (P = 0.308). Comparable results were obtained when analysing SAAw9 data (H = 14.68, P = 0.001), with SDPS-prone animals showing reduced approach behaviour as compared with both controls (P = 0.005) and the SDPS-resilient group (P < 0.001). No difference in SAA performance between the latter groups was observed (P = 0.185). A positive correlation between w5 and w9 SAA tests further validated the stability of performance in SDPS rats over-time, namely, a sustained avoidance response in SDPS-prone rats vs. intact social approach in the SDPS-resilient group (Supplementary Fig. S3).
At ~6 months from the last defeat exposure, a significant group effect (F(2,43) = 3.53, P = 0.038) confirmed that SDPS-prone animals continued displaying reduced social approach as compared with controls and SDPS-resilient rats (P = 0.046 and P = 0.017, respectively, Fig. 3a). Control and SDPS-resilient animals exhibited similar approach-avoidance performance (P = 0.634).
Development of deficits in short-term object place memory
Similar to SAA, the performance of the SDPS-prone, SDPS-resilient and control groups at the OPR task was used to illustrate the temporal profile of depression-associated cognitive deficits. No between group effects were observed in short-term memory retention before the start of the SDPS paradigm (FOPR-bl(2,41) = 0.43, P = 0.651, Fig. 3b). Following social defeat, overall OPR performance (repeated measures ANOVA: OPRw5, OPRw9) showed significant effects of time (FOPR(1,42) = 5.83, P = 0.020), group (FGROUP(2,42) = 20.95, P < 0.001) and interaction (FOPRxGROUP(2,42) = 5.06, P = 0.011). This indicated differential OPR performance of each group over the course of the two months after defeat (Fig. 3b).
In particular, at one month following defeat, a trend for a group effect (FOPRw5(1,42) = 2.92, P = 0.065) was observed, which was driven from the considerably poorer OPR scores of SDPS-prone rats compared with the other two groups (P = 0.031 vs. control; and P = 0.060 vs. SDPS-resilient). As with the previous tests, SDPS-resilient animals did not differ from controls (P = 0.769). Likewise, a significant group effect was observed at the OPRw9 test (FOPRw9(1,43) = 42.76, P < 0.001), with SDPS-prone rats displaying a significantly lower exploration index compared with both control and SDPS-resilient groups (P < 0.001 vs. both). This confirmed the consolidation of cognitive deficits in the SDPS-prone group at two months following exposure to defeat stress, which coincided with impaired social behaviour (Supplementary Fig. S4). Surprisingly, a significant group effect was observed between control and SDPS-resilient rats (P < 0.001), reflecting a slight improvement of OPR performance in the SDPS-resilient group (paired t-test OPRw5,w9, t(14) = −1.58, P = 0.136) together with a decrease in performance of controls (paired t-test OPRw5,w9, t(14) = 2.58, P = 0.022) (Fig. 3b).
Together, the SAA and OPR data indicated the formation of two distinct subpopulations following social defeat, which was independent of baseline performance. The SDPS-resilient population coped with defeat and isolation stress and did not develop any of the affective or cognitive deficits commonly seen after SDPS42, 43. In contrast, the SDPS-prone population showed long-lasting deterioration of affective performance, reflected in social withdrawal, and was accompanied by severe impairments in spatial memory, which worsened over time. Individual-based analysis of the temporal progression of the depressive-like state argued in favour of early establishment of impairments in social behaviour and later coincidence of the affective-cognitive symptoms.
Submission latency
Coping strategies during exposure to defeat stress, e.g., counter-attacks or freezing, predict the duration and severity of the psychobiological effects of defeat47. Therefore, we examined latency for first submission during defeat in the two subpopulations identified as SDPS-prone and SDPS-resilient. Analysis of submission latency over the five defeat episodes revealed a significant between-group effect (Friedman’s χ2(4) = 45.89, P < 0.001), as SDPS-prone rats submitted faster vs. their SDPS-resilient counterparts (Fig. 4a). Notably, a positive correlation between submission latency during the first defeat session and performance at SAAw5 was observed that was specific for the SDPS-prone group: SDPS-prone, Spearman’s r(15) = 0.53, P = 0.042; and SDPS-resilient, Spearman’s r(15) = −0.12, P = 0.677 (Fig. 4b). No correlation between submission latencies and any of the other behavioural tests (SAAw1, SAAw9, OPRw5, OPRw9) was seen.
Submission latency during social defeat. Latency to assume the first submissive posture during the five defeat sessions was documented for each SDPS animal. (a) Following the two-steps cluster analysis, average latency to first submission was calculated for each of the emerging SDPS-prone and SDPS-resilient subgroups. SDPS-prone animals displayed shorter submission latency over the five defeat sessions and this effect was most pronounced in day 3 of the social defeat period. (b) In the SDPS-prone group, submission latency during the first defeat episode was positively correlated with social avoidance as assessed at the SAAw5 test, which was the most prominent predictor of SDPS-proneness in the affective domain. No such correlation was observed for the SDPS-resilient rats. Spearman’s rank correlation coefficient (r) and the correspondent P-value is indicated for each group. *P < 0.05.
Discussion
In the present study, a large group of rats (n = 48) was subjected to the SDPS paradigm, with 5 daily defeat sessions followed by prolonged single-housing (6 months). The depressive-like state, which is known to be long-lasting41,42,43, was assessed at different time points after social defeat while animals remained in isolation. This entailed repeated measuring of social approach-avoidance behaviour (SAA) and performance in a short-term spatial memory task (OPR) with sufficient inter-test time interval. By adopting this approach, we examined how affective deficits, in the form of reduced motivation for social interaction, and cognitive deficits, seen as failure in memory retention, develop over time with a focus on the individual.
Individual variability in the effects of SDPS on each parameter was assessed by cluster analysis, revealing two distinct subpopulations of SDPS-prone and SDPS-resilient rats. This was in absence of pre-existing behavioural differences, suggesting that reactivity to social stress determines later depression vulnerability. SDPS-proneness was associated with persistent social withdrawal and a progressive decline in spatial memory. Furthermore, temporal profiling of SDPS effects showed that affective deficits emerged first, whereas aberrant memory processes developed later. SDPS-resilience was associated with absence of depression-like deficits.
Individual variability in the effects of SDPS on affective behaviour
Reduced interest in social activities is one of the core symptoms of depressive pathologies2, whereas loneliness and perceived isolation from social contexts contribute to chronic depression in humans48, 49. Reduced social motivation and increased social anxiety are risk factors for the onset and the duration of depression50, 51. Likewise, avoidance response, which precedes social withdrawal, confers vulnerability to the development and persistence of the disorder9. As such, social withdrawal, in the form of reduced interaction with an unfamiliar social target, has been extensively used to assess the development and magnitude of depressive-like states at the preclinical level52.
We previously reported that a general population of SDPS rats exhibits persistent social withdrawal that lasts up to 6 months43. This reliably resembles social withdrawal during development, in which severe reticence is longitudinally present53. Here we confirmed the negative effects of SDPS on affective behaviour, showing its importance in the development of depression vulnerability. SDPS-prone rats showed decreased approach behaviour immediately after the defeat week (SAAw1) and displayed reduced social motivation up to 6 months following the last defeat exposure (SAA6mth). The acute effect of social stress (SAAw1) in the SDPS-resilient rats was mild, as their interaction scores reached midway that of the control and SDPS-prone groups. Thereafter, the SDPS-resilient population showed stable approach behaviour throughout the experimental design, similar to controls. Together, these data suggest a disrupted affective response in SDPS-prone rats that leads to permanent social deficits, an effect absent in SDPS-resilient animals.
Individual variability in the effects of SDPS on cognition
In recent years, impaired cognition in MDD, including attentional bias and poor working memory11, 54, has gained growing attention in the clinic, as it is thought to perpetuate the depressive state and to hamper recovery3, 55. Longitudinal studies support a unidirectional relation between depression and cognitive dysfunction, with pre-existing depressive symptoms accelerating global cognitive deficits and episodic memory problems18. In addition, depression recurrence predicts failure in recollection memory56 and a parallel reduction in hippocampal volume15, 57, indicating that the duration and persistence of the depressive state negatively impact on brain morphology and cognitive function.
We previously showed that SDPS induces prolonged cognitive dysfunction, reflected in deficits in hippocampus-mediated spatial memory42, 43. Here, we validated the detrimental impact of depression-triggering stressors on hippocampal function58. Similar to approach-avoidance behaviour, only the subpopulation of SDPS-prone animals showed this inability to retain short-term information with regard to the spatial location of an object. SDPS-resilient animals were protected from SDPS-induced memory deficits, showing a relative improvement in OPR performance at two months following defeat.
The most prominent reduction in spatial memory performance was observed in the SDPS-prone group at two months after defeat, mimicking progressive cognitive decline in presence of a sustained depressive-state. Cognitive deficits following acute social defeat stress include reduced memory performance at the novel object recognition task59, which is largely independent of hippocampal function60, 61. In contrast, performance at the Morris water maze task, which, similar to OPR, examines hippocampus-mediated spatial memory, is not affected in the first two weeks following exposure to defeat stress59. In addition, in rats exposed to social defeat stress, long-term spatial memory deficits, as examined in the radial arm water maze, appear following >1 month from the last defeat exposure62.
In the present study, we did not assess OPR performance acutely following a defeat episode. Thus, based on our data we cannot exclude effects of acute stress on short-term recognition memory. In fact, it is very likely that spatial memory is affected at these early time-points after stress, as reviewed for several other stressful paradigms in rodents63. It is worth noting that these acute stress effects are mediated via glucocorticoid signaling64, which is altered shortly following exposure to stressful stimuli, including social defeat stress65, 66. However, as we did not observe any difference in basal glucocorticoid levels long-term after defeat41, we consider that the progressively deteriorating spatial memory performance we report here is mediated via divergent mechanisms, which apparently develop over time in absence of stress.
Taken together, it is plausible that maintained disruption of hippocampus-mediated cognition necessitates the presence of a chronic depressive-like state, just as observed in humans. Furthermore, our data indicate that, similar to vulnerability, resilience to depression-induced cognitive disturbances is an active process relying on adaptations that evolve over lengthy periods of time.
Temporal profiling of depressive-like symptoms in SDPS-prone individuals
Currently, profiling of depressive symptoms is limited to the prerequisite of experiencing severe mood- or anhedonia-associated impairments that persist for more than 2 weeks2. This neglects temporal aspects of depression occurrence and disease trajectory67. Likewise, most preclinical research relies on acute one-off behavioural assessments of the depressive-like state, seemingly overlooking empirical data that suggest that depressive pathology and the related burden intensify with time, including frequency of depressive episodes and their duration68.
Here, by employing repeated measurements of depression-associated deficits in two distinct behavioural domains, we provide evidence for a unique temporal profile in the development of the depressive-like state in SDPS-prone individuals. As the predictor efficacy of the cluster analyses revealed (cf. Fig. 1), there is a clear distinction in the development of the affective and cognitive phenotypes over time. Deficits in affective behaviour, i.e., reduced interest for social interaction (SAA test) appeared first, and were able to distinguish depression-prone animals already at week 5 following exposure to defeat stress. Cognitive impairments, i.e., reduced short-term spatial memory retention, developed later, as depicted by the strong influence of OPRw9 in predictor efficacy. This temporal profile was verified by a significant positive correlation between early affective and late cognitive deficits (Supplementary Fig. S5), indicating that the magnitude of impairments in social behaviour could predict the severity of cognitive symptoms in depressed individuals. From a clinical perspective, our results argue in favour of early identification of patients with mood-related symptoms and their recruitment for specific programs, such as prevention of social isolation and stimulation of cognitive capacity.
In inbred mice that are identified as susceptible based on increased avoidance behaviour at the SAA task shortly after social defeat, exposure to social stress halts normal hippocampal growth compared with the resilient subpopulation69. In addition, susceptible mice show pre-existing hippocampal volume differences that correlate with post-stress avoidance performance69. Together these data suggest that epigenetic, stress-induced hippocampal susceptibility can confer depression vulnerability. Although cognitive function was not assessed in these animals, these results fit in the temporal profile of the depressive state illustrated in our study, with the effects of SDPS first manifested in social avoidance and later, possibly following structural and functional reorganization of the hippocampus, in cognitive decline. This is in accordance with the idea that affective disturbances precede, or might even promote, deficits in cognitive processes in depression22, 70.
Individual differences in coping styles during social defeat
Coping strategies highly influence one’s ability to adapt during exposure to severe stress, and trigger allostatic mechanisms serving resilience or promoting vulnerability27. At the preclinical level, during social defeat, active (confrontation, defensiveness) or passive (immobility, submission) coping styles have been reported71,72,73 and are considered to be closely associated with responsivity to social defeat stress74 and to subsequent stressors75. A well-established measure of coping style during defeat stress is the latency to assume a subordinate posture47, 76, which has been used before in order to distinguish defeat-prone from defeat-resilient individuals30. In the present study, SDPS rats that were identified as prone following cluster analysis showed faster submission latency vs. their resilient counterparts. Our data are in agreement with the notion that rodents that exhibit passive coping styles, such as quick subordination during defeat, display vulnerability to depression, just like humans31.
Behavioural readouts that promote initiative and free choice are most discriminative of a proactive vs. a reactive coping style in face of stress32, granting the SAA task with high face value in categorizing active vs. passive copers. Following SDPS, latency to first submission predicted avoidance performance at the five weeks SAA task. Notably, this positive correlation was selective to the SDPS-prone subpopulation, further supporting an interplay between passive coping strategies and later vulnerability to the depression-triggering effects of stress31, 71.
Conclusions
In order to further elucidate the underlying causes of depression and to provide successful therapeutic options to treatment-resistant individuals77,78,79,80 preclinical models should prioritize on individual variability to the lasting effects of stress. Our data argue for the need of a temporal analysis of both affective and cognitive disturbances in paradigms that model (vulnerability to) depression. Finally, our data suggest that affective/motivational deficits precede cognitive decline in depression, which could prove useful in designing preventive and treatment strategies against this debilitating disorder.
Animals, Methods and Materials
Animals and social defeat-induced persistent stress (SDPS)
SDPS was carried out with male Wistar rats (n = 48 defeat, n = 32 controls, 9–10 weeks of age) as described before42, 43 (Supplemental Methods). In brief, SDPS rats were exposed to five 15-minute daily social defeat sessions as follows: rats were transported to the residents’ housing room and placed inside the residents’ cages (defeat cage). A transparent, perforated plexi-glass partition wall was used to separate the residents from the intruders, allowing for sensory exchange, but not for physical contact (pre-fight phase, 5 minutes). The wall was removed and Wistar rats were then exposed to a 5-minute fight phase, during which they were forced into submission. The defeat session concluded with an additional 5-minute period, during which the partition wall was placed back, separating the resident from the intruder (post-fight phase). A different resident was matched to each Wistar rat per day. From the first defeat session onwards, all animals were single-housed and remained in social isolation for the rest of the experimental manipulations, in absence of further sensory interaction with the stressor (residents), in a separate housing room. Two researchers monitored the social defeat sessions and the latency to submission during the fight phase was recorded for each rat in each of the five sessions provided. Experiments were divided over 3 independent batches, separated by 1 week each. Animals were housed on a reversed 12-h light-dark cycle (lights on 19.00 h) and all experiments were conducted during the dark phase. Rooms were equipped with infrared lights, which Wistar rats cannot detect. Animals received food and water ad libitum. All experiments were approved by the VU University Amsterdam Animal Users Care Committee, and were performed in accordance with the relevant guidelines and regulations.
Assessment of the depressive-like state
Social approach-avoidance test (SAA)
Approach-avoidance behaviour was estimated using an unfamiliar Long-Evans adult male rat (resident) as previously described42, 43 (Supplemental Methods). Interaction index was calculated as time spent in active zone (resident zone)/total exploration time (resident + neutral zone), in a 5-minute test. In order to examine the development and progression of social withdrawal the weeks after social defeat, all animals were exposed to 5 consecutive SAA tests: the week before social defeat (baseline, bl); following the defeat week (acute, w1); at week 5 (w5); at week 9 (w9) and at 6 months (6mth) following the last defeat exposure.
Object place recognition (OPR)
Hippocampus-dependent short-term memory was assessed by the object place recognition task using a 15-minute retention interval as previously described42, 43. Discrimination between the spatial locations of the two objects was used to assess spatial memory (exploration index = time spent in novel location/total exploration time (novel + familiar location)) in a 4-minute test. In order to examine the development and progression of cognitive impairments after SDPS, all animals participated in three OPR tests given the week before social defeat (baseline, bl); at week 5 (w5) and at week 9 (w9) following the last defeat exposure.
Statistical analyses
Analysis of behavioural readouts
All behavioural data collected from SAA, OPR were analysed using repeated measures analysis of variance (ANOVA), with test (time-points) as within- and group as between-subject factors. When P-values reached level of significance (P < 0.05), further analysis was performed using one-way ANOVA, paired or unpaired student’s t-test and post-hoc Tukey-HSD multiple comparisons. Homogeneity of variance, sphericity and normality assumptions were estimated and Huynh-Feldt correction or the non-parametric Kruskal-Wallis H, Mann-Whitney U and Friedman χ2 tests were implemented in case of violation. Preference in interaction and exploration indexes (SAA, OPR) was estimated against a fictive group representing performance at chance levels, while retaining the same variation as the experimental groups81. Correlation between different experimental readouts was estimated using Spearman’s correlation coefficient (r). All statistics were performed using IBM SPSS Statistics 21. All group data are depicted as mean ± SEM.
During assessment of the depressive-like state, the tracking software was erroneously terminated, leaving datasets for the following tests incomplete: ORRbl, n = 2; OPRw5, n = 3.
Selection procedure
SDPS rats were assigned to either SDPS-prone or SDPS-resilient subgroups following a two-step cluster analysis of individual performance in the social-approach avoidance test (SAA), and object place recognition test (OPR) at two time points, namely at week 5 (w5) and week 9 (w9) after the last defeat exposure. Both behavioural readouts (SAA, OPR) were weighted equally for final group assignment, as the criterion for susceptibility or resilience required to include both affective and cognitive aspects of the depressive-like state. Cluster analysis was performed using IBM SPSS Statistics 21, based on the Schwarz’s Bayesian criterion35 and with automatic generation of cluster numbers to avoid biased subject selection. First, we performed cluster analysis using the SAA data and SDPS animals were classified as prone or resilient based on their motivation to interact with the social target (affective domain). Subsequently, OPR data were used for cluster analysis in order to identify prone vs. resilient individuals in respect to spatial memory retention (cognitive domain). Animals that showed overlapping clustering in the two cluster analyses were finally identified as SDPS-prone (n = 15) and SDPS-resilient (n = 15).
Control animals were divided in two equally performing groups (balanced average performance in SAA and OPR tests43). Thus, a total of 16 control rats participated in the experiments described above, whereas the other 16 served as the control group in our previous study43. Data obtained from the final SDPS-prone and -resilient groups were re-analysed together with controls to validate the particular approach, i.e. post-hoc fitting of the clustering method (cf. Fig. 2).
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Belmaker, R. H. & Agam, G. Major depressive disorder. The New England journal of medicine 358, 55–68, doi:10.1056/NEJMra073096 (2008).
Association, A. P. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th edn, (American Psychiatric Publishing, Inc., 2013).
McIntyre, R. S. et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depression and anxiety 30, 515–527, doi:10.1002/da.22063 (2013).
Wade, T. D. & Kendler, K. S. The relationship between social support and major depression: cross-sectional, longitudinal, and genetic perspectives. The Journal of nervous and mental disease 188, 251–258 (2000).
Henriques, J. B. & Davidson, R. J. Decreased responsiveness to reward in depression. Cognition and Emotion 14, 711–724 (2000).
Pechtel, P., Dutra, S. J., Goetz, E. L. & Pizzagalli, D. A. Blunted reward responsiveness in remitted depression. Journal of psychiatric research 47, 1864–1869, doi:10.1016/j.jpsychires.2013.08.011 (2013).
Sloman, L., Gilbert, P. & Hasey, G. Evolved mechanisms in depression: the role and interaction of attachment and social rank in depression. Journal of affective disorders 74, 107–121 (2003).
Girard, J. M. et al. Nonverbal Social Withdrawal in Depression: Evidence from manual and automatic analysis. Image Vis Comput 32, 641–647, doi:10.1016/j.imavis.2013.12.007 (2014).
Trew, J. L. Exploring the roles of approach and avoidance in depression: an integrative model. Clinical psychology review 31, 1156–1168, doi:10.1016/j.cpr.2011.07.007 (2011).
Radke, S., Guths, F., Andre, J. A., Muller, B. W. & de Bruijn, E. R. In action or inaction? Social approach-avoidance tendencies in major depression. Psychiatry research 219, 513–517, doi:10.1016/j.psychres.2014.07.011 (2014).
Gotlib, I. H. & Joormann, J. Cognition and depression: current status and future directions. Annual review of clinical psychology 6, 285–312, doi:10.1146/annurev.clinpsy.121208.131305 (2010).
Campbell, S. & Macqueen, G. The role of the hippocampus in the pathophysiology of major depression. Journal of psychiatry & neuroscience: JPN 29, 417–426 (2004).
Hickie, I. et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. The British journal of psychiatry: the journal of mental science 186, 197–202, doi:10.1192/bjp.186.3.197 (2005).
Bremner, J. D. et al. Hippocampal volume reduction in major depression. The American journal of psychiatry 157, 115–118 (2000).
MacQueen, G. M. et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences of the United States of America 100, 1387–1392, doi:10.1073/pnas.0337481100 (2003).
Gould, N. F. et al. Performance on a virtual reality spatial memory navigation task in depressed patients. The American journal of psychiatry 164, 516–519, doi:10.1176/ajp.2007.164.3.516 (2007).
Cornwell, B. R. et al. Abnormal hippocampal functioning and impaired spatial navigation in depressed individuals: evidence from whole-head magnetoencephalography. The American journal of psychiatry 167, 836–844, doi:10.1176/appi.ajp.2009.09050614 (2010).
Panza, F. et al. Temporal relationship between depressive symptoms and cognitive impairment: the Italian Longitudinal Study on Aging. J Alzheimers Dis 17, 899–911, doi:10.3233/JAD-2009-1111 (2009).
Darcet, F., Gardier, A. M., Gaillard, R., David, D. J. & Guilloux, J. P. Cognitive Dysfunction in Major Depressive Disorder. A Translational Review in Animal Models of the Disease. Pharmaceuticals (Basel) 9, doi:10.3390/ph9010009 (2016).
Femenia, T., Gomez-Galan, M., Lindskog, M. & Magara, S. Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain research 1476, 58–70, doi:10.1016/j.brainres.2012.03.053 (2012).
Rock, P. L., Roiser, J. P., Riedel, W. J. & Blackwell, A. D. Cognitive impairment in depression: a systematic review and meta-analysis. Psychological medicine 44, 2029–2040, doi:10.1017/S0033291713002535 (2014).
Joormann, J. & Gotlib, I. H. Emotion regulation in depression: relation to cognitive inhibition. Cognition & emotion 24, 281–298, doi:10.1080/02699930903407948 (2010).
Gold, P. W. The organization of the stress system and its dysregulation in depressive illness. Molecular psychiatry 20, 32–47, doi:10.1038/mp.2014.163 (2015).
Assari, S. & Lankarani, M. M. S. L. Events and Risk of Depression 25 Years Later: Race and Gender Differences. Front Public Health 4, 49, doi:10.3389/fpubh.2016.00049 (2016).
Feder, A., Nestler, E. J. & Charney, D. S. Psychobiology and molecular genetics of resilience. Nature reviews. Neuroscience 10, 446–457, doi:10.1038/nrn2649 (2009).
Hammen, C. Stress and depression. Annual review of clinical psychology 1, 293–319, doi:10.1146/annurev.clinpsy.1.102803.143938 (2005).
Franklin, T. B., Saab, B. J. & Mansuy, I. M. Neural mechanisms of stress resilience and vulnerability. Neuron 75, 747–761, doi:10.1016/j.neuron.2012.08.016 (2012).
Miczek, K. A., Nikulina, E. M., Shimamoto, A. & Covington, H. E., 3rd. Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci 31, 9848–9857, doi:31/27/9848 [pii] 10.1523/JNEUROSCI.0637-11.2011 (2011).
Renstrom, F. et al. Genetic predisposition to long-term nondiabetic deteriorations in glucose homeostasis: Ten-year follow-up of the GLACIER study. Diabetes 60, 345–354, doi:10.2337/db10-0933 (2011).
Wood, S. K., Walker, H. E., Valentino, R. J. & Bhatnagar, S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology 151, 1795–1805, doi:10.1210/en.2009-1026 (2010).
Blalock, J. A. & Joiner, T. E. Interaction of cognitive avoidance coping and stress in predicting depression/anxiety. Cognitive Therapy and Research 24, 47–65 (2000).
Koolhaas, J. M., de Boer, S. F., Coppens, C. M. & Buwalda, B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Frontiers in neuroendocrinology 31, 307–321, doi:10.1016/j.yfrne.2010.04.001 (2010).
Cohen, H. et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biological psychiatry 59, 1208–1218, doi:10.1016/j.biopsych.2005.12.003 (2006).
Jakovcevski, M., Schachner, M. & Morellini, F. Individual variability in the stress response of C57BL/6J male mice correlates with trait anxiety. Genes, brain, and behavior 7, 235–243, doi:10.1111/j.1601-183X.2007.00345.x (2008).
Der-Avakian, A., Mazei-Robison, M. S., Kesby, J. P., Nestler, E. J. & Markou, A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biological psychiatry 76, 542–549, doi:10.1016/j.biopsych.2014.01.013 (2014).
Anacker, C. et al. Neuroanatomic Differences Associated with Stress Susceptibility and Resilience. Biological psychiatry. doi:10.1016/j.biopsych.2015.08.009 (2015).
Bagot, R. C. et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun 6, 7062, doi:10.1038/ncomms8062 (2015).
Cao, J. L. et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. The Journal of neuroscience: the official journal of the Society for Neuroscience 30, 16453–16458, doi:10.1523/JNEUROSCI.3177-10.2010 (2010).
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404, doi:10.1016/j.cell.2007.09.018 (2007).
Wilkinson, M. B. et al. A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci 31, 9084–9092, doi:10.1523/JNEUROSCI.0039-11.2011 (2011).
Van Bokhoven, P. et al. Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. The European journal of neuroscience 33, 1833–1840, doi:10.1111/j.1460-9568.2011.07668.x (2011).
Riga, D., Theijs, J. T., De Vries, T. J., Smit, A. B. & Spijker, S. Social defeat-induced anhedonia: effects on operant sucrose-seeking behavior. Front Behav Neurosci 9, 195, doi:10.3389/fnbeh.2015.00195 (2015).
Riga, D. et al. A sustained depressive state promotes a guanfacine reversible susceptibility to alcohol seeking in rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 39, 1115–1124, doi:10.1038/npp.2013.311 (2014).
Reijmers, L. G., Hoekstra, K., Burbach, J. P., van Ree, J. M. & Spruijt, B. M. Long-term impairment of social memory in the rat after social defeat is not restored by desglycinamide-vasopressin. Neuroscience letters 305, 145–148 (2001).
Artola, A. et al. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. The European journal of neuroscience 23, 261–272, doi:10.1111/j.1460-9568.2005.04552.x (2006).
Joormann, J. & Quinn, M. E. Cognitive processes and emotion regulation in depression. Depression and anxiety 31, 308–315, doi:10.1002/da.22264 (2014).
Meerlo, P., Sgoifo, A., De Boer, S. F. & Koolhaas, J. M. Long-lasting consequences of a social conflict in rats: behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behavioral neuroscience 113, 1283–1290 (1999).
Cacioppo, J. T., Hawkley, L. C. & Thisted, R. A. Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychology and aging 25, 453–463, doi:10.1037/a0017216 (2010).
Witvliet, M., Brendgen, M., van Lier, P. A., Koot, H. M. & Vitaro, F. Early adolescent depressive symptoms: prediction from clique isolation, loneliness, and perceived social acceptance. Journal of abnormal child psychology 38, 1045–1056, doi:10.1007/s10802-010-9426-x (2010).
Stein, M. B. et al. Social anxiety disorder and the risk of depression: a prospective community study of adolescents and young adults. Arch Gen Psychiatry 58, 251–256 (2001).
Cruwys, T., Haslam, S. A., Dingle, G. A., Haslam, C. & Jetten, J. Depression and Social Identity: An Integrative Review. Personality and social psychology review: an official journal of the Society for Personality and Social Psychology, Inc 18, 215–238, doi:10.1177/1088868314523839 (2014).
Toth, I. & Neumann, I. D. Animal models of social avoidance and social fear. Cell and tissue research 354, 107–118, doi:10.1007/s00441-013-1636-4 (2013).
Rubin, K. H. & Burgess, K. In The developmental psychopathology of anxiety (eds M.W. Vasey & M.R. Dadds) 407–434 (Oxford University Press, 2001).
Marazziti, D., Consoli, G., Picchetti, M., Carlini, M. & Faravelli, L. Cognitive impairment in major depression. European journal of pharmacology 626, 83–86, doi:10.1016/j.ejphar.2009.08.046 (2010).
Murrough, J. W., Iacoviello, B., Neumeister, A., Charney, D. S. & Iosifescu, D. V. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem 96, 553–563, doi:S1074-7427(11)00117-1 [pii] 10.1016/j.nlm.2011.06.006 (2011).
Basso, M. R. & Bornstein, R. A. Relative memory deficits in recurrent versus first-episode major depression on a word-list learning task. Neuropsychology 13, 557–563 (1999).
Sexton, C. E., Mackay, C. E. & Ebmeier, K. P. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry 21, 184–195, doi:10.1016/j.jagp.2012.10.019 (2013).
Buwalda, B. et al. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neuroscience and biobehavioral reviews 29, 83–97, doi:10.1016/j.neubiorev.2004.05.005 (2005).
Jin, H. M. et al. The effects of social defeat on behavior and dopaminergic markers in mice. Neuroscience 288, 167–177, doi:10.1016/j.neuroscience.2014.12.043 (2015).
Broadbent, N. J., Squire, L. R. & Clark, R. E. Spatial memory, recognition memory, and the hippocampus. Proceedings of the National Academy of Sciences of the United States of America 101, 14515–14520, doi:10.1073/pnas.0406344101 (2004).
Broadbent, N. J., Gaskin, S., Squire, L. R. & Clark, R. E. Object recognition memory and the rodent hippocampus. Learn Mem 17, 5–11, doi:10.1101/lm.1650110 (2010).
Patki, G. et al. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiology & behavior 130, 135–144, doi:10.1016/j.physbeh.2014.04.011 (2014).
Cazakoff, B. N., Johnson, K. J. & Howland, J. G. Converging effects of acute stress on spatial and recognition memory in rodents: a review of recent behavioural and pharmacological findings. Prog Neuropsychopharmacol Biol Psychiatry 34, 733–741, doi:10.1016/j.pnpbp.2010.04.002 (2010).
Cazakoff, B. N. & Howland, J. G. Acute stress disrupts paired pulse facilitation and long-term potentiation in rat dorsal hippocampus through activation of glucocorticoid receptors. Hippocampus 20, 1327–1331, doi:10.1002/hipo.20738 (2010).
Keeney, A. et al. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol 18, 330–338, doi:10.1111/j.1365-2826.2006.01422.x (2006).
Marini, F. et al. Single exposure to social defeat increases corticotropin-releasing factor and glucocorticoid receptor mRNA expression in rat hippocampus. Brain Res 1067, 25–35, doi:10.1016/j.brainres.2005.10.002 (2006).
Fried, E. I. & Nesse, R. M. Depression is not a consistent syndrome: An investigation of unique symptom patterns in the STAR*D study. J Affect Disord 172C, 96–102, doi:10.1016/j.jad.2014.10.010 (2014).
Chen, L. S., Eaton, W. W., Gallo, J. J., Nestadt, G. & Crum, R. M. Empirical examination of current depression categories in a population-based study: symptoms, course, and risk factors. The American journal of psychiatry 157, 573–580, doi:10.1176/appi.ajp.157.4.573 (2000).
Tse, Y. C. et al. A longitudinal study of stress-induced hippocampal volume changes in mice that are susceptible or resilient to chronic social defeat. Hippocampus 24, 1120–1128, doi:10.1002/hipo.22296 (2014).
De Raedt, R. & Koster, E. H. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, affective & behavioral neuroscience 10, 50–70, doi:10.3758/CABN.10.1.50 (2010).
Koolhaas, J. M. et al. Coping styles in animals: current status in behavior and stress-physiology. Neuroscience and biobehavioral reviews 23, 925–935 (1999).
Blanchard, R. J., McKittrick, C. R. & Blanchard, D. C. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiology & behavior 73, 261–271 (2001).
Ebner, K., Wotjak, C. T., Landgraf, R. & Engelmann, M. Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Hormones and behavior 47, 14–21, doi:10.1016/j.yhbeh.2004.08.002 (2005).
Walker, F. R., Masters, L. M., Dielenberg, R. A. & Day, T. A. Coping with defeat: acute glucocorticoid and forebrain responses to social defeat vary with defeat episode behaviour. Neuroscience 162, 244–253, doi:10.1016/j.neuroscience.2009.04.041 (2009).
Narayanan, V. et al. Social defeat: impact on fear extinction and amygdala-prefrontal cortical theta synchrony in 5-HTT deficient mice. PloS one 6, e22600, doi:10.1371/journal.pone.0022600 (2011).
Walker, F. R., Hinwood, M., Masters, L., Deilenberg, R. A. & Day, T. A. Individual differences predict susceptibility to conditioned fear arising from psychosocial trauma. Journal of psychiatric research 42, 371–383, doi:10.1016/j.jpsychires.2007.01.007 (2008).
Anisman, H. & Matheson, K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev 29, 525–546, doi:10.1016/j.neubiorev.2005.03.007 (2005).
Caldwell, E. E. & Riccio, D. C. Alcohol self-administration in rats: Modulation by temporal parameters related to repeated mild social defeat stress. Alcohol 44, 265–274, doi:10.1016/j.alcohol.2010.02.012 (2010).
Schmidt, M. V. et al. High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology 35, 635–643, doi:10.1016/j.psyneuen.2009.10.002 (2010).
Krishnan, V. Defeating the fear: new insights into the neurobiology of stress susceptibility. Experimental neurology 261, 412–416, doi:10.1016/j.expneurol.2014.05.012 (2014).
Akkerman, S., Prickaerts, J., Steinbusch, H. W. & Blokland, A. Object recognition testing: statistical considerations. Behavioural brain research 232, 317–322, doi:10.1016/j.bbr.2012.03.024 (2012).
Acknowledgements
The authors thank Sophie van der Sluis for her valuable assistance with the statistical analysis, and Yvar van Mourik for his excellent technical assistance. ABS and SS received support from HEALTH-2009-2.1.2-1 EU-FP7 ‘SynSys’ (#242167); D.R., L.J.M.S. and A.B.S. received support from NBSIK PharmaPhenomics grant L.S.H. framework FES0908; DR, L.J.M.S. and S.S. were supported by an NWO VICI grant (ALW-Vici 016.150.673/865.14.002).
Author information
Authors and Affiliations
Contributions
D.R., W.J.G.H., A.B.S., S.S. designed behavioural experiments. D.R., L.J.M.S. executed behavioural experiments. D.R., L.J.M.S. analysed behavioural data. D.R., S.S. made the figures. D.R., A.B.S., S.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Riga, D., Schmitz, L.J.M., Hoogendijk, W.J.G. et al. Temporal profiling of depression vulnerability in a preclinical model of sustained depression. Sci Rep 7, 8570 (2017). https://doi.org/10.1038/s41598-017-06984-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06984-5
This article is cited by
-
Effects of Exercise Training on Executive Functioning in Adults with Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Sports Medicine (2023)
-
From stress to depression: development of extracellular matrix-dependent cognitive impairment following social stress
Scientific Reports (2020)
-
Carbamoylated erythropoietin modulates cognitive outcomes of social defeat and differentially regulates gene expression in the dorsal and ventral hippocampus
Translational Psychiatry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.