Abstract
Arbuscular mycorrhizal fungi (AMF) and dark septate endophytes (DSE) form symbiotic relationships with plants influencing their productivity, diversity and ecosystem functions. Only a few studies on these fungi, however, have been conducted in extreme elevations and none over 5500 m a.s.l., although vascular plants occur up to 6150 m a.s.l. in the Himalayas. We quantified AMF and DSE in roots of 62 plant species from contrasting habitats along an elevational gradient (3400–6150 m) in the Himalayas using a combination of optical microscopy and next generation sequencing. We linked AMF and DSE communities with host plant evolutionary history, ecological preferences (elevation and habitat type) and functional traits. We detected AMF in elevations up to 5800 m, indicating it is more constrained by extreme conditions than the host plants, which ascend up to 6150 m. In contrast, DSE were found across the entire gradient up to 6150 m. AMF diversity was unimodally related to elevation and positively related to the intensity of AMF colonization. Mid-elevation steppe and alpine plants hosted more diverse AMF communities than plants from deserts and the subnival zone. Our results bring novel insights to the abiotic and biotic filters structuring AMF and DSE communities in the Himalayas.
Similar content being viewed by others
Introduction
Arbuscular mycorrhizal fungi (AMF, sub-phylum Glomeromycotina1) are a crucial part of soil microbiota across major biomes and associate with the vast majority of terrestrial plant species2. These fungi are obligate plant root symbionts that gain photosynthetic carbon from their host plant while providing it with improved nutrient uptake, resistance to pathogens and abiotic stress tolerance3, 4. As a result, the diversity of plants and AMF can be inter-related5, and AMF can have a profound effect on plant community composition and dynamics4. To better understand the role of AMF in shaping plant communities, more information is needed on their diversity and distribution along environmental gradients. Despite recent effort in addressing global patterns of AMF6, much less is known about AMF communities in extreme environments.
Some of the most extreme conditions occur in high mountains where plants are limited by low temperature, desiccation and a short growing season7. Although it seems that associations with AMF can be especially beneficial for plants under limited resources, previous research indicates that symbiosis is rare or dysfunctional in adverse conditions, mostly due to constraints of the carbon economy at lower temperatures8, 9. More recent studies have, however, found AMF colonization in host plants and diverse spore populations as high as 5200 m a.s.l.10,11,12 as well as in some of the coldest places in the world8, 13. Even if high-altitude mountain regions harbour relatively diverse AMF communities, it remains unclear exactly which host plants are colonized and which AMF taxa are able to persist in extreme environments. In addition to AMF, dark-septate endophytes (DSE) are often recorded in the roots of high-elevation plants14, 15, where they can partially or completely replace AMF16. DSE comprises a miscellaneous group of root-inhabiting fungi with notoriously obscure taxonomic and functional affinities. Although they can have a positive effect on plant growth17,18,19, the symbiotic status of DSE remains uncertain20.
Although AMF community patterns have been quite well studied in grasslands, forests and agricultural land21, little work has been conducted at high elevations or along elevation gradients. In addition, most studies have been concentrated in the Alps while very few reports are from elevations above 5000 m a.s.l. from the Andes and the Himalayas. Those from Andes show a rapid decrease of AMF abundance in plant roots with increasing elevation14, 22 while a study from an elevation gradient of 1990 to 4648 m a.s.l. in the Himalayas found no effect of elevation on AMF diversity, a significant effect on taxonomic composition and a negative effect on the abundance of AMF23. The highest record of AMF in the Andes comes from an elevation of about 5200 m a.s.l.10, however there were no studies conducted above this elevation. Similarly, there is no work from the Himalayas from elevations above 5500 m a.s.l., although there are reports about the occurrence of vascular plants up to 6150 m a.s.l.24,25,26. The studies from the Himalayas were mainly targeted on spore populations of Glomeromycotina in the soil and showed the dominance of the Glomus 11, 23, 27 or Acaulospora 28 genera. It therefore remains unknown whether AMF and DSE occur in these extreme elevations and help vascular plants to cope with the harsh environmental conditions.
We address the knowledge gap concerning AMF distribution along elevation gradients and its relation to the ecological properties of host plants by exploring the composition of mycorrhizal fungal communities associated with vascular plants growing in Ladakh, an arid high-elevation region in the NW Himalayas. Due to the dry continental climate, the mountains of Ladakh are often unglaciated up to 6200–6400 m a.s.l. allowing plant species to grow in extreme elevations, even up to 6150 m a.s.l.7. Environmental conditions change along the elevational gradient, ranging from a dry and hot desert, over a wide steppe belt, a water- and temperature-limited alpine belt and up to a cold, sparse subnival vegetation zone. Based on previous reports27, we can expect more diverse AMF communities in less stressful steppe habitats with the highest vegetation cover, and a decrease in AMF diversity towards more extreme habitats - deserts, alpine and subnival zones - with lower plant abundance. On the other hand, DSE are often recorded in the roots of plants from harsh conditions as high-elevation29 or arctic tundra30. Existing data suggest that DSE are more frequent in the plant roots in these habitats than AMF10, 30. Although a significant gap in knowledge exists about the functions of DSE in cold-stressed habitats, the phosphorus uptake is most probably enhanced by fine endophytes31 and they could be surrogate mycorrhizas in these ecosystems32.
In addition to environmental conditions, functional traits of host plant species, in particular those related to the carbon and water economies might influence the formation of mycorrhizal symbiosis as well as AMF community composition in plant roots33. Laboratory experiments showed that increasing the plant’s carbon supply to the AMF increases the uptake and transfer of phosphorus from fungi to plant34. Likewise, phosphorus uptake and transfer are lowered when the photosynthate supply to the fungi is decreased. Additionally, some species of AMF are poor symbionts, providing little phosphorus while taking relatively high amounts of carbon, resulting in a negative growth response35. Therefore, applying detailed analysis of AMF composition and diversity in tandem with host plant functional traits reflecting the carbon and water economy, may offer better insights into the processes of AMF community formation under varying environmental conditions.
One of the critical features of comparative studies on mycorrhizal association with plants and their functional traits and environmental preferences is the extent of phylogenetic relatedness among plant taxa36. Part of the explanatory power uncovered by relating the fungal community variation to plant ecological preferences might be alternatively explained by host plant phylogenetic inertia affecting both the similarity of fungal communities among closely related host taxa and the similarity of ecological niches that such taxa occupy. Understanding the interspecific differences in fungal communities between host species therefore requires separation of the evolutionary inertia of host plants from their true adaptation to the environment. This is commonly done by comparing analyses made with and without phylogenetic corrections37. This approach is based on discounting all of the variation that could possibly be explained by phylogenetic relatedness of the host species, before studying the effect of other potential predictors such as the species’ ecological niches or functional traits.
This study aimed to explore the variation in AMF community diversity and composition as well as DSE occurrence in relation to factors including altitude, habitat type, and phylogenetic relatedness as well as functional traits of host plants. We hypothesized that unlike the temperate plants that rely more on AMF in stressful and nutrient poor conditions, high-elevation plants from arid Himalayan regions will host more diverse and abundant AMF communities when they are in mesic steppe or alpine environments rather than in the extreme habitats represented by either deserts at low elevations or the cold subnival zone at high elevation. Specifically, we asked: what is the uppermost limit for AMF in the arid Himalayas? Do the habitats and their specific plants harbor specific taxa of AMF? Which abiotic and biotic factors are influencing AMF community richness and composition? Are DSE associated with the highest elevations? To answer these questions, we analyzed the AMF communities in plant roots from contrasting habitats using 454 sequencing of the SSU rRNA gene, and evaluated intra-radical AMF and DSE colonization by traditional microscopic quantification. Analyzing the roots and not the soil is an advantage in this case, as it indicates functionality rather than just identifying spores in the soil, which could very well be dormant. Although next generation sequencing (NGS) has been previously used to assess the taxonomic diversity of AMF6, it has not been used so far in studies from high-elevation regions. Additionally, we built a phylogenetic tree for host plant species to relate the variation in AMF community diversity and composition to host plant phylogeny. Finally, we compared in detail the variation of plant traits between mycorrhizal (Poa attenuata) and non-mycorrhizal (Waldheimia tridactylites) plant species as well as an intraspecific variation of AMF and DSE in the roots of Poa attenuata, both along a substantial elevational gradient.
Results
Plant mycorrhizal status
Out of 62 plant species studied, roots of 32 species were colonized by AMF structures (hyphae, vesicules and arbuscules) and were therefore classified as mycorrhizal plants. Two plant species (Carex sagaensis and Polygonum viviparum) had a very low level of hyphal colonization (less than 15%) with no vesicles or arbuscules present, and these were classified as non-associated with AMF. DSE was detected in 40 plant species (for details see Table S1).
The highest-living plants with AMF in their roots were Poa attenuata individuals from 5800 m a.s.l., and Saxifraga cernua and Saxifraga nanella individuals from 5740 m a.s.l. (Fig. 1). From 5800 up to 6150 m a.s.l no AMF was detected, however several plant species’ roots (P. attenuata, S. nanella and Stellaria decumbens) were colonized by DSE fungi.
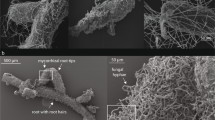
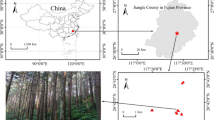
(a) Map of sampling sites (red point) in Ladakh, NW Himalaya. (b) Desert in lower part of Markha valley (3 400 m a.s.l.). (c) Steppe nearby the Tso Moriri Lake (4 800 m a.s.l.). (d) Alpine graslands nearby the Tso Moriri Lake (5 200 m a.s.l.). (e) Subnival vegetation on the eastern slope of the Shukule peak (5 800 m a.s.l.). (f) Habitat of Poa attenuata at 5800 m a.s.l. (g) Hyphae and arbuscules of AMF in a Poa attenuata root sample from 5800 m a.s.l. Map was generated in ArcGIS 10.2.1 (www.esri.com).
Molecular identification of AMF community
We obtained a total of 52 264 quality-filtered pyrosequencing reads which belonged to 46 OTUs of Glomeromycotina (Table S2). Out of these, 43 OTUs matched (97% similarity) previously-known virtual taxa (VT; i.e a molecular operational taxonomic unit) from the MaarjAM database21 of published Glomeromycotina SSU rRNA gene sequences, and 3 OTUs were treated as novel VT (94–96% similarity to previously known VT belonging to Glomeraceae, Paraglomeraceae and Claroideoglomeraceae).
From 46 OTUs, the majority (33 OTUs) belonged to Glomeraceae (Glomus group A). In addition, members of Claroideoglomeraceae (Glomus group B, 5 OTUs), Diversisporaceae (4 OTUs), Acaulosporaceae (1 OTU), Gigasporaceae (1 OTU), Pacisporaceae (1 OTU) and Paraglomeraceae (1 OTU) were found (Figs 2, 3 and S1).
Two cladograms of Bayesian majority-rule consensus tree showing the position and relationship between recorded AMF (cladogram on left site, based on partial sequence of SSU r RNA) and host plants (cladogram on right site, based on ITS, trnT-trnL, matK + trnK, and rbcL). Connections between them are colored by fungal family: Claroideoglomeraceae (yellow), Paraglomeraceae (white), Pacisporaceae (red), Gigasporaceae (blue), Diversisporaceae (green), Acaulosporaceae (violet) and Glomeraceae (gray with black lines). Interrupted lines indicate connection between fungi and putative non-mycorrhizal plants. The AMF tree includes the detected OTUs together with the most closely related virtual taxa (VTX) from MaarjAM database. The full version of the AMF cladogram with Bayesian posterior probabilities is in Fig. S1. Potentilla pamirica (associated with 6 OTUs) and Tanacetum stolickae (associated with 2 OTUs) is omitted in the plant cladogram due to the lack of their sequences in GenBank.
Relationships between AMF diversity, colonization and elevation
The highest AMF richness was found in the roots of Potentilla multifida (Rosaceae, 22 OTUs), Leontopodium ochroleucum (Asteraceae, 18 OTUs) and Tanacetum pyrethroides (Asteraceae, 16 OTUs). We found on average 6.5 OTUs per sample (Fig. 2 and Table S1). AMF richness did not significantly differ between plant families (Chi2 11 = 11.669, P = 0.389). AMF richness and Shannon diversity, as well as AMF colonization in plant roots had a unimodal relationship with elevation, exhibiting a peak at mid-elevations (Fig. 4). AMF richness was positively related to the intensity of AMF colonization (r = 0.1421, F1,30 = 4.80, P < 0.05). DSE colonization intensity, however, was not significantly related to elevation (Fig. 4)
Relationships between AMF, plant traits, environmental conditions and plant phylogeny
The phylogeny-corrected RDA analyses showed a significant effect of elevation on AMF community composition in plant roots. Elevation explained 9.2% of the total variation in fungal species composition (P < 0.05, Table 1). The results of the analyses without phylogenetic corrections (hereafter ahistorical comparison) of host plant relatedness and the phylogeny-corrected analyses showed similar patterns and so only the results from the latter ones are presented in the RDA ordination diagram (Fig. 5A). The differences in the OTU composition along the first axis correspond to elevation. Members of Glomeraceae occur more frequently at lower elevation steppes and deserts. Conversely, members of Clarodieoglomeraceae and most of Diversisporaceae prevail in the higher elevations. In addition, there was a significant, unique effect of habitat type (after accounting for the effect of elevation) on AMF community composition, however, only in the analyses without phylogenetic corrections. Indicator species analyses (Fig. 5B, see also Fig. 2) identified a significant relationship between one OTU (OTU 11) and desert habitat (P < 0.01), and between 5 OTUs (OTU 10, 18, 36, 39 and 40) and steppe habitat types (P < 0.05). Two OTUs (OTU 26 and 28) were related to both of these habitats and the alpine habitat lacked indicator OTUs.
Analysis of AMF community patterns. (A) Ordination diagram with the first two partial RDA axes (axes explain 9.2% of the variance left after accounting for phylogenetic relations) showing the relationship between OTU composition and elevation. Small arrows point in the direction of increasing expected values of OTUs occurrence, the large arrow points in the direction of increasing expected value of elevation. The twenty best-fitted plant species are shown. AMF families are indicated by arrow type: Glomeraceae (empty grey arrows), Gigasporaceae (filled grey arrow), Acaulosporacea (filled black arrow with dashed line), Claroideoglomeraceae (empty black arrows), Pacisporaceae (empty grey arrow with dotted line), Diversisporaceae (filled black arrows). For plant taxa, the first four letters from genus and species names are used in diagram, full names are in Table S1. (B) Presence-absence heat map showing the occurrence of individual OTUs in different habitats.
In the phylogeny-corrected RDA analysis of the relationship between a set of plant traits and elevation, the first ordination axis (horizontal) separated taller mycorrhizal species (i.e. Potentilla multifida or Tanacetum fruticulosum) typical for lower elevation desert and steppe habitat from smaller, non-mycorrhizal species (e.g. Ladakiella klimesii or Arenaria bryophylla) occuring at higher elevation alpine and subnival zones (Fig. 6, Table 1). The high-elevation plants had significantly higher concentrations of phosphorus in their leaves, nitrogen and phosphorus in their roots, higher root non-structural carbohydrates and higher water use efficiencies (more negative leaf δ13C), compared to the plants at lower elevations (see also Fig. 4). There was a significant, unique effect of habitat type (after accounting for the effect of elevation; explained 9.3% of variability) on the distribution of mycorrhizal plants, that tended to be in deserts and steppes while non-mycorrhizal plants tended to occur in the alpine and subnival zones (Table 1).
Analysis of plant functional traits. The first two partial RDA axes (after accounting for phylogenetic relations among species) showing the relationship between plant functional traits (ecophysiological parameters, AMF and DSE colonization) and elevation. Displayed axes explain 9.5% of the variance left after accounting for phylogenetic relations. Small arrows point in the direction of increasing expected values of corresponding plant traits, the large arrow points in the direction of increasing expected value of elevation. The twenty best-fitted plant species are shown. First four letters from genus and species names of host plants are used in diagram, full names are in Table S1. Full names of ecophysiological parameters are in the Table 1 legend.
Poa attenuata and Waldheimia tridactylites along the elevational gradient
Although Poa attenuata and Waldheimia tridactylites both belong to potentially mycorrhizal plant families, only the roots of P. attenuata were intensively colonized by AMF and DSE, and no structures of AMF and a very low abundance of DSE were detected in the roots of W. tridactylites. While the intensity of AMF colonization in the roots of P. attenuata significantly decreased with elevation, the opposite pattern occurred for DSE (Fig. 7). DSE colonization was significantly negatively correlated with AMF colonization in the roots of P. attenuata (r = −0.745, n = 20, P < 0.001). Plant height of both species decreased with elevation. There were large differences in nutrient and carbohydrate concentrations between both species (Fig. 7). Namely, while the concentration of phosphorus, nitrogen and soluble sugars in the roots of W. tridactylites significantly increased with elevation, the opposite pattern was evident for P. attenuata. In addition, root nitrogen concentration of P. attenuata was positively correlated with the intensity of DSE colonization, and root phosphorus concentration with the intensity of AMF colonization (Fig. 8).
The effect of intensity of AMF and DSE colonization on nutrient content in Poa attenuata roots. (A) Correlation between root phosphorus concentration and intensity of AMF colonization (r = 0.578, n = 22, p = 0.005). (B) Correlation between root nitrogen concentration and intensity of DSE colonization (r = 0.572, n = 22, p = 0.005).
Discussion
In order to improve the understanding of AMF distribution and diversity in extreme high-elevation environments, we analyzed AMF communities in the roots of 62 plant species from contrasting habitats along a prominent elevation gradient (3400–6150 m a.s.l.) in the dry mountains of Ladakh using a combination of optical microscopy and next generation sequencing.
As the region of Ladakh encompasses a wide range of conditions with low-elevation sites being dry and warm and high-elevation sites cold and relatively moist, plant responses bear a strong abiotic signal due to very low vegetation cover and the marginal role of interspecific interactions. These qualities of our study region allowed us to filter out major confounding factors, providing more reliable and general inferences about plant-fungal interactions along environmental gradients.
We found that high-elevation plants from the arid Himalayas host more diverse and abundant AMF communities in moderately stressful mesic steppes and alpine environments rather than in extreme habitats such as deserts at the low elevations or cold subnival zones at the highest elevations with plant life. The AMF OTU richness and intensity of colonization of plant roots declined both towards dry and cold ends of the elevational gradient. The phylogeny-corrected RDA analyses of relations between environmental conditions and intensity of colonization of mycorrhizal fungi showed significant, unique effects of habitat after accounting for elevation. Members of Glomeraceae prevailed more frequently at lower elevation steppes and deserts, while members of the Claroidieoglomeraceae and most of the Diversisporaceae were at the higher elevation alpine habitat. These results correspond with the habitat hypothesis, which postulates that both plant and AMF communities follow changes in abiotic conditions38.
There are several lines of evidence in our results supporting this view. For instance, AMF richness in plant roots exhibited a mid-elevation peak, similar to that previously observed for vascular plants39. The multivariate analyses with phylogenetic correction of host plant relatedness showed that the AMF community composition, and host plant traits covary with elevation across different habitats. Most of the results stay significant after the correction for phylogenetic relatedness of host plants, suggesting that the compositional differences of AMF are driven by host plant adaptations to temperature and moistureconstraints along the elevational gradient. Also, we detected AMF up to 5800 m a.s.l. in roots of Poa attenuata, but the individuals of the same species occurred without AMF up to 6150 m a.s.l., indicating that AMF are more limited by extreme conditions than their host plants13. This is in accordance with the positive correlation between the phosphorus content in roots of Poa attenuata and the intensity of mycorrhizal colonization, which corresponds to our present knowledge about AMF functioning where fungi contribute phosphorus to the plants2.
The rapid decrease of AMF abundance in plant roots along the elevational gradient corresponds with previous reports from high elevations10, 14, 22, 23. Nevertheless, all records from these studies come from elevations up to 4000 m a.s.l. and the elevation maximum of AMF occurrence is at 5500 m a.s.l.12. Comparing such results with studies from the Himalayas is difficult, because there are only a few reports from high elevations and most of them were focused on the diversity of AMF in the soil11, 23, 27, 40 that do not allow one to clarify the AMF abundance in plant roots. Analyzing the roots indicates more functional mycorrhizal associations than simply identifying spores in the soil, which could be dormant. We recorded the presence of AMF in plant roots up to 5800 m a.s.l. by using both methods - microscopy and 454 sequencing. Between 5800 and 6150 m elevation, only the two most common AMF OTUs were recorded by molecular methods in very low abundance. Simultaneously, in these samples, no arbuscules or vesicles were recorded using the optical microscope and only hyphae belonging to DSE were recorded. The positive results are probably caused by very low levels of AMF colonization. A similar case was Polygonum viviparum, which has been previously reported as an ectomycorrhizal (ECM)41, or in some cases ECM + AM plant (Wang & Qiu 2006). However, we did not detect any typical ECM nor AM structures in the roots of P. viviparum, but instead some hyphae. Simultaneously, we detected two OTUs from the same individuals, but of very low abundance. By combining optical microscoping with molecular methods we were able to clarify the mycorrhizal status of some plant taxa in our study area.
As expected, OTUs richness of AMF was influenced by the limitation of extreme conditions. The highest richness and abundance of AMF communities were found in mesic steppes with the most optimal conditions for plant growth compared to other habitat types (Fig. 5B). Diversity and abundance of AMF were lower in alpine and deserts habitats, and AMF presence was not recorded in the subnival zone with the most extreme conditions at 6150 m a.s.l.24. The absence of AMF in plant roots from the highest subnival habitats is likely caused by the conditions7, 25 being too extreme to allow mycorrhizal structures to establish, or by the absence of AMF in the soil. A likely reason is the short vegetation season, declining from 120 days at 5200 m a.s.l. to 40 days at 6150 m a.s.l., and a higher frequency of freeze-thaw cycles occurring7. This effectively reduces the time for plant assimilation, resulting in an insufficient photosynthate supply to the fungi. Moreover, phosphorus is readily available in the subnival zone, because the substrate is much wetter than in the lower elevation steppes and deserts. Elevated soil moisture, together with intensive bedrock weathering in the periglacial zone7 results in higher soil phosphorus concentrations, which reduces the plants’ need for AMF symbiosis. Low-temperatures in high-elevation zones restrict sink (growth) processes in plants more than the uptake of water and nutrients. Hence, the plants’ inability to use the absorbed resources for growth and tissue formation due to low-temperature restriction on enzymatic processes leads to high nutrient but also carbohydrate concentrations in alpine and subnival plants42. These processes could explain why our high-elevation plants have low AMF richness and abundance but high root phosphorus concentrations.
AMF absence in roots from the highest subnival habitats could also be the result of the dispersal limitation of AMF. Their large spores (up to 500 µm diameter) are only partly able to be dispersed by air, with hyphal growth allowing them to reach new substrates better than wind dispersal2. Due to this, AMF are often absent on initial successional stages in the glacier forefront with undeveloped soils43, 44, similar to the soil in subnival habitats of the studied Himalayan transect. In contrast to the glacier forefront, which predominantly connects into later successional sites occupied by AMF, the subnival zones are often fragmented by unsuitable habitats, making the colonization of new sites more difficult. On the other hand, Oehl and Körner8 recorded AMF in the roots and rhizospheres of isolated plants of Saxifraga oppositifolia on the summit of Dom in Valais (Switzerland). They suppose that wind dispersal of untypically small spores (50–90 µm) could lead to the successful AMF colonization of an unoccupied isolated site8, 44. Based on these controversial records, we are not able to make firm conclusion and research focusing on the dispersal of AMF and their spores in the soil is much needed.
The genus Glomus dominated in all studied habitats, which corroborates the other studies from the high elevation of Himalayas and elsewhere6. Out of 46 detected OTUs, we were unable to find a match from known VTs for three OTUs, others belonged to known virtual taxa (VT) already recorded in the MaarjAM database which includes world-wide accessions of AMF taxa21. In two previous studies from the Tibet plateau, 8 new AMF phylotypes (out of 21 detected) from high elevation (4500 m a.s.l.)27 and 8 new phylotypes (out of 38 detected) from lower elevation (3500 m a.s.l.)45 were reported. In comparison with the results of Gai et al.11, 23, we noted an absence of the genera Scutellospora and Ambispora and a low occurrence of genus Acaulospora in our samples. Although a comparison of our dataset based on molecular analyses with morphospecies detected by Gai et al.11, 23 is difficult, and the absence of Ambispora was most likely caused by the poor amplification of Archaeosporales by the AM1 primer46, the main differences in genus composition are recognizable. In addition, Liu et al.27 mentioned a similar absence of genus Scutellospora in their results. From the results of RDA, the Glomeraceae tend to occupy lower elevations and other families prefer higher elevations (see Fig. 5A). This is in accordance with the results of Li et al.47, who found that Glomeraceae abundance decreased with elevation while the other families increased.
We found high abundance of DSE in the roots of P. attenuata from alpine and subnival habitats. In addition, we found DSE reaching higher elevations than AMF. Although, the relationship between DSE root colonization intensity and elevation was not significant in our study, several studies43, 48 have reported an existing relationship. However, there was a significant correlation between nitrogen content in roots of P. attenuata and the intensity of DSE colonization, as was reported by Newsham19. Simultaneously, there was a correlation between nitrogen content and elevation and between DSE colonization and elevation. Therefore, our observation matches with the hypothesis that DSE might help with nutrient acquisition and could be surrogate mycorrhizas in these ecosystems32, although our data do not provide direct evidence about the positive effect of DSE on P. attenuata at extreme elevations. On the other hand, our observation is not consistent with some other studies, which show that phosphorus uptake is most probably enhanced by fine endophytes30. Although it could be very interesting, molecular identification of the fungal species of DSE is currently not feasible due to the absence of DSE-specific primers. Taxa of DSE could be amplified by universal fungal primers (e.g. ITS 1F49) together with most of the other fungal species in the plant roots, however, distinguishing between DSE and other fungi is difficult.
In summary, the highest diversity and abundance of AMF communities along the elevational gradient in the dry Himalayas were found in mesic steppes with the most suitable conditions for plant growth. Additionally, our results showed that AMF was limited by extreme conditions more than their host plants, which have their upper elevation limits at higher elevations than AMF. Species richness and colonization intensity of AMF were lower in alpine and desert habitats, and no AMF was recorded in the subnival zone above 5800 m a.s.l. with most extreme conditions. Although the mycorrhizal symbiosis is considered beneficial in stressful environments, it was absent at the highest elevations. This may be caused by the dispersal limitation of AMF in soils or by conditions too extreme to allow the establishment of the mycorrhizal structures. Conversely, DSE were found rather evenly throughout the whole elevation gradient and in all habitats, however, their benefits for plants remain unclear.
Material and Methods
Study area
The study was conducted in the Himalayan Mts. of Ladakh, Jammu and Kashmir State, India. The Ladakh region is arid (Leh: 115 mm/yr, 3514 m a.s.l., ca. 170 km NW of the study region, Gar: 54 mm/yr, 4232 m a.s.l., ca. 160 km SE of the study region). The studied localities ranged from 3400 m in Markha Valley to 6150 m in Tso Moriri Lake area (Fig. 1). Desert and semi-desert occupy the (relatively) lowest elevations of the Indus Valley and its major tributaries (ca. 2900–3800 m a.s.l.). The subalpine vegetation belt stretches from approximately 3800 up to 5000 m a.s.l. in east Ladakh, and is widely dominated by cold steppe vegetation. The alpine belt extends between ca. 4500 and 5200 m (occasionally to 5500 m) a.s.l., on the Tibetan border, and hosts alpine grasslands, including the characteristic moist alpine turf of Kobresia pygmaea. A very sparse subnival vegetation zone is developed up to 6150 m a.s.l.
The elevational gradient in our study region is tightly related to a temperature/drought gradient39, 50. It is therefore warmer at low-elevation deserts, with intense drought due to lower precipitation and colder and more humid at high-elevation alpine and subnival zones. The length of the growing season decreases linearly with increasing elevation, from 270 to 30 frost-free days, while mean growing season temperature decreases from 13 to 3 °C between 3400 and 6150 m50. Daily temperatures in the study area vary from 0 to 30 °C in summer and from −40 to −10 °C in winter. Annual precipitation is about 50 mm in the desert and semi-desert belt (falling mostly during the Indian summer monsoon), 100 mm in the alpine steppes, and 150–250 mm in the alpine and subnival zones50, 51. Precipitation above 5000 m a.s.l. is mostly falling as snow and its more frequent in summer than in winter7.
Soils have a coarse-grained structure, with a high percentage of large gravel, low water and organic matter content, high pH (7–8) and relatively high concentrations of total N and P52. The organic matter content and concentrations of total nitrogen, ammonia, nitrates and phosphorus increase with increasing elevation, with the highest values in the alpine zone and the lowest in the desert and semi-desert7, 53. The concentration of calcium and magnesium and the pH decreases with increasing altitude. The coarsest soils are found in subnival zone, where the fraction >0.5 mm represents up to 60% of the soil particles; the soil with highest proportion of fine particles is from alpine meadows.
Plant sampling
From each site we collected one to twelve common species, represented usually by three individuals per species. In addition, Poa attenuata (Poaceae) and Waldheimia tridactylites (Asteraceae) were sampled at 150–200 m intervals on an elevation gradient from 5050 to 6150 m a.s.l. on the eastern slope of Shukule Peak above lake Chilling Tso. Fresh, non-damaged and fully developed plants at a comparable phenological stage were collected during the peak of the growing season, weighed fresh and then sun-dried in situ. Altogether, 219 individual plants of 62 plant species were collected. For the details see Table S1 (Supporting information).
AMF and DSE quantification
We collected fine roots from each sampled plant and stored the samples in silica gel until processing. Approximately one-half of each sample was used for the quantification of the intra-radical AMF and DSE, whereas the other half was stored for DNA-based AMF taxon identification. The roots for fungal quantification were stained with Chlorazol Black E (Sigma-Aldrich, USA) using a modified protocol of Vierheilig et al.54: rehydrated roots were cleaned in 10% KOH at room temperature for 16 h, neutralized in 3.5% HCl for 2 min, and stained using 0.03% w/v of Chlorazol Black E in lactoglycerol (14:1:1 lactic acid, glycerol and deionized water) in a 90 °C water bath for an hour. Root AMF and DSE colonisation was quantified under a light microscope BX-50 (Olympus, Japan) in a standard light field at ×40–200 magnification, with the morphological details of AMF structures observed at ×400 magnification. To record the intensity of root colonization, we estimated a four categorical variable: total mycorrhizal colonization (six classes), the abundance of arbuscules and vesicles (four classes for both), and DSE colonization (six classes); and used them to calculate the intensity of root colonization according to a modified formula of Trouvelot et al.55, for details see supporting information A1. DSE structures were defined as clusters of inflated, rounded and thick-walled cells (microsclerotia) and melanized and septate hyphae within the cortical cells of roots as described in Jumpponen and Trappe56. The abundance of arbuscules (r = 0.693, n = 84, P < 0.001) and vesicles (r = 0.517, n = 84, P < 0.001) significantly positively correlated with mycorrhizal colonization, so only the total mycorrhizal colonization was used for statistical analyses. Plants with hyphal colonization more than 15% and presence of arbuscules or vesicles were considered as mycorrhizal. All samples from plant species where mycorrhiza was observed were further used for molecular identification of AMF taxa.
Molecular identification of AMF
To assess the taxonomic composition of AMF, the collected root samples of the same species and from the same site were pooled (42 pooled samples in total, belonging to 30 plant species). Total DNA was extracted by CTAB method as described by Doyle & Doyle57 and purified using the PowerClean DNA Clean-Up Kit (MO BIO Laboratories, USA). A partial sequence of the small subunit (SSU) rRNA gene was amplified using primers NS31 (universal eukaryotic58) and AM1 (specific to AMF59). PCR products were used as a template for a secondary, semi-nested PCR using the universal eukaryotic primer WANDA60 fused with 454 Adaptor A and multiplex identifier (published by 454 Life Sciences Corporation/Roche Life Science, USA, http://www.454.com/), and the reverse primer AM1 fused with 454 Adaptor B. Both PCRs were conducted with Plain Combi PP Master Mix (Top-Bio, Czech Republic) under a temperature profile of 94 °C for 3 min, followed by 35 cycles at 94 °C for 30 s, 62 °C for 1 min, 72 °C for 40 s, and a final extension at 72 °C for 10 min for the first PCR and 94 °C for 3 min, followed by 10 cycles at 94 °C for 30 s, 60 °C for 1 min, 72 °C for 35 s, and a final extension at 72 °C for 10 min for the secondary PCR. We successfully amplified 40 samples belonging to 28 plant species. The PCR products were gel-purified using the UltraClean GelSpin DNA Extraction Kit (MO BIO Laboratories, USA), pooled at equimolar concentration, and subjected to unidirectional sequencing on a GS Junior System using Titanium Series reagents (454 Life Sciences Corporation ⁄ Roche Life Science, USA) at the Institute of Soil Biology (Biology Centre ASCR, Č. Budějovice, Czech Republic). To achieve directionality of the sequencing, DNA template was added to the A-beads only.
Sequence reads were processed using SEED 1.2.1_64bit61 and Mothur v.1.3662. Sequences were trimmed based on a minimum Phred score of 25, averaged over 50 bp moving window and chimeric sequences were detected by the UCHIME63. Only reads with a minimum of 400 bp were included and these were trimmed to a maximum length of 510 bp. Operational taxonomic units (OTUs) were delimited using USEARCH64 and 97% similarity threshold, and singletons were removed. Putative fungal species identity ware assigned using BLASTn search against the NCBI database (http://www.ncbi.nlm.nih.gov). Fungi belonging to Glomeromycotina were classified using BLASTn search against the MaarjAM database (November 2015)21. One representative sequence of each Glomeromycotina OTU was deposited in GenBank (Table S2). Samples which yielded more than 10 AMF sequences were used for further statistical analyses.
Phylogenetic analysis
Phylogenetic relationships were analyzed using Bayesian inference. The phylogenetic position of AMF symbionts was analyzed using the partial sequence of SSU rRNA of one representative sequence per OTU. The obtained sequences, their close BLAST matches from MaarjAM database, and AMF sequences from GenBank covering main phylogenetic group were used for the analysis (see Table S2 for GenBank accession numbers). In total 98 sequences were aligned using the E-INS-i algorithm in MAFFT v6 (http://mafft.cbrc.jp/alignment/server65) and adjusted manually, yielding an alignment of 506 positions. The GTR model with auto-correlated discrete gamma distribution66 was selected by evaluating the AIC values in Kakusan4 v267. Two runs of 5 × 106 generations with sampling every 1000th generation were conducted in MrBayes v3.2.568 under the best model assumption. The analysis was considered complete when the average standard deviation of split frequencies dropped below 0.01. The first 25% of trees were discarded as burn-in with the majority-rule consensus tree being built from the remaining trees. Branches with Bayesian posterior probabilities (BPP) below 0.95 were regarded as poorly supported.
For building the plant phylogeny tree, the sequences were extracted from GenBank (www.ncbi.nlm.nih.gov/nuccore/) and a combined multigene approach was applied. The maximum data coverage was achieved for the internal transcribed spacer (ITS) of nuclear ribosomal DNA and three chloroplast loci: trnT-trnL intergenic spacer, matK + trnK region, and the gene for rubisco large subunit (rbcL). The GTR model with autocorrelated discrete gamma distribution was selected as the best substitution model and two runs of 5 × 106 generations with sampling every 1000th generation were conducted. The analysis was considered complete when the average standard deviation of split frequencies dropped below 0.01. The first 25% of trees were discarded as burn-in with the majority-rule consensus tree being built from the remaining trees. The nomenclature follows the Ladakh plant list69.
Plant functional traits measurements
We measured several morphological and ecophysiological traits on the same plant individuals from which the fine roots were collected. These traits were: plant height, leaf carbon, nitrogen and phosphorus concentrations, leaf δ 13C, root nitrogen and phosphorus concentrations, and the content and composition of nonstructural carbohydrates. After transport to the laboratory the leaves and roots were oven dried (60 °C) until constant weight, ground and analyzed. Phosphorus was determined colourimetrically after digestion in HClO4 using a UV - 1650PC spectrophotometer (Shimadzu, Japan). The δ13 C in the plant leaves, as well as total carbon and nitrogen in the plant leaves and roots, were measured using an elemental analyser coupled to an IRMS at the Stable Isotope Facility, UC Davis, USA (http://stableisotopefacility.ucdavis.edu/). The δ13C values were used as an integrated, long-term measure of the ratio between internal and ambient CO2 concentrations (Ci/Ca), which reflects the intrinsic water use efficiency of the plants70. Fructan and starch contents were determined colourimetrically, and free sugars were quantified using a high-performance anion exchange chromatography with a pulsed amperometric detection71.
Statistical analyses
The relationship between the AMF community parameters (OTU composition) and host plant species habitat parameters (elevation and habitat type) was evaluated using multivariate redundancy analysis (RDA72) fitted for OTU community data as a response variable in a global multivariate test. The relationship between plant functional traits (expressed by ecophysiological parameters of leaves and roots, and binary values of AMF and DSE colonization) and habitat parameters (elevation and habitat type) were evaluated using RDA fitted for trait data as a response variable in a global multivariate test. Elevation or habitat with elevation as a covariate were used as explanatory variables. Type I errors were estimated using non-parametric Monte Carlo permutation tests, based on the F statistic, with 1999 random permutations. We used the method by Diniz-Filho et al.73 as modified by Desdevises et al.37 for phylogenetic corrections. There, the variation explained by the phylogenetic relatedness of host species was removed from the model, using species coordinates on selected axes of a principal coordinate analysis (PCoA, calculated for both models separately) calculated from a patristic distance matrix corresponding to the MrBayes phylogenetic tree described above. Selected principal coordinates, which were used as covariates in tests including phylogenetic correction, were also used as predictors for fungal community composition (first model), as well as for functional traits and AMF and DSE colononization (second model), to estimate the amount of variation in the trait values explained by species evolutionary history37. All multivariate analyses were done using Canoco 574.
All other analyses were performed by R software, version 3.2.375. The Kruskal-Wallis test was used to compare OTU richness between plant families. Correlation between nutrient content and intensity of DSE and AMF colonization were quantified by Pearson linear correlation. The ape 76 package was used for the creation of phylogenetic trees. Indicator species analyses was performed with the indicspecies 77 package using the multipatt function with 999 permutations. To assess whether the attribute (OTU richness, OTU diversity - based on Shannon diversity index computed from OUT abundance, intensity of AMF and DSE colonization, functional traits) responses to elevation gradient were linear or nonlinear, a first (straight line) and second (quadratic model) order polynomial was fitted and the trend (increase, decrease, hump, or valley shape) was recorded. Response curves of the individual parameters were fitted using RLM (Robust Fitting of Linear Models). This was conducted by iterated re-weighted least squares using R package MASS78. Statistical differences between tested categories (e.g. age classes, NSC compounds) were based on 95% confidence intervals calculated for each the RLM line, with non-overlapping intervals indicating statistically significant differences.
References
Spatafora, J. W. et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108, 1028–1046 (2016).
Smith, S. E. & Read, D. J. Mycorrhizal Symbiosis. Soil Science Society of America Journal 137 (2008).
Rillig, M. C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. soil Sci. 84, 355–363 (2004).
van der Heijden, M. Ga., Boller, T., Wiemken, A. & Sanders, I. R. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79, 2082–2091 (1998).
Hiiesalu, I. et al. Species richness of arbuscular mycorrhizal fungi: Associations with grassland plant richness and biomass. New Phytol. 203, 233–244 (2014).
Davison, J. et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science (80-.). 349, 970–973 (2015).
Doležal, J. et al. Vegetation dynamics at the upper elevational limit of vascular plants in Himalaya. Sci. Rep. 6, 24881 (2016).
Oehl, F. & Körner, C. Multiple mycorrhization at the coldest place known for Angiosperm plant life. Alp. Bot. 124, 193–198 (2014).
Ruotsalainen, A. L., Väre, H. & Vestberg, M. Seasonality of root fungal colonization in low-alpine herbs. Mycorrhiza 12, 29–36 (2002).
Schmidt, S. K., Sobieniak-Wiseman, L. C., Kageyama, Sa., Halloy, S. R. P. & Schadt, C. W. Mycorrhizal and Dark-Septate Fungi in Plant Roots Above 4270 Meters Elevation in the Andes and Rocky Mountains. Arctic, Antarct. Alp. Res. 40, 576–583 (2008).
Gai, J. P. et al. Occurrence and distribution of arbuscular mycorrhizal fungal species in three types of grassland community of the Tibetan Plateau. Ecol. Res. 24, 1345–1350 (2009).
Pan, J. et al. Arbuscular mycorrhizal and dark septate endophytic fungi at 5,500 m on a glacier forefront in the Qinghai-Tibet Plateau, China. Symbiosis 60, 101–105 (2013).
Upson, R., Newsham, K. K. & Read, D. Root-fungal associations of Colobanthus quitensis and Deschampsia antarctica in the maritime and subantarctic. Arctic, Antarct. Alp. Res. 40, 592–599 (2008).
Casanova-Katny, M. A., Torres-Mellado, G. A. & Palfner, G. & Cavieres, L. a. The best for the guest: High Andean nurse cushions of Azorella madreporica enhance arbuscular mycorrhizal status in associated plant species. Mycorrhiza 21, 613–622 (2011).
Urcelay, C., Acho, J. & Joffre, R. Fungal root symbionts and their relationship with fine root proportion in native plants from the Bolivian Andean highlands above 3,700 m elevation. Mycorrhiza 21, 323–330 (2011).
Ruotsalainen, A. A. L., Väre, H., Oksanen, J. & Tuomi, J. Root Fungus Colonization along an Altitudinal Gradient in North Norway Root Fungus Colonization along an Altitudinal Gradient in North Norway. 36, 239–243 (2004).
Haselwandter, K. & Read, D. J. The significance of a root fungus association in 2 Carex species of high alpine plant communities. Oecologia 53, 352–354 (1982).
Wu, L. & Guo, S. Interaction between an isolate of dark-septate fungi and its host plant Saussurea involucrata. Mycorrhiza 18, 79–85 (2008).
Newsham, K. K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 190, 783–793 (2011).
Jumpponen, A. Dark septate endophytes - Are they mycorrhizal? Mycorrhiza 11, 207–211 (2001).
Öpik, M. et al. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 188, 223–241 (2010).
Kessler, M., Güdel, R., Salazar, L., Homeier, J. & Kluge, J. Impact of mycorrhization on the abundance, growth and leaf nutrient status of ferns along a tropical elevational gradient. Oecologia 175, 887–900 (2014).
Gai, J. P. et al. Arbuscular mycorrhizal fungal diversity along a Tibetan elevation gradient. Pedobiologia (Jena). 55, 145–151 (2012).
Angel, R. et al. The root-associated microbial community of the world’s highest growing vascular plants. Microb. Ecol. 72, 394–406 (2016).
Dvorský, M. et al. Vascular plants at extreme elevations in eastern Ladakh, northwest Himalayas. Plant Ecol. Divers. 8, 571–584 (2015).
Dvorský, M. et al. Gardening in the zone of death: an experimental assessment of the absolute elevation limit of vascular plants. Sci. Rep. 6, 24440 (2016).
Liu, Y. et al. Diverse communities of arbuscular mycorrhizal fungi inhabit sites with very high altitude in Tibet Plateau. FEMS Microbiol. Ecol. 78, 355–365 (2011).
Sundqvist, M. K., Liu, Z., Giesler, R. & Wardle, D. A. Plant and microbial responses to nitrogen and phosphorus addition across an elevational gradient in subarctic tundra. Ecology 95, 1819–1835 (2014).
Mandyam, K. & Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 53, 173–189 (2005).
Newsham, K. K., Upson, R. & Read, D. J. Mycorrhizas and dark septate root endophytes in polar regions. Fungal Ecol. 2, 10–20 (2009).
Crush, J. R. Signifi cance of endomycorrhizas in tussock grassland in Otago, New Zealand. New Zeal. J. Bot. 11, 645–660 (1973).
Bledsoe, C., Klein, P. & Bliss, L. A survey of mycorrhizal plants on Truelove lowland, Devon island, NWT, Canada. Can. J. Bot. 68, 1848–1856 (1990).
Pfeffer, P., Douds, D., Becard, G. & Shachar-Hill, Y. Carbon Uptake and the Metabolism and Transport of Lipids in an Arbuscular Mycorrhiza. Plant Physiol. 120, 587–598 (1999).
Bücking, H. & Shachar-Hill, Y. Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol. 165, 899–912 (2005).
Kiers, E. T. et al. Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis. Science (80-.). 333, 880–882 (2011).
Ackerly, D. D. T. S. Correlated Evolution, and Independent Contrasts. Evolution (N. Y). 54, 1480–1492 (2000).
Desdevises, Y., Legendre, P., Azouzi, L. & Morand, S. Quantifying phylogenetically structured environmental variation. Evolution (N. Y). 57, 2647–2652 (2003).
Zobel, M. & Öpik, M. Plant and arbuscular mycorrhizal fungal (AMF) communities - which driveswhich? J. Veg. Sci. 25, 1133–1140 (2014).
Dvorský, M., Doležal, J., De Bello, F., Klimešová, J. & Klimeš, L. Vegetation types of East Ladakh: Species and growth form composition along main environmental gradients. Appl. Veg. Sci. 14, 132–147 (2011).
Chaurasia, B., Pandey, A. & Palni, L. M. S. Distribution, colonization and diversity of arbuscular mycorrhizal fungi associated with central Himalayan rhododendrons. For. Ecol. Manage. 207, 315–324 (2005).
Mundra, S. et al. Arctic fungal communities associated with roots of Bistorta vivipara do not respond to the same fine-scale edaphic gradients as the aboveground vegetation. New Phytol. 205, 1587–1597 (2015).
Körner, C. Alpine Plant Life - Functional Plant Ecology of High Mountain Ecosystems. (Springer, 2003).
Cázares, E., Trappe, J. M. & Jumpponen, A. Mycorrhiza-plant colonization patterns on a subalpine glacier forefront as a model system of primary succession. Mycorrhiza 15, 405–416 (2005).
Oehl, F., Schneider, D., Sieverding, E. & Burga, Ca. Succession of arbuscular mycorrhizal communities in the foreland of the retreating Morteratsch glacier in the Central Alps. Pedobiologia (Jena). 54, 321–331 (2011).
Liu, Y. et al. Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytol. 194, 523–535 (2012).
Schüßler, A., Schwarzott, D. & Walker, C. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 105, 1413–1421 (2001).
Li, X. et al. Contribution of arbuscular mycorrhizal fungi of sedges to soil aggregation along an altitudinal alpine grassland gradient on the Tibetan Plateau. Environ. Microbiol. 17, 2841–2857 (2015).
Jumpponen, A. Spatial distribution of discrete RAPD phenotypes of a root endophytic fungus Phialocephala fortinii, at a primary successional site on a glacier forefront. New Phytol. 141, 333–344 (1999).
Gardes, M. & Bruns, T. D. ITS primers with enhanced specificity for basidiomycetes, application to the identification of mycorrihiza and rusts. Mol. Ecol. 2, 113–118 (1993).
Dvorský, M., Macek, M., Kopecký, M., Wild, J. & Doležal, J. Niche asymmetry of vascular plants increases with elevation. J. Biogeogr. doi:10.1111/jbi.13001 (2017).
Karger, D. N. et al. Climatologies at high resolution for the earth’s land surface areas (2016).
Janatková, K. et al. Community structure of soil phototrophs along environmental gradients in arid Himalaya. Environ. Microbiol. 15, 2505–2516 (2013).
Řeháková, K., Chlumská, Z. & Doležal, J. Soil cyanobacterial and microalgal diversity in dry mountains of Ladakh, NW Himalaya, as related to site, altitude, and vegetation. Microb. Ecol. 62, 337–346 (2011).
Vierheilig, H., Schweiger, P. & Brundrett, M. An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol. Plant. 125, 393–404 (2005).
Trouvelot, A., Kough, J. & Gianinazzi-Pearson, V. In Physiological and Genetical Aspects of Mycorrhizae (eds Gianinazzi-Pearson, V. & Gianinazzi, S.) 217–221 (INRA Press, 1986).
Jumpponen, A. & Trappe, J. M. Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol. 140, 295–310 (1998).
Doyle, J. J. & Doyle, J. L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15 (1987).
Simon, L., Lalonde, M. & Bruns, T. D. Specific amplification of 18s fungal ribosomal genes from vesicular–arbuscular endomycorrhizal fungi colonizing roots. Appl. Environ. Microbiol. 58, 291–295 (1992).
Helgason, T., Daniell, T. J., Husband, R., Fitter, A. H. & Young, J. P. W. Ploughing up the wood-wide web? Nature 394, 431 (1998).
Dumbrell, A. J. et al. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol. 190, 794–804 (2011).
Větrovský, T. & Baldrian, P. Analysis of soil fungal communities by amplicon pyrosequencing: Current approaches to data analysis and the introduction of the pipeline SEED. Biol. Fertil. Soils 49, 1027–1037 (2013).
Schloss, P. D. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Katoh, K. & Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 9, 286–298 (2008).
Yang, Z. A space-time process model for the evolution of DNA sequences. Genetics 139, 993–1005 (1995).
Tanabe, A. S. Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol. Ecol. Resour. 11, 914–921 (2011).
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001).
Klimeš, L. & Dickoré, W. B. Flora of Ladakh (Jammu & Kashmir, India). A preliminary checklist published at http://www.butbn.cas.cz/klimes. Accessed 4 October 2010 (2006).
Farquhar, G. D., Ball, M. C., Voncaemmerer, S. & Roksandic, Z. Effect of salinity and humidity on delta-C-13 value of halophytes - evidence for diffusional isotope fractionation determined by the ratio of inter-cellular atmospheric partial pressure of CO2 uncer different environmental conditions. Oecologia 52, 121–124 (1982).
Chlumska, Z., Janecek, S. & Dolezal, J. How to Preserve Plant Samples for Carbohydrate Analysis? Test of Suitable Methods Applicable in Remote Areas. Folia Geobot. 49, 1–15 (2013).
Legendre, P. & Legendre, L. Numerical Ecology (Elsevier Science, 1998).
Diniz-Filho, J. A. F., de Sant Ana, C. E. R. & Bini, L. M. An eigenvector method for estimating phylogenetic inertia. Evolution (N. Y). 52, 1247–1262 (1998).
ter Braak, C. J. F. & Šmilauer, P. Canoco reference manual and users’s guide: sofware for ordination (version 5.0). (Microcomputer Power, 2012).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2015).
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004).
De Caceres, M. & Jansen, F. INDICSPECIES: Relationship Between Species and Groups of Sites. R package version 1.7.5. https://cran.r-project.org/web/packages/indicspecies/indicspecies.pdf (2015).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S. (Springer-Verlag, 2002).
Acknowledgements
We thank our technicians K. Kunertova and M. Prusova for laboratory work and T. Těšitelová and J. Š Farská for helpful comments. This research was supported by The Czech Science Foundation (grant no. GACR 13-13368S and 17-19376S) and RVO 67985939. M. Kotilínek was supported by grant GACR 31-14-21432S. I. Hiiesalu was supported by the grant from the Estonian Research Council (PUT-1170) and by the European Regional Development Fund (Center of Excellence EcolChange).
Author information
Authors and Affiliations
Contributions
M.Kot. and J.D. were responsible for the overall direction of the research. The data were collected by M.Kot., J.A., M.D., M.Kop. and J.D. M.Kot. and M.Š. performed microscopic quantification and M.Kot. and J.K. performed molecular analyses. M.Kot., I.H., P.Š. and J.D. conducted the statistical analyses. The paper was written by M.Kot., J.D. and I.H. with contributions from all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kotilínek, M., Hiiesalu, I., Košnar, J. et al. Fungal root symbionts of high-altitude vascular plants in the Himalayas. Sci Rep 7, 6562 (2017). https://doi.org/10.1038/s41598-017-06938-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06938-x
This article is cited by
-
Arbuscular mycorrhizal fungal diversity associated with an endangered species, Chamaecyparis formosensis, in the nature habitat
Tropical Ecology (2024)
-
Mycorrhizal symbioses in the Andean paramo
Mycorrhiza (2024)
-
Soil conditions regulate the patterns of root colonization by fungal endophytes in Guinea grass (Megathyrsus maximus)
Symbiosis (2023)
-
Species composition of root-associated mycobiome of ruderal invasive Anthemis cotula L. varies with elevation in Kashmir Himalaya
International Microbiology (2023)
-
Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: resilience to biotic and abiotic stresses
World Journal of Microbiology and Biotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.