Abstract
NFκB signaling has a pivotal role in regulation of development, innate immunity, and inflammation. Ikk2 is one of the two critical kinases that regulate the NFκB signaling pathway. While the role of Ikk2 in immunity, inflammation and oncogenesis has received attention, an understanding of the role of Ikk2 in vertebrate development has been compounded by the embryonic lethality seen in mice lacking Ikk2. We find that despite abnormal angiogenesis in IKK2 zygotic mutants of zebrafish, the maternal activity of Ikk2 supports embryogenesis and maturation of fertile animals and allows to study the role of IKK2 in development. Maternal-zygotic ikk2 mutants represent the first vertebrates globally devoid of maternal and zygotic Ikk2 activity. They are defective in cell proliferation as evidenced by abnormal cytokinesis, nuclear enlargement and syncytialisation of a significant portion of blastoderm. We further document that reduced phosphorylation of Aurora A by Ikk2 could underlie the basis of these defects in cell division.
Similar content being viewed by others
Introduction
A number of cellular and extracellular signals trigger the activation of the NFκB transcription factors1, 2. The IκB kinase (IKK) complex consisting of core components such as IKK1 (IKBKA) and IKK2 (IKBKB) and non-catalytic components such as NEMO (IKBKG), Rap13 and ELKS4, 5 plays a critical role in activation of NFkB. Signaling downstream of IKK2 is largely termed as the canonical NFκB signaling, whereas IKK1-mediated regulation of NFkB pathway is classified as the noncanonical NFκB signaling6,7,8,9. Kinase activity of IKK2 is known to be required for transcription of genes involved in innate immunity, inflammation, as well as development6, 10,11,12,13,14,15. While IκB proteins are the key targets of IKK2, other signaling proteins such as p5316, p653 and Aurora A17 have been linked to key physiological processes downstream of IKK2. It has been documented that Ikk2 regulates cytokinesis by stabilizing Aurora A and Kif11 to maintain the bipolar spindle in vitro 17.
Although IKK2 loss-of-function (LOF) mutations in human patients have been documented18,19,20, effects of pan-embryonic deficiency of Ikk2 in vertebrates remains poorly characterized as severe liver apoptosis in mice precludes their analysis6. Hence the roles ascribed to IKK2 are largely extrapolated from in vitro experiments or tissue-specific mutagenesis21, 22. In particular, epidermis-specific deletion of Ikk2 causes severe inflammatory skin disease23, while deficiency of Ikk2 in endothelium results in vascular permeability and reduces cell migration traced to transcriptional regulation of HIF-1α24, 25. Despite these results, a thorough understanding of the developmental function of IKK2 in a global is lacking.
Zebrafish has been established as a useful vertebrate model to study a role of genes during development26,27,28. Recent introduction of efficient methods of gene-specific mutagenesis has made zebrafish particularly useful for analysis of mutations, which lead to embryonic lethality in mice since the early development of zebrafish is less dependent on circulation29,30,31. At midblastula transition (MBT) cells of early zebrafish embryo segregate into three different lineages – deep cells, which form the embryo proper, and two extraembryonic lineages – yolk syncytial layer (YSL) and enveloping layer (EVL)32. EVL forms an external layer of epidermis (periderm), which is thought to play an evolutionarily conserved role in communication with epidermis. Stabilization of both differentiating epidermis and epithelial–mesenchymal interaction with the TNF signaling plays a pivotal role in periderm homeostasis33,34,35,36,37. Microtubules are involved in the intracellular transfer of vesicle-packed membrane components towards the furrow and, thus, play an important role in blastomeres cohesion during first cell divisions. Microtubules deficiency causes the premature and excessive formation of the YSL and axis deficiency38, 39.
Previously, the inhibition of the Ikk2 action towards NFκB in zebrafish revealed its role in axis formation40. To analyze the NFκB-dependent and -independent roles of Ikk2 during early development in an unbiased manner, we used the zinc-finger nuclease (ZFN)-mediated mutagenesis to generate the two different mutant alleles of Ikk2. Their analysis demonstrates that the zygotic function of Ikk2 is required during embryogenesis to establish vascular integrity. Some of the zygotic Ikk2 mutants survive until sexual maturity. A significant portion of these animals develops skin lesions and deformed body axis. Further analysis of their progeny (MZikk2 −/−) totally devoid of Ikk2 activity reveals a role of Ikk2 in cytokinesis, epiboly (gastrulation) and axis development.
Results and Discussion
To study the role of Ikk2 in development, we cloned it from total RNA of 96 hpf zebrafish. Zebrafish Ikk2 is homologous to the mammalian Ikk2 with 75% identity (Fig. S1A). The genomic locus of ikk2 contains 22 exons spanning 87.61 kb. It encodes a polypeptide of 779 aa residues that contains evolutionarily conserved kinase, ubiquitine-like (ULD), leucine zipper (LZ), helix-loop-helix (HLH) and NEMO-binding (NBD) domains (Figs 1A; S1A). Notably, lysine 44 of the catalytic active site and the two serine residues (S177/S181) of the activation loop are conserved between zebrafish and other species Ikk2s. Lentivirus-mediated reconstitution of zebrafish Ikk2 in Ikk2-null murine fibroblasts led to IκBα phosphorylation and subsequent degradation upon induction with physiological stimuli TNFα, with kinetics similar to that seen in mammalian cells (Fig. 1B). This result suggested that the zebrafish Ikk2 is physiologically functional as a kinase and operates with similar efficiency as murine Ikk2 in activating the canonical NFκB signaling.
The two alleles of zebrafish ikk2 (ikbkb) mutant made using zinc-finger nuclease (ZFN)-mediated mutagenesis. (A) Genomic organization of the zebrafish ikk2 locus. It contains 22 exons (numbered). The domain organization of a putative Ikk2 of 779 aa derived by homology comparison with human IKK2. Numbers define amino acid residues. Abbreviations: ULD – ubiquitin-like domain; LZ - leucine zipper; HLH - helix-loop-helix domain; and NBD - NEMO-binding domain. The critical amino acid residues conserved in evolution are shown in red. (B) Zebrafish Ikk2 restored phosphorylation of murine IκBα in IKK2−/− immortalized MEFs upon treatment with TNFα for the indicated time period. Actin was used as loading control. Band intensities were quantified by densitometry in ImageJ and normalized to the most intensive band for each target. n.d., not detected. Data are representative of three experiments. (C) The first ZFN-mutation upstream of the kinase domain (ikk2 m10) caused an indel mutant with a frame shift from 10 aa onwards and a truncated polypeptide of 60 aa. (D) The second ZFN-mutation designed within the ubiquitin-like domain (ikk2 m379) caused an indel mutant with a frame shift from 379 aa onwards and a truncated polypeptide of 402 aa.
ikk2 transcript is maternal suggesting its role during early development. It expresses throughout embryogenesis and in adults (Fig. S1B,C). To address a developmental role of Ikk2 and increase our chances to cause a developmental defect linked to Ikk2, we generated two mutant alleles using the zinc-finger nuclease (ZFN)-mediated mutagenesis29, 30. The first mutation was designed to be in the second exon, which corresponds to the N-terminal of the kinase domain. In this position, the 2-base pairs (bp) insertion typical of non-homologous end joining (NHEJ) repair was detected. This resulted in a shift of the reading frame causing an amino acid (aa) residue replacement (Q10C) and termination of a polypeptide after 60 aa (Figs 1C; S1D). This mutation (ikk2 m1 °) probably represents a null mutation (sq302). The second mutation was designed to be in the ULD. Analysis of this mutant allele demonstrated a 7 bp deletion, resulting in a frame shift, causing replacement of aa residue (N379S) that may terminate the polypeptide after 402 aa resulting in a truncated Ikk2 containing an intact kinase domain (ikk2 m379, sq303; Figs 1D; S1D), but without the important C terminal domain required for regulation of IKK2 activity41. When crossed to homozygosity, we observed no obvious difference in recessive mutant phenotypes displayed by the two mutant alleles and mutants generated by complementation crosses (ikk2 m10/m379). This is probably due to the fact that the loss of C-terminal domain in ikk2 m379renders the truncated kinase domain almost inactive. This is consistent with the extensively analyzed truncated human patient allele (Q432, premature stop) resulting in complete loss of IKK2 in fibroblasts and peripheral peripheral-blood mononuclear cells and impaired NFκB signaling downstream of TNFα/TLR518. Therefore, unless otherwise stated, our analyses of zebrafish ikk2 −/− is based on the putative null allele (ikk2 m10).
A significant number of ikk2 −/− embryos (18.5%) developed hemorrhage at 48 hpf, with 82.2% of cases being cranial (Fig. 2A–D). We crossed the ikk2 m10 mutant to Tg(fli:EGFP)/Tg(gata:DsRed) background, which labels endothelial cells and erythrocytes respectively, for detailed confocal analysis of vessel patterning in the hindbrain. The major arteries (BA) and veins (PHBC) were intact, hence vasculogenesis in the mutant was not affected (Fig. 2E–F). However, capillaries (CtAs) sprouting from existing veins (PHBC) were reduced in both number (Fig. 3A–C) and complexity (Fig. 3D–H; Fig. S2A–F). This implies that the hemorrhage observed in mutants could be due to reduced sprouting of capillaries and insufficient development of capillary network, a phenotype normally associated with angiogenesis defects. Previously, NFκB activation has been linked to maintaining blood vessel integrity in zebrafish through Birc2/TNF receptor complex42. In mice, deficiency of Ikk2 function in endothelium causes vascular permeability and reduces cell migration traced to regulation of HIF-1α24, 25. Cranial hemorrhages have also been described in human patients with IKK2 mutation18. Our observation of endothelial defects in zygotic Ikk2 mutants supports these observations and links this phenotype to several genes of angiogenesis (see below).
Loss of Ikk2 caused hemorrhage. (A,B) Bright-field images of wild-type and ikk2 −/− (zygotic) embryos obtained from heterozygotic parents at 48 hpf. Red arrows - the hemorrhage site. (A,A′) Ventral view. (B,B′) dorsal view. (C) Quantification of hemorrhaging embryos. (D) Quantification of embryos with cranial hemorrhage vs that in other parts of the body. (C,D) Unpaired Student’s t test. (E,F) Confocal Z-projection shows wild type (E) and ikk2 −/− (F) embryos of the Tg(fli:EGFP)/Tg(gata:DsRed) background at 48 hpf. (E′,F′) Confocal Z-projection shows Tg(fli:EGFP) embryos without the dorsal most MtA, MsA and DLV. EGFP was pseudo-colored as black. PHBC (blue), BA (red), and CtAs (purple) were marked. * - the affected CtAs with cranial hemorrhage in (F). All data expressed as mean ± SEM; scale bar in all panels: 100 μm. Abbreviations – PHBC: primordial hindbrain channels; BA: basilar artery; CtAs: central arteries (51).
Loss of Ikk2 affects angiogenesis. (A,B) Confocal horizontal section of ventral hindbrain in wild-type and ikk2 −/− (zygotic) embryos obtained from heterozygotic parents of Tg(fli:EGFP) background at 48 hpf. CtA (purple arrowhead) stem sprouting from PHBC in each rhombomere (r) was marked accordingly. At this stage, the bilateral CtAs in the r3 formed two connections with lumen to BA (yellow arrow) in wild-type and only one in ikk2 −/− embryo. Connections of CtAs in r5 (yellow) and r6 (blue) marked. (C) Quantification of CtA sprouting from PHBC in each rhombomere. P value calculated by paired Student’s t test. (D,E) Confocal projection of CtA angiogenic vessels in Tg(fli:EGFP)/Tg(gata:DsRed) 48 hpf embryos. (J′,K′) Blowup shows the hindbrain of Tg(fli:EGFP)- labelled CtA vessels. Numbers correspond to the rhombomeres containing CtA sprouts. Note that 1*, 2*, 3* marked the CtA vessels that were reduced both in size and connectivity in ikk2 −/− embryos. This is the area of hemorrhage (E). (F–H) Quantification of angiogenic index of hindbrain CtAs. P value calculated by Student’s t test. All data are expressed as mean ± SEM; scale bar in all panels: 100 μm. Abbreviations – PHBC: primordial hindbrain channels; BA: basilar artery; CtAs: central arteries (51).
A small percentage of ikk2 −/− embryos (~5%) showed phenotype similar to that of the ntl mutants, which have truncated body axis due to deficiency of the notochord43, with various degree of penetrance (Fig. S3A–H). They develop loss of cells during late epiboly, abnormal Kuppfer’s vesicle, ntl phenotype and vacuolated notochord (Fig. S3A–H and not shown). Similar phenotype was detected using the dominant-negative approach9 and transient morpholino-mediated knockdown of Ikk2 (Table S1). Hence it seems that the ntl-like phenotype develops due to incomplete inhibition of Ikk2-NFκB activity. In both embryos and adult mutants neither hepatic apoptosis nor liver failure were observed in ikk2 −/− zebrafish. This finding is in accordance with IKK2-deficient human patients where no liver damage is reported18,19,20. It further confirms that unlike loss of NEMO or p65 lack-of-function that of IKK2 function could be tolerated. Embryonic apoptosis of hepatocytes resulting in lethality in Ikk2 KO mice could simply be due to excessive sensitivity of hepatocytes to TNFα during embryogenesis in mice. However, a significant reduction in mutant fish population was observed and the one year survival rate calculated to be mere 21.7% (Fig. 4A). Adult ikk2 −/− fish were short, hypo-pigmented and prone to skin lesions (Figs 4B–F; S4A–F). Frequency of skin lesions increased during mating (Fig. 4Q), probably due to mechanical stress of copulation. Skin lesions in a significant number of fishes fail to heal and they die after a couple of weeks post injury. Injury sites showed pathological changes, including loss of scales, reduced skin parenchyma and hyper-proliferation of keratinocytes (Figs 4E–H; S4E,F). Lateral body muscles of the mutant fish showed less myofibrils and signs of inflammation with infiltration of blood cells (Fig. 4G,H). In addition, mutant males developed deformed and reduced in number breeding tubercles (keratinized cuticles present on dorsal pectoral fin of male zebrafish for grasping and positioning of females during mating, Fig. 4I,J). The pharyngeal teeth of mutants were also reduced in number (Fig. 4K–P,S). These signs indicated systemic deficiency of integument derivatives, including skin and skin appendages. This is consistent with published analyses of Ikk2-deficiency in mammals associated with severe inflammatory skin disease18,19,20, 23. This function seems to be different from that of Ikk1, which in zebrafish and mice controls epidermal differentiation independent of NFκB activation6,7,8, 44. Interestingly, in all IKK2 human patients analyzed so far, no anhidrotic ectodermal dysplasia (EDA) was reported18,19,20, suggesting a dispensable role of IKK2 for development of skin appendages. The skin phenotype of Ikk2 zebrafish mutant together with that in conditional KO mice23 probably implicates a more precise level of Ikk2/NFκB activity required for skin development in animals, which could somehow be tolerated in human. It is also possible that some of these skin defects could be due to defects in immunodeficiency (manuscript in preparation) or a combination of both.
Ikk2 regulates development of skin and its appendages. (A) Kaplan-Meier analysis of survival of ikk2 −/− fish during one-year period. P < 0.001, by Logrank test. (B) Nine months-old adult ikk2 −/− (zygotic) mutants obtained from heterozygotic parents are small. P < 0.001, unpaired t test. (C–F) Adult ikk2 −/− fish are hypopigmented (boxed area and inset, E,F). They succumb to skin injuries without or with mating, with an average frequency of 11% and 21.5%, respectively. Scale bar in (C,D): 1 cm; in (E,F): 1000 µm. (Q). (E,F) blowup of white rectangle area in (C,D). In severe cases, the injuries cause skin lesion. (G,H) H&E stained cross-section at the level of skin lesion area (dashed line in C,D). Scale bar: 500 µm. (I,J) Epidermal tubercles (ET) were reduced in ikk2 −/−. (I′,J′) Blowup of white rectangle area in (I,J). Quantifications by unpaired t test were shown in (R) Scale bar in I,J: 500 µm; in (I′,J′): 100 µm. (K–N) Alizarin red staining of teeth. Scale bar: 500 µm. (O,P) H&E stained sagittal section at the Vth branchial arch. Teeth numbers were marked by *. Note that in ikk2 −/− fish, 4 V* was absent, 3 V* was defective, and blood supply to the dentation area (arrow) compromised. Scale bar: 100 µm. (S) Teeth number was significantly reduced in adult mutants. P = 0.0058, paired t test.
Ikk2-deficient adults could generate maternal-zygotic embryos (MZikk2 −/−) which allow us to study the global function of Ikk2 in the context of null background. When crossed, the ikk2 −/− adults produce progenies with clutch size significantly smaller than controls (Fig. 5P), suggesting a functional role of Ikk2 in oogenesis. Indeed, real-time PCR (qPCR) indicated presence of maternal transcripts at as early as 1-cell stage (Fig. S6A). In such 8-cell stage embryos the cell boundaries were blurred (Fig. 5A′) and at the 32-64-cell stage cells were found to detach from each other (Fig. 5B′,C′). At the sphere stage, the mutant yolk syncytial layer (YSL) was abnormally large and cells formed a “cellular island” (Fig. 5D′), a characteristic phenotype of embryos with cell division defects45. By the time when the stage-matched controls complete epiboly, some of the MZikk2 −/− embryos reached 50% epiboly and developed a ectopic yolk constriction that broke the yolk cell and caused early death (Fig. S5A–H). In summary, these phenotypes are observed consistently in crosses from different batches and usually 100% of MZikk2 −/− embryos are affected. Occasionally (in one out of 10 crosses), a minority of MZikk2 −/− embryos (less than 5%) develop past epiboly and develop the embryonic lethal ntl-like phenotype indicative of gastrulation deficiency (ref. 43; Fig. S4J–N; Table S1). Hence it is possible that this phenotype could also be due to incomplete inhibition of both maternal and zygotic Ikk2 activities.
Cell adhesion and cytokinesis are affected in MZikk2 −/− embryos. (A–D) Early cell adhesion and cytokinesis defects in MZikk2 −/− embryos (obtained from homozygotic parents, arrow) were followed by the ‘cellular island’ phenotype (arrow) at sphere stage. (E,F) (H,E) stained sections showing abnormal nuclear clumps in the EVL (arrow) and enlarged YSL (arrowhead) of MZikk2 −/− embryos. (G-I) krt8 WISH reveals multinuclear cells in MZikk2 −/− embryos. (I,I′) Blowup of boxed areas in (H,H′), respectively. (J–L) Whole-mount immunostaining using Phalloidin and DAPI shows cell boundaries (K,K′) and nuclei (L,L′) in MZikk2 −/− embryos (animal pole view, sphere stage). (M,N) Cell division furrows stained by E-cadherin, 8-cell stage. (O) Quantification of embryos with mild and severe division furrow defects based on E-cadherin staining. (P) A number of embryos in ikk2 −/− crosses as compared to wild-type; p < 0.001, Student’s t test. (Q) Ikk2-dependent phosphorylation of Aurora-A in extracts of ikk2 −/− and controls. Actin and Coomassie blue, loading control. All data are expressed as mean ± SEM; Scale bar in all panels: 100 μm.
Using whole-mount in situ hybridization (WISH) analysis we found relatively normal distribution of transcripts of a number of dorsal-ventral (flh, chd and bmp2b) and mesodermal markers (ntl, papc, eve1) in MZikk2 −/− embryos at shield stage, suggesting that early embryonic patterning and differentiation still took place in the MZikk2 −/− mutants (Fig. S5I–T). Interestingly, epidermal marker, krt8, which expression was absent in ikk1 mutant44 was expressed (Fig. 5G,H). This provides evidence of keratinocytes differentiation in MZikk2−/− embryos. As Ikk1 controls epidermal differentiation independent of canonical NFκB activation7, 44, the phenotype observed here could be attributed to the lack of Ikk2 function towards NFkB or other substrates, that the presence of Ikk1 catalytic activity cannot compensate for. Importantly, although the enveloping layer (EVL, primitive epidermis) was present, its cells were multinuclear (Fig. 5I,I′). Immunostaining with phalloidin and DAPI further revealed blurred cell boundaries and fused nuclei in the EVL (Fig. 5J–L). Sections of the sphere and shield stage MZikk2 −/− embryos detected cytokinesis defects in deep cells also. The acellular area corresponding to YSL increased dramatically resulting in formation of the ‘cellular island’ phenotype (Fig. 5E,F). Taken together, these results suggest that cytokinesis defects could be the underlying primary defect in MZikk2 −/− embryos.
Anti-Ecad immunohistochemistry revealed two distinct Ecad-positive subcellular signals in early WT embryos: a) the cell division furrow and, b) multiple small vesicles (with diameter below 10 μm) in close proximity to the furrow (Fig. 5M). In the MZikk2 −/− mild mutant the small Ecad-positive vesicles were significantly reduced (Fig. 5N) and in the severe mutants the signals associated with cell division furrow was greatly reduced (Fig. 5N′,O). Formation of the cell division furrow depends upon vesicular transport and its irregularities were linked to deficient cytokinesis and enlarged YSL38.
Thus, the deficiency of cytokinesis led to expansion of the YSL. Although the molecular mechanism underlying cytokinesis has been a matter of intense investigation, the one underlying YSL formation has just begun to be elucidated32, 46. In embryonic zebrafish, the YSL forms due to regression of preexistent marginal cells membranes and depends on coordinated activity of different elements of the cytoskeleton. It was demonstrated that a disturbance of the cytoskeleton in embryonic zebrafish results in conversion of the blastoderm into syncythium. In particular, deficiency of several maternal proteins, including, centriolar protein Sas-6, Aurora-B kinase, and components of Rho signaling (Rho-kinase 1 and Slc3a2) was linked with excessive syncitialization of blastoderm45, 47,48,49.
The phenotype of MZikk2 −/− embryos is strikingly similar to that of the MZ mutant of Aurora B45. It has been shown previously that a related protein Aurora-A plays a role in maintaining microtubule-based cytoskeleton during cell division and its inhibition decreases cell proliferation by inducing cell cycle arrest, polyploidy and affects the NFκB pathway50. To analyze a role of Ikk2 in zebrafish in vivo, Ikk complex was precipitated from adult ikk2 −/− fish using anti-NEMO antibodies and Ikk activity was determined in an kinase assay using the recombinant full-length His-Aurora-A as substrate. Indeed kinase activity towards Aurora-A was reduced by 25% in the Ikk2 mutants suggesting subnormal functioning of the Ikk2-Aurora-A axis (Fig. 5Q). Deficiency of cytokinesis could be caused by abnormal microtubule-based cytoskeleton in MZikk2 −/− mutants resulting in the “mitotic catastrophe” and termination of development.
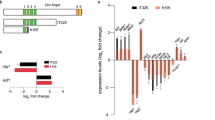
Since MZikk2 −/− embryos lack global Ikk2 activity, it opened a possibility to use RNAseq and identify the genes regulated by Ikk2 during oogenesis and early embryogenesis. We performed this analysis on the MZ ikk2 m10 mutants and controls at 2 hpf (prior to MBT, genes transcribed during oogenesis) and 4 hpf (post-MBT, same plus early embryonic genes; Fig. 6). Our analyses revealed that expression of genes related to angiogenesis (ptena, angpt4, vegfaa, flt4; Fig. 6B), skin development (anxa6, tgm2b, Fig. 6C), and cytokinesis (junbb, etc; Fig. 6D) were significantly affected. Expression of some of these genes was further validated by qPCR analyses (Fig. 6E). Some genes in these groups are known NFκB targets (ptena, tgm2b, junbb). In addition, this analysis revealed a number of other genes/transcripts, which were not known previously as NFκB targets and yet their expression was significantly affected by the Ikk2 mutation (fhad1). Interestingly, the expression of three transcripts of aurka encoding the zebrafish homologue of Aurora kinase A (Aurora-A) changed in the MZikk2 mutants along with expression of the negative regulator of aurka - aurkaip1 (Fig. S6B). This suggested that regulation of Aurora-A kinase by Ikk2 probably takes place not only at the level of posttranslational regulation of Aurora-A (Fig. 5Q), but also at the level of transcription of aurka and aurkaip1. Amongst other regulators of mitosis Cdk4 plays a role in G-S1 phase51. Expression of cdk4 was up-regulated significantly in MZ mutant embryos (Fig. 6D; Supplemental Table 1). It has been shown that early genes regulated by the NFκB-dependent TNFα-signaling in epidermal keratinocytes include BMP252. This is consistent with the RNAseq data in MZikk2 showing an up-regulation of bmp2b (GEO submission no. GSE90971). These preliminary observations defined tentative points of interest for future studies of the cytokinesis defect caused by a global deficiency of Ikk2 in vivo in zebrafish embryos.
RNAseq analysis of transcriptome of wild-type and MZikk2 −/− embryos (obtained from homozygotic parents) reveals novel molecular targets regulated by Ikk2. (A–D) Green - down regulated in ikk2 −/− as compared to wild type control, red - up regulated in ikk2 −/− as compared to wild type control. (A) Heat maps illustrating total stage- and gene-specific expression trends. (B) Comparison of expression of angiogenesis-related genes. (C) Comparison of expression of skin-related genes. (D) Comparison of expression of cytokinesis-related genes. (E) Real-time PCR (qPCR) verification of selected target genes identified by RNAseq. Two internal reference, actb (actin) and gapdh were used for normalization. Data quantification was done by using the ΔΔCt method and the result was presented as a relative fold change compared to wild-type embryos at 2 and 4 hpf. Data are expressed as mean ± SEM, p < 0.05 for all four genes listed.
A complex signalling pathway that controls many aspects of cellular functions and molecular interactions, NFκB pathway and its key molecular inter-players had been the focus of intensive studies over the last decades. Among them, the IKK complex which directly phosphorylate IκBα and allow NFκB protein translocation into nucleus has been one of them. Evidence from animal genetics (mainly mice) and cell-based biochemical studies point at IKK2 as the main catalytic active kinase, whereas IKK1 has some level of kinase activity with its own independent function and IKKγ functions as an essential regulator. Recent advance in human genetics leads to the identification human patients with IKK2 mutations18,19,20 and studies based on their phenotype seems to reinforce its role in immunity as patients all develop Severe Combined Immunodeficiency (SCID) within the first 6 month of age, which is attributed to the loss of function of canonical NFκB signalling. These data supports our previous understanding of IKK2 function gained from mice studies, but also emphasizes certain discrepancies. For example, none of patients display any hepatic problems whereas Ikk2 knockout mice is embryonically lethal due to TNFα-induced severe liver apoptosis, hence the prenatal function cannot be addressed. Although conditional knockout mice with immunodeficiency has been described21, 22, the exact molecular mechanism and pathological consequence due to loss of IKK2 at a systemic level remain to be elucidated. Our analysis of zebrafish Ikk2 mutant fits nicely into this gap. Zebrafish ikk2 mutants develop haemorrhage at early embryonic stage and later succumb to systemic infection (our unpublished data) hence poor survivability, a phenotype very similar to that described in human patients. With large fecundity (~150–250 embryos per clutch) and ease of maintenance, zebrafish ikk2 mutants represent an attractive vertebrate model for drug screening to be carried at at Ikk2/NFκB deficient background.
In Drosophila, the immune deficient (IMD) pathway (canonical NFκB pathway equivalent), is triggered by Gram-negative bacteria and activates Relish, a p100-like NFκB precursor protein, resulting in induction of antimicrobial peptide genes. The Drosophila Ikk complex contains 2 subunits, a catalytic kinase subunit encoded by ird5 (IKK2)53 and a regulatory subunit encoded by kenney (IKKγ)54. The lack of IKK1 homologue in Drosophila reiterates the evolutionary conserved function of IKK2 as the key kinase that activates canonical NFκB signalling, yet calls for a more complex system with IKK1 functional interplay. Our zebrafish analysis fits nicely here. Our studies of MZikk2 −/− mutants at a completely null background reveals a novel function of Ikk2 in regulating cytokinesis early in development. As zebrafish ikk1 −/− mutants survive to adulthood without obvious morphological problem, whereas MZikk1 −/− cannot differentiate into a proper embryonic ectoderm44, it is therefore obvious that Ikk1 and Ikk2 have their own individual roles in epidermal development, which possibly explains the skin phenotype observed at later stages in zygotic mutants as well as other in other model systems. Hence, the availability of both ikk1 and ikk2 maternal zygotic mutants in zebrafish offers us an unique peek into the function of these two kinases in a very clean background that other model systems do not offer. Taken together, the establishment of zebrafish ikk2 mutants and their functional analysis offer an attractive alternative approach in understanding the physiological function of IKK2 that complements current knowledge based on mammalian models and changes our perspective on a developmental role of the NFκB signalling pathway in vertebrates.
Material and Methods
Zebrafish husbandry and transgenic lines
Wild-type zebrafish (AB strain) were maintained as described previously55. All animal experiments were carried according to the regulations of Institutional Animal Care and Use Committee (Biological Resource Center of Biopolis, license no. 120787), which approved this study. Developmental stages are in hours post fertilization (hpf) at 28.5 °C. Transgenic lines: Tg(fli:EGFP) and Tg(gata:DsRed) that label zebrafish vasculature and erythrocytes, respectively, were used for analyzing angiogenesis defects.
Generation of zebrafish ikk2 mutants using zinc-finger nuclease (ZFN)
ZFN design, assembly and validation
The two ZFN pairs used in this study were purchase from CompoZr Custom Zinc finger nuclease (Sigma-Aldrich, St. Louis, USA), with design, assembly and validation performed by the manufacturer. ZFN activities were measured using yeast MEL-I reporter assay by the manufacturer and were reported to be 68.3% and 118.2% relative to positive control for ZFN pair 1 (ZFN1, Fig. 1C) and ZFN pair 2 (ZFN2, Fig. 1D) respectively. Experiments were performed according to manufacturer’s guidelines.
Preparation and microinjection of ZFN mRNA
All pZFN plasmids were linearized with XbaI, for synthesis of ZFN encoding mRNA using mMessenger kit (Ambion) according to manufacturer’s protocols. Synthesized mRNAs were Poly (A) tailed with Poly (A) tailing kit (Ambion) and purified by phenol/Chloroform extraction. Paired ZFN mRNAs were mixed at a 1:1 ratio and 400 pg of RNA was injected into 1-cell staged zebrafish embryos. Embryos that survived into adulthood were screened for mutation in the respective ZFN target site.
ZFN induced mutation detection using SURVERYOR nuclease
The Surveyor Mutation Detection Kit (Transgenomic) was used to detect ZFN-induced mutation within ikk2 exon 2. PCR reactions (50 µl) were carried out using DreamTaq polymerase (Fermentas) in the manufacturer-supplied buffer. Primer pairs flanking the ikk2 exon 2 ZFN 1 target site (5′-GGGTAAATACAATTCAGAGCATG-3′, 5′-CTTTTACTGCTGAACTACACC T-3′) and ikk2 exon 11 ZFN 2 target site (5′-GCCATAAGTCACATGTCATGTT-3′, 5′-GTT AAAGCAACAGCTAGAGAGG-3′) were used. PCR reaction program: denature at 95 °C for 2 min followed by 35 cycles consisting of 95 for 40 s, 55 for 30 s and 72 for 30 s; 72 for 10 min final extension. PCR product was hybridized in a thermocycler following the protocol in the Surveyor manual. The hybridized DNA was assayed for inhomogeneity by adding 1 µl Surveyor Enhancer S, 1 µl Surveyor nucleases S and 2 µl of 0.15 M MgCl2 per 20 µl of DNA and incubating the reaction at 42 °C for 1hr. After incubation, treated DNA was assayed by 2% agarose TBE gel electrophoresis. Identified founders were out-crossed with wild-type AB fish to establish F1 generations. Exact indel mutant sequences were confirmed by cloning and sequencing. F1 generations were then subsequently out-crossed to wild-type AB strain for at least four generations for clearance of potential off-target background until linked mutant phenotype were confirmed. All genotyping were performed by sequencing (Fig. S1B).
Cell lines, antibodies and other reagents
Wild type (WT) and Ikk2 −/− mouse embryonic fibroblast (MEFs) were grown in DMEM supplemented with 10% heat inactivated fetal bovine serum, 2 mM L-glutamine, 2 mM Na pyruvate and 1x PSF (penicillin, streptomycin and fungizone) at 37 °C with 5% CO2. Antibodies against IKKα (Sc7182, Sc7218), IKKβ (Sc7607), IKKγ (Sc8330) were from Santa Cruz Biotechnology and Biosource. The anti-IKKα/β rabbit IgG antibody (sc-7607, Santa Cruz Biotechnology) recognizes human IKK1 and IKK2 both. IKKγ specific antibody was obtained from Biosource and Millipore. TNFα (Calbiochem) were used at concentration of 10 ng/ml.
Lentivirus Production and Transduction
Production and purification of lentiviral vectors has been described previously. Briefly, lentiviral vector encoding the zebra fish Ikk2 and other control molecules and packaging plasmids (µg) were co-transfected into 293 T cells using calcium phosphate method. Culture media was changed after 12 hours and harvested at 24 and 48 hours before centrifuging to pellet the release virus. Viruses were re-suspended in 1.6 ml of 1x HBSS and aliquoted before freezing at 80 °C. Cells in 24-well plate was transduced with the lentivirus (50–100 µl/well) for 24 hours before changing media.
Western Blot Analysis
Total protein was extracted with TOTEX buffer (20 mM Hepes pH 7.9, 0.35 M NaCl, 20% glycerol, 1% NP-40, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA, 50 mM NaF and 0.3 mM NaVO3) containing a mixture of protease inhibitors (Roche). 20 µg of total protein were separated on NuPAGE Novex 4–12% Bis-Tris gels (Invitrogen) and transferred to polyvinylidene difluoride membrane (Biorad). Immunoblotting were performed with specific antibodies and visualized using ECL Western Blotting detection kit (Amersham Bioscience).
Co-Immunoprecipitation
At a desired point after infection/transfection, cells were washed with ice-cold PBS, and lysed in 10 mM Tris pH8, 170 mM NaCl, 0.5% NP40 and protease inhibitors for 30 minutes on ice. Cell lysates were cleared by centrifugation and the supernatants were incubated with anti-p65 antibody overnight at 4 °C and with protein-G sepharose for a further 2 hour. Beads were washed 4x with 1 ml of wash buffer (200 mM Tris pH 8.0, 100 mM NaCl and 0.5% NP-40). Bound proteins were eluted with SDS sample buffer and separated on NuPAGE Novex 4–12% Bis-Tris gels before immunoblotting with specific antibodies.
Quantitative real-time PCR
Total RNA were isolated using RNeasy Kit (Qiagen) as per manufacturer’s protocol. cDNA were prepared from 1–2 µg of RNA using the Superscript III or superscript Vilo reverse transcriptase (Invitrogen) with random hexamers primers. Real time PCR reactions were performed in duplicates using SYBR Green (Biorad) or SYBR GreenER (Invitrogen) as per manufacturer’s instructions. Cycles for BioRad 60–95 °C for 30 sec followed by 40 cycles of 95 °C 15 Sec and 60 °C for 30 seconds. Cycles for SYBR GreenER: 7.5 mins at 95 °C for the initial denaturation, followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 30 seconds. Primer sequences are available upon request56.
In-vitro Kinase Assay
Total lysates prepared from female WT and ikk2 −/− tissue were immunoprecipitated with anti-IKKγ/NEMO (sc-8330, FL-419, Santa Cruz) overnight at 4 °C. After extensive washing of the beads, kinase activity was determined by incubating beads with 1 μml−1 (32P) ATP and 2 μg of purified recombinant His-aurora kinase A (plasmid kindly provided from Edward Manser’s lab, IMCB, Singapore) for 30 minutes at 30 °C. Reactions were resolved on 15% PAGE gel and visualized by autoradiography as previously described57.
Protein Expression and Purification
Proteins were expressed in BL21 cells grown at 37 °C to an OD600 = 0.6, then induced with 0.5 mM IPTG and incubated for a further 9 hours at 20 °C as previously described57. The GST-fusion proteins were purified on Glutathione Sepharose resin (Amersham Bioscience). The resin was washed with buffer A (50 mM Tris pH7.5, 1 mM EGTA, 1 mM EDTA and 2 mM DTT) followed by buffer B (50 mM Tris pH7.5, 200 mM NaCl and 2 mM DTT). Bound protein was eluted with elution buffer (50 mM Tris pH8.5, 10 mM glutathione). Eluted protein was filtered, concentrated and frozen in aliquots before use.
Whole mount in situ hybridization (WISH), histology and immunohistochemistry
WISH, histology and immunohistochemistry were performed following established protocols. Briefly, for WISH, embryos were fixed in 4% paraformaldehyde in PBS for 4 hours at room temperature, and hybridized with specific probes in hybridization buffer (50% formamide, 5X SSC, 50 mg/ml Heparin, 500 μg/ml tRNA and 0.1% Tween20 in PBS) at 68 C. Stain was developed with NBT/BCIP (3 μl/ml of 50 mg/ml stock). All washings were done in PBS with 0.1% Tween20. Specifically, embryos at blastula and gastrula stages were fixed within their chorion on, whereas embryos after 24 hpf were de-chorionated and fixed. Anti-E-cadherin (mouse, #610181, BD transduction laboratories) primary antibodies were used for immunohistochemistry (1:200 in PBS), followed by secondary antibodies with AlexaFluor-488 (Invitrogen) (1:500 in PBS). AlexaFluor-555-conjugated Phalloidin and DAPI were used 1:500 in PBS. Confocal images were taken with Zeiss LSM710 confocal systems and processed using ImageJ.
Skin and skin appendages analysis
To quantify ET units per segment, one pectoral fin from each animal was collected and regions of four segments proximal to the bifurcation point of the third and fourth fin rays were assessed. To visualize adult teeth, anesthetized fish were fixed in 4% PFA overnight and transferred into 0.01% alizarin red in 0.5% KOH for staining at 4 °C overnight. Following rinsing in 0.5% KOH, teeth from both side were removed from the fifth branchial arches and transferred into 50% glycerol for imaging.
Survival curve, standard length and statistical analysis
Survival of individual fish was monitored daily for a year. From birth onwards, number of embryos derived from heterozygotes inter-crosses were counted and each dead embryos/larvae were collected and soaked immediately in DNA extraction buffer for subsequent genotyping. On 60 dpf, all fish survived were genotyped by fin-clipping, grouped into WT, ikk2 +/− and ikk2 −/− categories and individual death was recorded daily thereafter. Number of fish were re-counted every 5 days and any fish missing was censored (marked as a tick in Fig. 4A). Kaplan-Meier survival curve was plotted from data pooled from three independent repeats using Prism Graphpad and p values were computed using the log-rank test. For measurement of standard length, the linear distance between the snout and the caudal peduncle was taken to the nearest millimeter using a plastic ruler. All data are expressed as mean ± SEM. Unless otherwise stated, statistical differences were assessed by either paired or unpaired Student’s t-test using Prism Graphpad 4.0.
Transcriptome analysis by RNAseq and bioinformatics
150 embryos for each sample were collected at 2 and 4 hpf (wild type control and MZikk2 −/− for both stages, correspondingly) and RNA preparation was done as published before58. mRNA quantification data was obtained using a standard pipeline based on TopHat-cufflinks59 with the Danio rerio gene annotation file (assembly GRCz10) from Ensembl database. For differential expression analysis, gene-level FPKM values were converted into log ratios (KO/WT, base 2) for 2 h and 4 h data and the threshold for differential expression was determined to meet 1% FDR by a mixture model-based approach called EBprot60. The transcripts whose largest FPKM values was below 25 percentile of all FPKM values across the sample were considered unreliable for quantification and removed from subsequent analysis. For the genes that were quantified in either KO or WT but not both, a gene was considered differentially expressed if the quantified FPKM value61 was above the 25 percentile of the whole transcriptome in the given sample. Test of functional enrichment (Gene Ontology) was performed using an in-house program that computes the significance of enrichment by hypergeometric test, using the GO annotation of genes from ZFIN database (http://zfin.org). The list of NFkB target genes for zebrafish was identified by first performing gene orthology mapping to human genes (ZFIN database) and matching them to a curated list of known NFkB target genes (http://www.bu.edu/nf-kb/gene-resources/target-genes). The RNAseq data were deposited online (GEO submission no. GSE90971).
References
Tergaonkar, V., Correa, R. G., Ikawa, M. & Verma, I. M. Distinct roles of IkappaB proteins in regulating constitutive NF-kappaB activity. Nat Cell Biol 7, 921–923, doi:10.1038/ncb1296 (2005).
Tong, L. & Tergaonkar, V. Rho protein GTPases and their interactions with NFkappaB: crossroads of inflammation and matrix biology. Bioscience reports 34, doi:10.1042/BSR20140021 (2014).
Teo, H. et al. Telomere-independent Rap1 is an IKK adaptor and regulates NF-kappaB-dependent gene expression. Nat Cell Biol 12, 758–767, doi:10.1038/ncb2080 (2010).
Wu, Z. H. et al. ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Molecular cell 40, 75–86, doi:10.1016/j.molcel.2010.09.010 (2010).
Ducut Sigala, J. L. et al. Activation of transcription factor NF-kappaB requires ELKS, an IkappaB kinase regulatory subunit. Science 304, 1963–1967, doi:10.1126/science.1098387 (2004).
Li, Z. W. et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. The Journal of experimental medicine 189, 1839–1845 (1999).
Hu, Y. et al. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature 410, 710–714, doi:10.1038/35070605 (2001).
Takeda, K. et al. Limb and skin abnormalities in mice lacking IKKalpha. Science 284, 313–316 (1999).
Correa, R. G. et al. Zebrafish IkappaB kinase 1 negatively regulates NF-kappaB activity. Current biology: CB 15, 1291–1295, doi:10.1016/j.cub.2005.06.023 (2005).
Kanegae, Y., Tavares, A. T., Izpisua Belmonte, J. C. & Verma, I. M. Role of Rel/NF-kappaB transcription factors during the outgrowth of the vertebrate limb. Nature 392, 611–614, doi:10.1038/33429 (1998).
Li, Q., Estepa, G., Memet, S., Israel, A. & Verma, I. M. Complete lack of NF-kappaB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev 14, 1729–1733 (2000).
Rudolph, D. et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev 14, 854–862 (2000).
Tickle, C. Worlds in common through NF-kappaB. Nature 392, 547–549, doi:10.1038/33276 (1998).
Hacker, H. & Karin, M. Regulation and function of IKK and IKK-related kinases. Science’s STKE: signal transduction knowledge environment 2006, re13, doi:10.1126/stke.3572006re13 (2006).
Tergaonkar, V. NFkappaB pathway: a good signaling paradigm and therapeutic target. The international journal of biochemistry & cell biology 38, 1647–1653, doi:10.1016/j.biocel.2006.03.023 (2006).
Xia, Y. et al. Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc Natl Acad Sci USA 106, 2629–2634, doi:10.1073/pnas.0812256106 (2009).
Irelan, J. T. et al. A role for IkappaB kinase 2 in bipolar spindle assembly. Proc Natl Acad Sci USA 104, 16940–16945, doi:10.1073/pnas.0706493104 (2007).
Pannicke, U. et al. Deficiency of innate and acquired immunity caused by an IKBKB mutation. The New England journal of medicine 369, 2504–2514, doi:10.1056/NEJMoa1309199 (2013).
Nielsen, C. et al. Immunodeficiency associated with a nonsense mutation of IKBKB. Journal of clinical immunology 34, 916–921, doi:10.1007/s10875-014-0097-1 (2014).
Senegas, A., Gautheron, J., Maurin, A. G. & Courtois, G. IKK-related genetic diseases: probing NF-kappaB functions in humans and other matters. Cellular and molecular life sciences: CMLS 72, 1275–1287, doi:10.1007/s00018-014-1793-y (2015).
Greten, F. R. et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 130, 918–931, doi:10.1016/j.cell.2007.07.009 (2007).
Li, Z. W., Omori, S. A., Labuda, T., Karin, M. & Rickert, R. C. IKK beta is required for peripheral B cell survival and proliferation. Journal of immunology 170, 4630–4637 (2003).
Pasparakis, M. et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417, 861–866, doi:10.1038/nature00820 (2002).
Ashida, N. et al. IKKbeta regulates essential functions of the vascular endothelium through kinase-dependent and -independent pathways. Nature communications 2, 318, doi:10.1038/ncomms1317 (2011).
Rius, J. et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453, 807–811, doi:10.1038/nature06905 (2008).
Korzh, V. P. Genetic control of early neuronal development in vertebrates. Current opinion in neurobiology 4, 21–28 (1994).
Haffter, P. et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36 (1996).
Langdon, Y. G. & Mullins, M. C. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annual review of genetics 45, 357–377, doi:10.1146/annurev-genet-110410-132517 (2011).
Doyon, Y. et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nature biotechnology 26, 702–708, doi:10.1038/nbt1409 (2008).
Meng, X., Noyes, M. B., Zhu, L. J., Lawson, N. D. & Wolfe, S. A. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nature biotechnology 26, 695–701, doi:10.1038/nbt1398 (2008).
Moore, F. E. et al. Improved somatic mutagenesis in zebrafish using transcription activator-like effector nucleases (TALENs). PLoS One 7, e37877, doi:10.1371/journal.pone.0037877 (2012).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Developmental dynamics: an official publication of the American Association of Anatomists 203, 253–310, doi:10.1002/aja.1002030302 (1995).
Armstrong, M. T. et al. A novel form of epithelial wound healing of the embryonic epidermis. Experimental cell research 312, 2415–2423, doi:10.1016/j.yexcr.2006.04.006 (2006).
M’Boneko, V. & Merker, H. J. Development and morphology of the periderm of mouse embryos (days 9–12 of gestation). Acta anatomica 133, 325–336 (1988).
Cui, C. Y. et al. Lymphotoxin-beta regulates periderm differentiation during embryonic skin development. Human molecular genetics 16, 2583–2590, doi:10.1093/hmg/ddm210 (2007).
Hardman, M. J., Moore, L., Ferguson, M. W. & Byrne, C. Barrier formation in the human fetus is patterned. The Journal of investigative dermatology 113, 1106–1113, doi:10.1046/j.1523-1747.1999.00800.x (1999).
Slanchev, K. et al. The epithelial cell adhesion molecule EpCAM is required for epithelial morphogenesis and integrity during zebrafish epiboly and skin development. PLoS Genet 5, e1000563, doi:10.1371/journal.pgen.1000563 (2009).
Jesuthasan, S. Furrow-associated microtubule arrays are required for the cohesion of zebrafish blastomeres following cytokinesis. Journal of cell science 111(Pt 24), 3695–3703 (1998).
Strahle, U. & Jesuthasan, S. Ultraviolet irradiation impairs epiboly in zebrafish embryos: evidence for a microtubule-dependent mechanism of epiboly. Development 119, 909–919 (1993).
Correa, R. G. et al. Characterization of NF-kappa B/I kappa B proteins in zebra fish and their involvement in notochord development. Molecular and cellular biology 24, 5257–5268, doi:10.1128/MCB.24.12.5257-5268.2004 (2004).
Delhase, M., Hayakawa, M., Chen, Y. & Karin, M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science 284, 309–313 (1999).
Santoro, M. M., Samuel, T., Mitchell, T., Reed, J. C. & Stainier, D. Y. Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nat Genet 39, 1397–1402, doi:10.1038/ng.2007.8 (2007).
Schulte-Merker, S., van Eeden, F. J., Halpern, M. E., Kimmel, C. B. & Nusslein-Volhard, C. no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 120, 1009–1015 (1994).
Fukazawa, C. et al. poky/chuk/ikk1 is required for differentiation of the zebrafish embryonic epidermis. Developmental biology 346, 272–283, doi:10.1016/j.ydbio.2010.07.037 (2010).
Yabe, T. et al. The maternal-effect gene cellular island encodes aurora B kinase and is essential for furrow formation in the early zebrafish embryo. PLoS Genet 5, e1000518, doi:10.1371/journal.pgen.1000518 (2009).
Carvalho, L. & Heisenberg, C. P. The yolk syncytial layer in early zebrafish development. Trends in cell biology 20, 586–592, doi:10.1016/j.tcb.2010.06.009 (2010).
Chu, L. T. et al. Yolk syncytial layer formation is a failure of cytokinesis mediated by Rock1 function in the early zebrafish embryo. Biology open 1, 747–753, doi:10.1242/bio.20121636 (2012).
Takesono, A. et al. Solute carrier family 3 member 2 (Slc3a2) controls yolk syncytial layer (YSL) formation by regulating microtubule networks in the zebrafish embryo. Proc Natl Acad Sci USA 109, 3371–3376, doi:10.1073/pnas.1200642109 (2012).
Yabe, T., Ge, X. & Pelegri, F. The zebrafish maternal-effect gene cellular atoll encodes the centriolar component sas-6 and defects in its paternal function promote whole genome duplication. Developmental biology 312, 44–60, doi:10.1016/j.ydbio.2007.08.054 (2007).
Chefetz, I., Holmberg, J. C., Alvero, A. B., Visintin, I. & Mor, G. Inhibition of Aurora-A kinase induces cell cycle arrest in epithelial ovarian cancer stem cells by affecting NFkB pathway. Cell cycle 10, 2206–2214, doi:10.4161/cc.10.13.16348 (2011).
Adon, A. M. et al. Cdk2 and Cdk4 regulate the centrosome cycle and are critical mediators of centrosome amplification in p53-null cells. Molecular and cellular biology 30, 694–710, doi:10.1128/MCB.00253-09 (2010).
Banno, T., Gazel, A. & Blumenberg, M. Pathway-specific profiling identifies the NF-kappa B-dependent tumor necrosis factor alpha-regulated genes in epidermal keratinocytes. J Biol Chem 280, 18973–18980, doi:10.1074/jbc.M411758200 (2005).
Silverman, N. et al. A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev 14, 2461–2471 (2000).
Rutschmann, S. et al. Role of Drosophila IKK gamma in a toll-independent antibacterial immune response. Nature immunology 1, 342–347, doi:10.1038/79801 (2000).
Westerfield, M. The Zebrafish Book. The guide of the laboratory use of zebrafish (Danio rerio). 5th edn, (University of Oregon Press, 2007).
Li, Y. et al. Non-canonical NF-kappaB signalling and ETS1/2 cooperatively drive C250T mutant TERT promoter activation. Nat Cell Biol 17, 1327–1338, doi:10.1038/ncb3240 (2015).
Shin, E. M. et al. DEAD-box helicase DP103 defines metastatic potential of human breast cancers. J Clin Invest 124, 3807–3824, doi:10.1172/JCI73451 (2014).
Winata, C. L. et al. Genome wide analysis reveals Zic3 interaction with distal regulatory elements of stage specific developmental genes in zebrafish. PLoS Genet 9, e1003852, doi:10.1371/journal.pgen.1003852 (2013).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols 7, 562–578, doi:10.1038/nprot.2012.016 (2012).
Koh, H. W. et al. EBprot: Statistical analysis of labeling-based quantitative proteomics data. Proteomics 15, 2580–2591, doi:10.1002/pmic.201400620 (2015).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature biotechnology 28, 511–515, doi:10.1038/nbt.1621 (2010).
Acknowledgements
We are thankful to Drs S. Fong, C.-M. Wen, H.-L. Ang involved in preliminary morpholino-based experiments; Drs M. Balasubramanian, S. Oliferenko, P. Kaldis, E. Manser for fruitful discussions; Drs E. Manser, T. Carney, S. Rastegar and U. Straehle for reagents; the personnel of the IMCB Fish Facility for fish maintenance; the personnel of the Advanced Molecular Pathology Laboratory (IMCB) for histological service.
Author information
Authors and Affiliations
Contributions
H.S. - designed and generated mutants and analyzed them by developmental biology methods, made most of Figures and wrote a paper; E.M.S. – performed biochemical experiments and made Figures; S.L. and S.M. – made RNAseq libraries and preliminary bioinformatics analysis; H.K. and H.C. – performed detailed bioinformatics analysis; M.O. – designed and supervised biochemical experiments; V.T. – designed mutants, designed and supervised biochemical experiments and wrote and approved a paper; V.K. – designed mutants, supervised developmental biology experiments, made Figures, wrote and approved a paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shen, H., Shin, E.M., Lee, S. et al. Ikk2 regulates cytokinesis during vertebrate development. Sci Rep 7, 8094 (2017). https://doi.org/10.1038/s41598-017-06904-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06904-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.