Abstract
Previous studies indicated that homocysteine (Hcy) is associated with higher peripheral systolic blood pressure (pSBP). There have been few data on the relationship between Hcy and central SBP (cSBP). A total of 4,364 Chinese subjects from the Shijingshan community in Beijing were included. cSBP and pSBP were measured with an Omron HEM-9000AI device. Subjects were 57.20 ± 8.9 years old, 37.9% were male. The median of Hcy was 11.96 μmol/L. The mean of cSBP and pSBP was 129.94 ± 18.03 mmHg and 133.25 ± 18.58 mmHg. lnHcy was associated with cSBP (adjusted β = 2.17, SE = 0.80, P = 0.007) and pSBP (adjusted β = 2.42, SE = 0.75, P = 0.001). With increasing Hcy, there were enhanced correlations of Hcy with pSBP and cSBP (p for trend between quartiles <0.01). Using Q1 for reference, the Q4 was associated with cSBP (adjusted β = 1.77, SE = 0.89, P = 0.047) and pSBP (adjusted β = 2.15, SE = 0.84, P = 0.011). The correlations were more significant in non-obese subjects than in obese subjects (cSBP: β = 4.30 vs 0.46, pSBP: β = 5.04 vs 1.18, P for interaction <0.001). Our study showed that Hcy was associated with higher cSBP and pSBP, especially in non-obese subjects.
Similar content being viewed by others
Introduction
Hyperhomocysteinemia (HHcy) has emerged as an independent risk factor for cardiovascular disease (CVD)1. However, whether homocysteine (Hcy) is a risk factor for hypertension still remains controversial. Several findings in conventional observational analyses supported a positive association between Hcy concentration and blood pressure (BP)2,3,4 as well as higher Hcy in hypertensive patients compared to normotensive patients in case-control studies5,6,7, but subsequent prospective studies yielded considerably weaker associations8,9,10. In contrast, observations that homocysteine-lowering therapies with folic acid-based treatments were associated with decreases in BP raise the possibility that the link between Hcy and BP is causal11,12,13,14.
Systolic pressure varies throughout the arterial tree; in most circumstances, aortic (central) systolic pressure (cSBP) is lower than the corresponding brachial values. However, some studies showed that more than 70% of individuals with high-normal BP had aortic systolic pressures that were similar to those of individuals with stage 1 hypertension15. Vital organs are exposed to the central rather than the peripheral BP although this difference is highly variable between individuals16. Emerging evidence now suggests that central pressure is better correlated with end-organ damage and cardiovascular events than peripheral systolic blood pressure (pSBP)17. Moreover, anti-hypertensive drugs can exert differential effects on brachial and central pressure18. Therefore, cSBP has different physiology and may offer improvements in CVD risk assessment compared to pSBP. Currently, cSBP can be assessed noninvasively through the use of several devices19. cSBP is associated with age, sex, brachial BP, heart rate, pulse wave velocity, and many other risk factors, such as body mass index (BMI), lipids and diabetes20. However there are few data about the relationship between Hcy and central BP.
The present study aims to elucidate the association of plasma Hcy with cSBP and pSBP in a Chinese community-based population.
Methods
Subjects
Participants were from the Gucheng and Pingguoyuan communities of the Shijingshan district in Beijing, China, and participated in an atherosclerosis cohort survey performed from December 2011 to April 2012. The methods and primary results of this survey have been reported elsewhere21,22,23. After excluding those with missing covariates, a total of 4,364 eligible participants aged ≥40 years old were included in this analysis. This study was approved by the ethics committee of Peking University and Peking University First Hospital, and each participant provided written informed consents before enrollment. We adhered to the principles of the Declaration of Helsinki. The procedures followed were in accordance with institutional guidelines.
Data collection
Baseline data were collected by trained research staff according to standard operating procedures. All participants were interviewed using a standardized questionnaire that was specifically designed for the present study, providing information including sociodemographic status, education, occupation, diet, lifestyle, health behavior, medical history and medication use. Anthropometric measurements were taken according to a standard operating procedure. Current smoking was defined as smoking one cigarette per day for at least half a year. Current drinking was defined as drinking once per week for at least half a year. Diabetes was defined from self-reported history or index abnormality(fasting blood glucose (FBG) ≥ 7 mmol/L or oral glucose tolerance test (OGTT) ≥ 11.1 mmol/L); hypertension was defined from self-reported history or SBP ≥ 140 mmHg or DBP ≥ 90 mmHg. Dyslipidemia was defined from self-reported history or abnormal lipid profiles. CVD was defined as any self-reported history of coronary heart disease, myocardial infarction, stroke, or transient ischemic attack. BMI was calculated as weight (kg)/height2 (m2).
Brachial blood pressure and central systolic blood pressure
Radial artery pressure waveforms and brachial BP were recorded simultaneously using a fully automated device (HEM-9000AI, Omron Healthcare, Kyoto, Japan) to calculate late systolic pressure in the radial artery (SBP2) and estimate central systolic BP. Brachial BP was measured with an oscillometric manometer and the radial pulse waveforms were recorded noninvasively using an applanation tonometer. Inflection points or peaks corresponding to early and late SBP were obtained from multidimensional derivatives of the original pulse waveforms. Then, the maximal SBP and DBP in the radial artery were calibrated with the brachial SBP and DBP. Finally, an estimate of cSBP was calculated by the pressure at the late systolic shoulder of the radial pressure waveform using linear regression with SBP2 as a major independent variable24.
Blood sample collection and laboratory methods
A venous blood sample was obtained from the forearm of each participant after an overnight fast of at least 12 hours. Serum or plasma samples were separated within 30 minutes of collection and were stored at −80 °C. Plasma Hcy was measured using an autobiochemical analyzer (Beckman Coulter AU480) with the enzymatic method. This method mainly uses the S-adenosylhomocysteine (SAH) hydrolase reaction principle, in which SAH is hydrolyzed by hydrolytic enzymes into adenosine and Hcy, adenosine is immediately hydrolyzed into ammonia and hypoxanthine, nicotinamide adenine dinucleotide (NADH) is converted to NAD with ammonia and glutamic dehydrogenase, and the concentration of Hcy in the sample is proportional to the NADH transformation rate. Folate was measured using an automated chemiluminescence immunoassay analyzer (MAGLUMI4000) with the electrochemiluminescence method. Hcy and folate were all tested at the core laboratory of the National Clinical Research Center for Kidney Disease, at the Nanfang Hospital in Guangzhou, China. FBG and the standard 75-g OGTT as well as the lipid profiles and serum creatinine (Scr) at baseline were measured on the Roche C8000 Automatic Analyzer in the laboratory of the Chinese PLA General Hospital.
Statistical analysis
Categorical variables were expressed as numbers and percentages. Continuous variables were described using means with standard deviations for data with normal distribution, and medians for non-normally distributed data. Univariate comparison were made between groups using ANOVA test for continuous variables and the χ2 test for categorical variables. A generalized additive model (GAM) with a spline smoothing function was applied to examine the relationship between cSBP, pSBP and Hcy, and a piecewise linear regression analysis was conducted to fit the smoothing curve, with adjustments for potential confounders, including age, sex, BMI, Scr, current smoking, current drinking, diabetes, dyslipidemia, CVD and antihypertension drug use. Univariate and multivariate analysis were performed to assess the associations between cSBP, pSBP and Hcy. The multivariable regression model was adjusted for other variables as well, including age, sex, BMI, Scr, current smoking, current drinking, diabetes, dyslipidemia, CVD and antihypertension drug use. Subgroup analyses examined the relationships of cSBP, pSBP and Hcy stratified by covariates, including sex, age, BMI, Scr, smoking, drinking, hypertension, diabetes mellitus, dyslipidemia, CVD, antihypertensive medication and folate. Tests for interactions in the linear regression model were used to compare β between the analyzed subgroups. Analyses were performed using Empower (R) (www.empowerstats.com, X&Y solutions, Inc. Boston MA) and R (http://www.R-project.org). A P-value < 0.05 was considered statistically significant.
Declarations
Ethics approval, accordance and informed consent to participate:The proposal was approved by the ethics committee of Peking University and Peking University First Hospital, and all subjects signed informed consent before enrollment. We adhered to the principles of the Declaration of Helsinki. The procedures followed were in accordance with institutional guidelines.
Results
The baseline characteristics of included participants stratified by Hcy quartiles are shown in Table 1. Subjects were 57.20 ± 8.91 years old, and 37.9% were male. The median value of Hcy was 11.96 (IQR: 10.03–14.92) μmol/L, and folate was 6.18 (IQR: 5.00–8.19) ng/ml. Of the subjects, 50.1% had hypertension, of whom 32.5% received antihypertensive medications. The mean value of cSBP and pSBP was 129.94 ± 18.03 mmHg and 133.25 ± 18.58 mmHg, respectively. To achieve an even distribution in each group, the subjects were divided into subgroups using Hcy quartiles: Q1: 8.87 (≤10.02) μmol/L; Q2: 11.02 (10.03–11.96) μmol/L; Q3: 13.16 (11.97–14.91) μmol/L; and Q4: 18.48 (≥14.92) μmol/L. Higher Hcy levels were significantly associated with female gender, older age, higher BMI, Scr, SBP, DBP and cSBP as well as a higher proportion of smokers, drinkers, subjects with hypertension, CVD and antihypertensive treatment usage. However, with increasing Hcy, folate levels have a downward trend (P < 0.001). No differences were observed for dyslipidemia or diabetes between groups. These data are presented in Table 1.
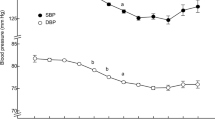
The smoothing curve showed that, after adjusting for confounders, including age, sex, BMI, smoking status, drinking status, Scr, DM, dyslipidemia, CVD and antihypertension drug use, there is a positive linear correlation between cSBP, pSBP and lnHcy, cSBP and pSBP were increasing linearly with lnHcy (Figs 1 and 2). Univariable and multivariable analyses were carried out to assess whether Hcy is independently associated with pSBP and cSBP after adjusting for likely confounders as mentioned above. For cSBP, lnHcy was positively associated with both cSBP and pSBP. A unit increase in lnHcy was associated with increases of 2.17 mmHg in cSBP (adjusted β = 2.17, SE = 0.80, P = 0.007) and increases of 2.42 mmHg in pSBP (adjusted β = 2.42, SE = 0.75, P = 0.001). With increasing Hcy, there were significantly enhanced correlations of Hcy with pSBP and cSBP (p for the trend between quartiles <0.01). Using Quartile 1(Q1) for reference, Quartile 4 (Q4) group was positively associated with both cSBP (adjusted β = 1.77, SE = 0.89, P = 0.047) and pSBP (adjusted β = 2.15, SE = 0.84, P = 0.011). These data are presented in Table 2. In addition to these confounders, we furtherly adjusted for folate, and the relationships of Hcy with pSBP and cSBP remained statistically significant. (for cSBP: adjusted β = 2.24, SE = 0.83, P = 0.007; for pSBP: adjusted β = 2.62, SE = 0.78, P < 0.001). Using Quartile 1(Q1) for reference, Quartile 4 (Q4) group was positively associated with both cSBP (adjusted β = 1.86, SE = 0.94, P = 0.047) and pSBP (adjusted β = 2.39, SE = 0.88, P = 0.007).
The interaction test showed no significant interactions between Hcy levels and the covariates mentioned above when different SBP traits were used to determine the outcomes, except for BMI (Table 3). The relationships were more significant in non-obese subjects than in obese subjects (for cSBP: β = 4.30 vs 0.46, P for interaction = 0.006; for pSBP: β = 5.04 vs 1.18, P for interaction = 0.004).
Discussion
The major findings of our study were that Hcy levels were independently associated with both pSBP and cSBP, especially in non-obese subjects.
The relationship between HHcy and hypertension has been proposed by multiple researchers, most of whom only used brachial BP as the BP parameter. The results of the present study are consistent with some of the results from prior studies. Cross sectional data from the Third National Health and Nutrition Examination Survey showed that one standard deviation (5 μmol/l) increase in Hcy was associated with increases of 0.5 and 0.7 mmHg in diastolic and systolic blood pressure, respectively, after adjusting for cardiovascular risk factors4. The Hordaland study examined a very large sample (16, 176 individuals) and reported a weak association of plasma Hcy with SBP and DBP that was confined to younger individuals2. Data from a total of 3,524 schoolchildren including children and adolescents in a study of cardiovascular health showed that Hcy was independently associated with SBP25. Regina reported that SBP was correlated with Hcy levels and inversely correlated with plasma folates in juvenile essential hypertension patients26. Similar findings concerning the relationship between plasma Hcy and BP were provided by the SHEP study, which showed a direct correlation between Hcy and SBP in the elderly population27. A cross sectional study including 7,130 Chinese participants showed that HHcy was independently associated with the risk of hypertension in males (OR = 1.501, 95%CI: 1.012–2.227, P = 0.001)3. The relationship between SBP and Hcy was also found in hypertensive patients from Chinese rural areas28, smokers29, diabetic patients30, hemodialysis patients31, patients with stroke32, and several other small sample studies5,6,7, 33. On the other hand, numerous studies have yielded conflicting results. The Framingham Heart Study investigated for the first time in a community–based setting the relationships between plasma Hcy levels and hypertension. However, in age- and sex-adjusted analyses, the association was not statistically significant34, 35. In addition, no associations between SBP and DBP with Hcy concentrations were found in the Iranian population36, in Chinese subjects without antihypertensive medication use37, in young African American women38 or in the Brazilian population39. There were few prospective studies that illustrated the causal association between Hcy and BP. In Framingham Heart Study, no major relationship between baseline Hcy levels and hypertension incidence or longitudinal BP progression was found8. Wang reported that Hcy is related to hypertension incidence, with the results approximating a U-shaped curve in the Chinese population9.
Most interestingly, we find that Hcy is independently associated with cSBP. The studies addressing the link between central arterial BP and Hcy were very limited. Xiao et al. reported that, in a cross-sectional study with a community-based sample of 1680 Chinese subjects, neither peripheral nor central BP differed according to Hcy levels in normotensive and hypertensive subjects40. The BROOF study demonstrated that lnHcy was strongly associated with PWV, but no significant association was observed for Aix and aortic pulse pressure41. To the best of our knowledge, this is the first study to report the positive relationship between the Hcy level and cSBP. The results may have some instructive significance. First, mechanisms that could explain the relationship between Hcy and BP include homocysteine-induced arteriolar constriction, renal dysfunction, increased sodium reabsorption, and increased arterial stiffness42, 43. Compliance of central artery is one of the most important factors that influence cSBP. Thus, the results of the study indicate that the arterial stiffness might be an important issue linking HHcy and hypertension. In addition, it has been established that HHcy is a risk factor for CVD, and the association between Hcy and cSBP may contribute to the elevated CVD risks that HHcy induced.
We also found that Hcy levels were more associated with both cSBP and pSBP in the non-obese subgroup. There were very few prior studies that could explain these results, so we raise some hypotheses. First, obesity is considered a risk factor for hypertension and other CVD related factors, therefore, in higher BMI groups, the association between Hcy and SBP might be negated by other factors. Second, studies have reported that Hcy is associated with insulin resistance44, 45, which hypertension is one of the features of this syndrome. And this may link the Hcy and hypertension in non-obese population. Third, The sympathetic nervous system is an important regulator of blood pressure, especially in non-obese subjects, but the effects of Hcy on its activity do not appear to have been studied. The interference of BMI to Hcy and hypertension needs more basic and independent sample researches to be further verified.
Compared to prior studies, the population of our study was from a Chinese urban community, which is not covered as much in previous studies. The sample size of our study is relatively larger. The median level of Hcy was 11.98 μmol/L, which was comparable to the other data46. The prevalence of hypertension is similar to that reported in prior research3. However, the proportion of diabetes is relatively higher. Moreover, as previous observation studies have shown that the relationship between BP and Hcy attenuated after adjustments, it is possible that plasma Hcy is a marker for age, age-related renal dysfunction and hypertensive drugs with Hcy-elevating effects47. Thus, many factors that might have contributed to hypertension and HHcy were taken into count in the present study, and after adjustments and subgroups analyses, the association remained statistically significant. Furthermore, we assessed the association using both pSBP and cSBP, which provided more solid evidence in support of the findings from previous studies.
The present study has several limitations. First, it was a cross-sectional study, and thus, predictions about the incidence of hypertension due to HHcy in the general population cannot be extrapolated from these data. Longitudinal studies are required for the further investigation of these findings. Second, the data are not necessarily representative of populations in other locations within China, but many studies conducted in different regions reported data that were consistent with ours. Third, pSBP and cSBP values were based on a single assessment, which may introduce variation, but the large sample of the study can also attenuate the variation. In addition, antihypertensive drugs, and particularly beta-blockers, exert differential effects on central blood pressure, but we weren’t able to detail the effects of antihypertensive drugs. Fourth, other Hcy related factors, including lifestyle factors such as coffee consumption and physical activity, genetics data, vitamin B intake except folate, that were not assessed in detail due to lack of such data.
Conclusion
In conclusion, we found that plasma Hcy levels are independently associated with pSBP and cSBP especially in non-obese subjects, which provide potential evidence that Hcy may play an important role in regulating blood pressure and hypertension. Large prospective studies and independent replications are required to elucidate these issues.
References
Ganguly, P. & Alam, S. F. Role of homocysteine in the development of cardiovascular disease. Nutrition journal 14, 6 (2015).
Nygard, O. et al. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. Jama 274, 1526–1533 (1995).
Li, Z. et al. Hyperhomocysteinemia independently associated with the risk of hypertension: a cross-sectional study from rural China. Journal of human hypertension 30, 508–512 (2016).
Lim, U. Homocysteine and Blood Pressure in the Third National Health and Nutrition Examination Survey, 1988-1994. American Journal of Epidemiology 156, 1105–1113 (2002).
Cai, W., Yin, L., Yang, F., Zhang, L. & Cheng, J. Association between Hcy levels and the CBS844ins68 and MTHFR C677T polymorphisms with essential hypertension. Biomedical reports 2, 861–868 (2014).
Golbahar, J. & Mostafavi, E. Association between low red blood cell 5-methyltetrahydrofolate and hyperhomocysteinaemia with hypertension: a cross-sectional study. High blood pressure & cardiovascular prevention: the official journal of the Italian Society of Hypertension 19, 229–235 (2012).
Muda, P. et al. Homocysteine and red blood cell glutathione as indices for middle-aged untreated essential hypertension patients. Journal of hypertension 21, 2329–2333 (2003).
Sundstrom, J. et al. Plasma homocysteine, hypertension incidence, and blood pressure tracking: the Framingham Heart Study. Hypertension 42, 1100–1105 (2003).
Wang, Y. et al. Homocysteine as a risk factor for hypertension: a 2-year follow-up study. PloS one 9, e108223 (2014).
Bowman, T. S., Gaziano, J. M., Stampfer, M. J. & Sesso, H. D. Homocysteine and risk of developing hypertension in men. Journal of human hypertension 20, 631–634 (2006).
van Dijk, R. A., Rauwerda, J. A., Steyn, M., Twisk, J. W. & Stehouwer, C. D. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: a 2-year, randomized, placebo-controlled trial. Arteriosclerosis, thrombosis, and vascular biology 21, 2072–2079 (2001).
Papandreou, D., Malindretos, P., Arvanitidou, M., Makedou, A. & Rousso, I. Homocysteine lowering with folic acid supplements in children: effects on blood pressure. International journal of food sciences and nutrition 61, 11–17 (2010).
McRae, M. P. High-dose folic acid supplementation effects on endothelial function and blood pressure in hypertensive patients: a meta-analysis of randomized controlled clinical trials. Journal of chiropractic medicine 8, 15–24 (2009).
Mangoni, A. A., Sherwood, R. A., Swift, C. G. & Jackson, S. H. Folic acid enhances endothelial function and reduces blood pressure in smokers: a randomized controlled trial. Journal of internal medicine 252, 497–503 (2002).
McEniery, C. M. et al. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension 51, 1476–1482 (2008).
McEniery, C. M., Cockcroft, J. R., Roman, M. J., Franklin, S. S. & Wilkinson, I. B. Central blood pressure: current evidence and clinical importance. European heart journal 35, 1719–1725 (2014).
Kollias, A., Lagou, S., Zeniodi, M. E., Boubouchairopoulou, N. & Stergiou, G. S. Association of Central Versus Brachial Blood Pressure With Target-Organ Damage: Systematic Review and Meta-Analysis. Hypertension 67, 183–190 (2016).
Manisty, C. H. & Hughes, A. D. Meta-analysis of the comparative effects of different classes of antihypertensive agents on brachial and central systolic blood pressure, and augmentation index. British journal of clinical pharmacology 75, 79–92 (2013).
O’Rourke, M. F. & Adji, A. Noninvasive studies of central aortic pressure. Current hypertension reports 14, 8–20 (2012).
Rosenwasser, R. F. et al. Baseline predictors of central aortic blood pressure: a PEAR substudy. Journal of the American Society of Hypertension: JASH 8, 152–158 (2014).
Cheng, G. et al. Different associations between blood pressure indices and carotid artery damages in a community-based population of China. Journal of human hypertension 30, 750–754 (2016).
Fan, F. et al. Noninvasive Central Systolic Blood Pressure Is More Strongly Related to Kidney Function Decline Than Peripheral Systolic Blood Pressure in a Chinese Community-Based Population. Hypertension 67, 1166–1172 (2016).
Momin, M. et al. Relationship between plasma homocysteine level and lipid profiles in a community-based Chinese population. Lipids in health and disease 16, 54 (2017).
Takazawa, K. et al. Estimation of central aortic systolic pressure using late systolic inflection of radial artery pulse and its application to vasodilator therapy. Journal of hypertension 30, 908–916 (2012).
Osganian, S. K. et al. Distribution of and factors associated with serum homocysteine levels in children: Child and Adolescent Trial for Cardiovascular Health. Jama 281, 1189–1196 (1999).
Kahleova, R. et al. Essential hypertension in adolescents: association with insulin resistance and with metabolism of homocysteine and vitamins. American journal of hypertension 15, 857–864 (2002).
Sutton-Tyrrell, K., Bostom, A., Selhub, J. & Zeigler-Johnson, C. High homocysteine levels are independently related to isolated systolic hypertension in older adults. Circulation 96, 1745–1749 (1997).
Qin, X. et al. Elevated Homocysteine Concentrations Decrease the Antihypertensive Effect of Angiotensin-Converting Enzyme Inhibitors in Hypertensive Patients. Arteriosclerosis, thrombosis, and vascular biology 37, 166–172 (2017).
Kennedy, B. P., Farag, N. H., Ziegler, M. G. & Mills, P. J. Relationship of systolic blood pressure with plasma homocysteine: importance of smoking status. Journal of hypertension 21, 1307–1312 (2003).
Neugebauer, S. et al. Total plasma homocysteine is associated with hypertension in Type I diabetic patients. Diabetologia 45, 1315–1324 (2002).
Tsai, J. C. et al. Correlation of plasma homocysteine level with arterial stiffness and pulse pressure in hemodialysis patients. Atherosclerosis 182, 121–127 (2005).
Mizrahi, E. H. et al. Further evidence of interrelation between homocysteine and hypertension in stroke patients: a cross-sectional study. The Israel Medical Association journal: IMAJ 5, 791–794 (2003).
Malinow, M. R. et al. Role of blood pressure, uric acid, and hemorheological parameters on plasma homocyst(e)ine concentration. Atherosclerosis 114, 175–183 (1995).
Jacques, P. F. et al. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. The American journal of clinical nutrition 73, 613–621 (2001).
Levy, D. et al. Associations of plasma natriuretic peptide, adrenomedullin, and homocysteine levels with alterations in arterial stiffness: the Framingham Heart Study. Circulation 115, 3079–3085 (2007).
Fakhrzadeh, H. et al. Plasma homocysteine concentration and blood pressure in healthy Iranian adults: the Tehran Homocysteine Survey (2003-2004). Journal of human hypertension 19, 869–876 (2005).
Zhan, S. et al. [A case-control study on the relationship between abnormal homocysteine metabolism and essential hypertension]. Zhonghua liu xing bing xue za zhi=Zhonghua liuxingbingxue zazhi 21, 194–197 (2000).
Dinavahi, R. Plasma homocysteine concentration and blood pressure in young adult African Americans. American journal of hypertension 16, 767–770 (2003).
Borges, M. C., Hartwig, F. P., Oliveira, I. O. & Horta, B. L. Is there a causal role for homocysteine concentration in blood pressure? A Mendelian randomization study. The American journal of clinical nutrition 103, 39–49 (2016).
Xiao, W. et al. Plasma homocysteine is associated with aortic arterial stiffness but not wave reflection in Chinese hypertensive subjects. PloS one 9, e85938 (2014).
van Dijk, S. C. et al. Homocysteine level is associated with aortic stiffness in elderly: cross-sectional results from the B-PROOF study. Journal of hypertension 31, 952–959 (2013).
Stehouwer, C. D. & van Guldener, C. Does homocysteine cause hypertension? Clinical chemistry and laboratory medicine 41, 1408–1411 (2003).
Dinavahi, R. & Falkner, B. Relationship of homocysteine with cardiovascular disease and blood pressure. Journal of clinical hypertension (Greenwich, Conn) 6, 494–498; quiz, 499–500 (2004).
Ustundag, S., Arikan, E., Sen, S., Esgin, H. & Ciftci, S. The relationship between the levels of plasma total homocysteine and insulin resistance in uncomplicated mild-to-moderate primary hypertension. Journal of human hypertension 20, 379–381 (2006).
Meigs, J. B. et al. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham offspring study. Diabetes care 24, 1403–1410 (2001).
Qian, G. et al. Associations between plasma total homocysteine, blood pressure stages and pulse wave velocity in Chinese rural community population. Blood pressure 24, 340–346 (2015).
Westphal, S., Rading, A., Luley, C. & Dierkes, J. Antihypertensive treatment and homocysteine concentrations. Metabolism: clinical and experimental 52, 261–263 (2003).
Acknowledgements
The authors thank all study team members for their participation. We are especially grateful to the site managers of Gucheng and Pingguoyuan Community Health Centers for their supports. This study was supported by a grant from the University of Michigan-Peking University Health Science Center (UM-PUHSC) joint institute for translational and clinical research (grant No: BMU20110177).
Author information
Authors and Affiliations
Contributions
Mohetaboer Momin participated in the design of the study, performed the statistical analysis, and drafted the manuscript. Jia Jia participated in the epidemical survey, data management, and helped to draft the manuscript. Jianping Li and Litong Qi participated in the study design and helped to draft the manuscript. Xianhui Qin participated in data management and helped with statistical analysis. Fangfang Fan participated in implementing the investigation, data analysis, and drafting the manuscript. Yong Huo and Yan Zhang, who are principal investigators of this study, conceived of the study, and participated in its design and coordination, and helped to analyze data and draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Momin, M., Fan, F., Li, J. et al. Associations of plasma homocysteine levels with peripheral systolic blood pressure and noninvasive central systolic blood pressure in a community-based Chinese population. Sci Rep 7, 6316 (2017). https://doi.org/10.1038/s41598-017-06611-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06611-3
This article is cited by
-
Associations between remnant lipoprotein cholesterol and central systolic blood pressure in a Chinese community-based population: a cross-sectional study
Lipids in Health and Disease (2021)
-
Kidney function predicts the risk of asymptomatic peripheral arterial disease in a Chinese community-based population
International Urology and Nephrology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.