Abstract
Human-induced forest fragmentation poses one of the largest threats to global diversity yet its impact on rattans (climbing palms) has remained virtually unexplored. Rattan is arguably the world’s most valuable non-timber forest product though current levels of harvesting and land-use change place wild populations at risk. To assess rattan response to fragmentation exclusive of harvesting impacts we examined rattan abundance, demography and ecology within the forests of northeastern, Australia. We assessed the community abundance of rattans, and component adult (>3 m) and juvenile (≤3 m) abundance in five intact forests and five fragments (23–58 ha) to determine their response to a range of environmental and ecological parameters. Fragmented forests supported higher abundances of rattans than intact forests. Fragment size and edge degradation significantly increased adult rattan abundance, with more in smaller fragments and near edges. Our findings suggest that rattan increase within fragments is due to canopy disturbance of forest edges resulting in preferential, high-light habitat. However, adult and juvenile rattans may respond inconsistently to fragmentation. In managed forest fragments, a rattan abundance increase may provide economic benefits through sustainable harvesting practices. However, rattan increases in protected area forest fragments could negatively impact conservation outcomes.
Similar content being viewed by others
Introduction
Deforestation of tropical rainforests rarely removes all pre-existing vegetation in a given area1, but leaves isolated fragments of the original vegetation surrounded by new habitat types2. Fragmentation of tropical forests is globally pervasive and increasing in extent3,4,5, with forest fragments now representing 46% of the remaining forested area6. Forest fragments support less species than comparable intact forest7, 8. The estimated 13–75% lost diversity7 that occurs in fragments has been associated with habitat alteration due to the degradation of a variety of biological and physical processes e.g. see reviews by: refs 8–11. For instance, one by-product of forest fragmentation is that it greatly increases the area of forest edge habitat12. In fact, current estimates suggest 70% of the world’s remaining forest is within 1 km from a forest edge7. Proximity to a newly-created forest edge exposes the surviving biota to numerous environmental changes associated with edges, such as: increased light levels, increased desiccation, and greater temperature variability11, 13, 14. These environmental changes are a consequence of increased disturbance found on forest edges due to mechanisms such as an increase in the rate of large tree loss and tree-turnover10, 15,16,17. In addition, forest fragmentation threatens species’ long-term persistence through the degradation of beneficial ecological interactions such as pollination and seed dispersal, between the remnant biota11, 18,19,20,21.
Despite their degraded state, forest fragments are often the sole means of preservation for many rare and endangered species and threatened ecosystems within heavily deforested regions22,23,24. Consequently, retention of forest fragments is of high importance for species and community conservation at regional spatial scales22,23,24. If the conservation values of forest fragments are to be preserved, fragments must not only be retained but effectively managed. This necessitates an understanding of their internal biota and ecology.
The majority of work on fragmentation has involved the study of trees. Indeed, the response of forest trees to fragmentation has received considerable focus e.g. refs 10, 11, 17, 25 and 26. However, despite the high diversity of non-tree life forms in tropical forests27 the potential impact of forest fragmentation on this forest component is less well known. For instance, even though rattans are one of the World’s most valuable non-timber forest products28, 29 and the existence of many wild populations is under threat30, 31, how rattans respond to forest fragmentation has yet to be explored.
“Rattan” is the generic term used to describe climbing species within the palm family Arecaceae (subfamily Calamoideae)32. Within Arecaceae, rattans represent roughly one fifth of the currently described taxa; comprising 13 genera and ~600 species33, 34. The majority of these species (~400 spp.) belong to the genus Calamus L.33, 35. Calamus is the most diverse genus within Arecaceae33 and one of the most diverse genera of all climbing plants36. Calamus is widely distributed throughout the Old World humid tropics ranging from Africa, through much of Asia to Australasia and parts of the Pacific region (e.g. Fiji). The Calamus genus attains maximum diversity in the closed-canopy forests of south-east Asia, where their predominance is a striking characteristic of Asian liana communities33, 36.
Economically, rattans are used extensively for furniture, basket making and construction making them a valuable non-timber forest product28, 29. The use of rattan by rural communities has persisted for centuries37, 38. Historically, most rattan has been harvested from wild populations in primary forests38, yet overharvesting along with continued land clearing has left many rattan species threatened with extinction30, 31. Understanding how rattan abundance responds to forest fragmentation exclusive of harvesting pressures would allow for increased effectiveness of rattan management for production39.
Few studies have explored the response of wild populations of rattans to the concurrent alteration of multiple environmental traits imposed by fragmentation. However, individual environmental traits are known to strongly influence rattan abundance. For example, in general, rattan abundance increases in moderate to high light conditions39, in well drained soils39,40,41,42 and peaks in abundance at mid-elevations (~1000 m)43,44,45. However, species-specific rattan responses have been identified for light-availability, soil type, elevation and soil moisture39, 40, 46 some of which are contradictory40, 45. For instance, in a study of two species of Calamus in Indonesia, Siebert40 identified C. zollingeri Becc. as displaying a positive relationship with light intensity whilst C. exilis Griff. abundance was negatively related to light intensity. Determining which environmental variables positively relate to rattan abundance and whether synergisms exist would allow for the improved conservation of wild rattan populations39.
Rattans are generally included in forest assessments as lianas sensu lato 47. While both rattans and lianas are climbing-plants, are structurally dependent on trees36, 43, 48, and proliferate in disturbed environments15, 40, 48,49,50 they differ in important ways. Within forests, rattans function differently from true lianas. As monocotyledons, they exhibit no secondary growth51 and rarely re-root their stems to the soil surface52. This lessens their ability for long-distance clonal colonization of tree-fall gaps53. Rattans also lack the capacity to branch52 resulting in difficulty maintaining canopy position during the stem elongation necessary for their leaf production48. Furthermore, rattans interact differently with their tree hosts. Unlike tendril-climbing or stem-twining lianas43, 54, rattans can utilize large diameter supports by embedding into tree branches or trunks55, 56 using recurved hooks on flagella (a modified inflorescence) or cirri (extensions of the leaf rachis)48, 55, 56. Thus rattans depend more on the proximity of supports rather than on the alignment of a series of successively taller, small diameter supports that are required by true lianas43. Rattans can also span larger inter-support gaps than most lianas48, 54. This is because a lack of secondary growth means young rattan stems are of a similar size to mature stems and are considerably more rigid than vine leader shoots (with additional rigidity provided by leaf sheaths)48, 57. Increased rigidity also means young rattan stems do not require structural support as early in plant development as vine leader shoots48. As a consequence, rattans generally access the canopy through smaller, more vertical openings in the overstorey39 and use larger supports over larger intra-support distances than many lianas could43. Therefore, despite the inclusion of rattans with lianas within forest assessments47, rattans are likely to respond differently to the enhanced disturbance within forest fragments10, 11, 26. Nevertheless, it is yet to be determined how rattans respond to forest fragmentation, and whether these responses differ from those of lianas15, 50, 58. Furthermore, a single rattan “response” to fragmentation may not be expected as adult rattans are reliant on structural hosts (trees) whilst juveniles are free-standing48. Consequently, juvenile rattans may respond differently to environmental and ecological variables than adult rattans46, 59. For instance, juvenile rattans in Indonesian forests were found to show a stronger relationship to ecological and spatial factors than adult rattans, possibly due to differential microhabitat preferences46. Juvenile arboreal palms have also been observed to display a greater sensitivity to edge effects than adults in a study of Ecuadorian forests59. These findings suggest that the demographic structure of rattan communities may be altered both temporally and spatially by forest fragmentation. As juvenile rattans constitute up to half the abundance of understory plants in some tropical forests39 it is important for both conservation and production values to ascertain whether their response to fragmentation is consistent with that of adult rattans.
Here, we examine the effect of forest fragmentation on total rattan community abundance and demographic structure at both a landscape level (comparing fragmented versus intact forests) and local level (within fragments), in a long-term (~100 years) fragmented-forest landscape of northeastern, Australia. We aimed to; a) determine the influence of fragmentation on total rattan abundance and rattan demographic structure (by looking at the component juvenile and adult rattan abundance separately), and b) identify the environmental and ecological predictors associated with these measures. We predicted that the highly-disturbed environmental conditions found within forest fragments would favor an increase in total community, juvenile and adult rattan abundance. However, we predicted that adult and juvenile rattan abundances would respond differently to both environmental factors and host (tree) abundance due to different responses to environmental conditions and the adult rattans reliance on hosts for structural support which is not required by their free-standing juveniles48.
Results
Rattan abundance and demography: intact vs fragmented forests
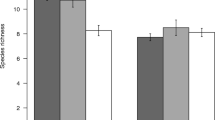
At a landscape level, we recorded a total relative rattan abundance of 3023 (n) stems ~70% of which were found in fragmented forests (n = 2128) and the remaining ~30% in intact forests (n = 895) (Fig. 1). Within the total rattan community, adult rattans (n = 2763) comprised >90% of the recorded stems, whilst juvenile rattans (n = 260) contributed <10% (Fig. 1). Despite considerable variation in environmental and ecological traits (Table 1, Supplementary Tables 1, 2 and 3), forest state (fragmented or intact) was the only significant predictor of total and adult rattan abundance within the landscape, with more rattans occurring in fragmented than intact forests (Fig. 1, Table 2). Additionally, adult and total rattan abundances displayed a positive relationship with distance from the forest edge whilst the relationship between juvenile rattan abundance and distance from the forest edge was negative, though these relationships were not significant.
Rattan abundance and demography: within forest fragments
Within fragmented forests, juvenile, adult and total rattan abundance was significantly and negatively related to: fragment area and canopy cover. The abundance of juvenile rattans was also significantly and negatively related to plot elevation and positively to liana abundance, whereas adult rattans were significantly and negatively influenced by tree abundance. Furthermore, total rattan abundance was positively associated with liana abundance and negatively with plot slope and tree abundance (Table 3). Interestingly, in contrast with the findings at the landscape level, within fragments, adult rattan abundance displayed a negative relationship to distance from the forest edge whilst the relationship with juvenile rattan abundance and distance from the forest edge was positive, though these relationships again were not significant (Table 3).
Environmental traits of fragmented and intact forests
Canopy cover was significantly lower in fragmented than intact forests and was lower on forest edges than forest interiors (Supplementary Table 3). This decreased canopy cover also penetrated significantly further into the edges of fragmented than intact forests (Supplementary Table A3). Canopy cover was also found to be significantly and negatively related to altitude (Supplementary Table 3).
Tree abundance was significantly lower in fragmented forests than in intact forests but was higher on forest edges than forest interiors (Supplementary Table 1). Furthermore, tree abundance was significantly and positively related to forest live carbon however it was significantly and negatively related to altitude (Supplementary Table 1).
Discussion
The fragmentation of the rainforests of the Atherton tablelands of north Queensland, Australia, has resulted in significantly higher total rattan abundance, and in particular, adult rattan abundance than similar, intact, forest locations. In fact, at a landscape level whether a forest was fragmented or not was the single best predictor of total and adult rattan abundance, in this study. The proliferation of rattans in response to forest fragmentation is similar to that found for woody-dicotyledonous lianas50, 60 and suggests that fragmentation promotes environmental or ecological changes which favor both types of climbing plants (rattans and lianas). However, juvenile rattan abundance was not significantly different between the two forest states, and forest type was not retained in any of the selected models used to describe juvenile rattan abundance. That no single model including forest type was retained (i.e. all had a ∆ AIC > 2) strongly suggests forest type (i.e. intact vs. fragmented) exerts very limited influence on the abundance of juvenile rattans.
Within forest fragments, light availability had a significant positive influence on rattan abundance. Sites with lower canopy cover had greater total, adult and juvenile rattan abundances than sites with high canopy cover. This finding supports previous reports of rattans proliferating in disturbed, high-light sites15, 49, 61 and the observations of Siebert39 who stated that “light is the most important determinant of rattan species composition, densities and growth rates” for South-East Asian rattan communities. Furthermore, we found that fragments had significantly lower canopy cover than intact forests and reduced canopy cover penetrated significantly further into the edges of fragmented than intact forests. The decreased canopy cover in fragments can result in changes to microclimatic conditions10, 11, 62, 63 including increased light availability64. This result also supports numerous studies which have shown that fragment edges experience higher levels of disturbance that those of intact forests10, 11, 65,66,67. Interestingly, however, when the response of rattans to forest edges was examined within individual demographic classes (adult and juveniles) the findings were not consistent across classes. For instance, at a landscape level, adult rattans displayed a positive relationship to forest edge distance and juveniles a negative relationship, whilst the reverse relationships were true for the abundances of both groups when examined in fragmented forests alone. Whilst these finding were non-significant, they suggest a potential that juvenile rattans may respond differently to adult rattans in how they react to the environmental and ecological alterations found on fragmented forest edges46, 59. However, further testing would be required to confirm the presence of these contrary responses to fragmentation by the adult and juvenile rattan age classes and if found to identify the underlying mechanisms (e.g. seed dispersal limitation, structural host limitation, climate change, survival differences between age classes). It can however be concluded that the increased disturbance of fragment edges leads to a general increase in rattan abundance, even though adult and juvenile rattan responses to fragmentation and edge effects may not be consistent.
Further support that forest disturbance drives an increase in rattan abundance in fragments was our finding that fragment area was significantly and negatively related to juvenile, adult and total rattan abundance. Fragment area is negatively correlated with tropical forest disturbance with smaller fragments likely to experience significantly higher levels of disturbance which is chronic10,11,12, 26, 68. This disturbance is the consequence of elevated rates of large tree mortality, turnover and treefall-gap creation17, 25, 26, 69, 70 mostly on fragment edges due to wind-disturbance, desiccation, and micro-climate alteration10, 14, 63, 71. In corroboration, there was a positive relationship between rattan abundance and fragment shape, where more dissected fragments with greater edge exposure12, 72, were found to display greater rattan abundances.
In our study, lianas and rattans appear to have similar habitat preferences, with both increasing in abundance in response to fragmentation. For instance, analogous with rattans, lianas are renowned for proliferation in response to forest disturbance50, 73, peaking in areas of high-light availability such as forest edges and treefall gaps74,75,76,77. These findings lend further credence to the assertion that rattans become more abundant in fragments due to disturbance and increased light availability15, 38, 39, 49, 61. However, though adult rattan abundance was positively related to liana abundance this relationship was not significant. It is plausible that whilst adult rattans increase in abundance in the disturbed and high-light environments within which lianas are found, there is considerable competition between these ecologues (functional ecological analogues) for essential structural supports (tree hosts) despite the difference in their preferential trellis morphology. For instance, the capacity of lianas to branch and their highly specialized climbing apparatus dedicated for attachment to smaller climbing trellises43, 54, 78, may provide a competitive advantage in areas with smaller climbing trellises54, 79, such as the edges of forests and regenerating treefall gaps74,75,76,77, areas in fragmented forests which have previously been found to exhibit increased liana abundances50, 58, 80, 81.
Rattan abundance would increase within fragments if altered environmental conditions provide them a competitive advantage for host trees colonization. Though speculative, this mechanism could explain why adult rattan abundance increased in forest fragments with respect to forest edges. Beyond a certain threshold the number of supports available (trees), not the access to sufficient light, becomes the limiting factor for both rattan and liana abundance82. We found fragments had significantly less trees than intact forests (however we did not examine trees <10 cm DBH) and thus potential structural hosts. A collapse in tree abundance often occurs within heavily disturbed forest fragments11, 26, 70 and this has previously been found to result in reduced liana abundance and diversity linked to increased competition for hosts83,84,85,86. Given lower tree abundances within fragments and their significantly lower canopy cover (Supplementary Tables 1 and 3), it is plausible that climbing plants must span larger distances between successive supports. Young rattans are comparatively rigid meaning they do not require structural support as early as vine leader shoots48. Rattans also possess flagella or cirri often several metres long48. As a consequence of both these traits, rattans possess a superior ability to span larger inter-support distances than lianas48. Furthermore, the ability of rattans to embed into tree branches and trunks55, 56, allows them to attach to and climb larger supports (which are themselves further apart) than could most lianas48, 54,55,56. If correct, this hypothesis would also explain the lack of any detectable response of juvenile rattan abundance to fragmentation as juvenile rattans being free-standing would not be affected by inter-host distances unlike adults. Whilst, this hypothesis of rattan and dicotyledonous liana competition and host distance is as yet un-tested, their specialized morphology and restricted monocotyledonous phylogeny55,56,57, 87, suggest that rattans function as a specialized sub-component within the broader climbing plant community.
In addition, the above hypothesized competition for climbing supports may be one of many as yet unknown ancillary processes contributing to the lack of response to fragmentation by juvenile rattans. For instance, there is considerable variation in light-level preferences of rattan species in some South-East Asian forests88. Unfortunately, there is very little known of the responses to light availability for the species occurring in this study. Furthermore, it is unclear whether differences in light-level preferences occur between the different age classes of rattan species or communities studied here or elsewhere in the world. Additionally, further insight into the response of the rattan community to fragmentation could be had by examining earlier life history stages. For example, we did not examine rattan seedling recruitment in this study. Rattans possess fleshy fruits whose principle means of dispersal are birds and mammals32, 39, 42, 89. Fragmentation and associated impacts e.g. increased hunting90; are known to differentially alter the populations of many birds and mammals e.g. refs 21, 91–93 and thus potential rattan dispersers. As such, patterns of dispersal of rattan propagules within-and-between forest fragments could also be influential in setting overall abundances.
Conclusion
Rattans are more abundant in the fragmented than intact rain forests of tropical north-eastern Australia. The increase in rattan abundance is underlain by an increase in adult rattans and likely due to greater canopy disturbance of fragmented forest edges leading to an increase in light availability. Adult rattans may also increase in abundance in fragments as their ability to span larger inter-support distances could allow them to better colonize the widely-spaced tree hosts that occur there. Finally, though requiring further examination, the response of adult and juvenile rattans to fragmentation and edge effects may not be consistent suggesting the underlying mechanisms that determine their distribution and abundances in forest fragments may be different.
Methods
Study area
Our study was located on the Atherton Tableland, north-eastern Queensland, Australia (Fig. 2a). The Atherton Tableland is a hilly upland plateau ranging in elevation from ~600–1100 m.a.s.l. Mean annual precipitation ranges from 1400 to 3000 mm due to a localized north-west (low) to south-east (high) rainfall gradient, with a pronounced wet season from January to April Bureau of ref. 94. The region is also prone to cyclones with 45 cyclonic impacts recorded for the region from the years 1858 to 201195. Cyclone impacts can range from elevated precipitation to severe canopy damage of forest trees96, 97.
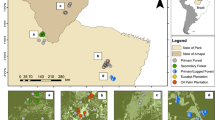
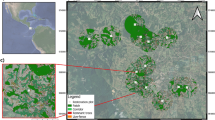
Field site location and experimental design. (a) Location of the ten study sites on the Atherton Tablelands, Australia. Study sites are indicated as triangles for intact forests and circles for fragmented forest. Malanda as the nearest town is indicated with an asterix. (b) Illustrates the design of vegetation sampling at each study site wherein five 20 × 20 m plots were stratified and randomly placed with respect to the position along the forest edge. The map (a) was generated using google earth version 7.1.8.3036 and the inset map was created using Esri ArcMap 10.2. (http://www.arcgis.com).
Forests in the study area are described as complex mesophyll and notophyll rainforests98, 99. These are structurally similar to those of the Indo-Malay region100 and contain abundant rattans. Four of the eight species of Calamus present in Australia are found in the area: C. australis Mart., C. caryotoides A.Cunn. ex Mart., C. moti F.M. Bailey, and C. radicalis H.Wendl. & Drude89, 101. These forests have not experienced rattan harvesting since harvesting is uncommon in the region and most forests are protected. Vegetation of the study area comprises primary remnants, secondary forests and large rain forest areas on surrounding mountain ranges. Deforestation here began in the early 1900’s and proceeded rapidly with most forest clearance occurring within three decades102,103,104,105. The study area is now heavily fragmented with remaining vegetation fragments spatially isolated by a predominantly agricultural land use matrix (Fig. 2a). Additionally, most of the remnant rain forest vegetation has, at some time in the past, been exposed to selective logging for valuable hardwood timber species such as Red Cedar (Toona ciliata)103, 104, 106.
Fragments are generally found overlying volcanic soils, namely krasnozems, and topographically occur on level to gently undulating plains and gently undulating to undulating rises107. Larger remnant intact forests are mostly located on steeper mountainous areas that were less conducive to logging and on poor nutrient granite and rhyolite-derived soils that restricted their suitability to agriculture107.
Site selection
Ten sites were selected for study, comprising five forest fragments and five sites in nearby intact rain forest (Fig. 2a). Forest fragments were selected to minimize variation in total area, ranging from 23–58 ha, and thus limit patch-area effects on rattan abundance19, 50. Intact-forest sites were selected to be as close as possible to the fragments, with the largest between-site distance for all sites being <23 km. Inter-site distance was minimized to lessen variation in environmental variables known to influence rattan abundance; in particular rainfall, elevation, and soil type39,40,41,42,43,44,45,46. Finally, fragments were selected to ensure that they were all created prior to 1950 (i.e. ≥60 years since isolation) and are currently surrounded by cattle pastures to lessen possible confounding effects of fragment age or surrounding matrix type.
Rattan measures
Over the period March 2012 to February 2014, rattan abundance was recorded at five 20 × 20 m plots in 10 forest sites (Fig. 2b) five in forest fragment sites and five in intact forest sites (N = 50 plots in total). At the four corners of each plot, line intercept transects of 3 m were established in the four cardinal directions. Along the transects, individual rattan stems that intercepted the line, including those up to 1.8 m in height above it, were counted (Fig. 3). For each plot, the 16 samples were summed to produce a relative abundance estimate of rattans. Any rattan stems that intercepted the line transect and could be distinguished as coming from a previously encountered rattan clump were disregarded. Finally, to ascertain rattan population demography, all sampled rattans were categorized as either juvenile (≤3 m) or adult (>3 m). We used a similar method of aging rattans as Thonhofer et al.46 in their study from central Sulawesi, however, we chose a 3 m cut off for the category of juvenile rattans rather than 1 m as this was the height at which rattans transitioned from free standing to utilizing tree hosts.
Representative rattan abundance measurement protocol. All rattan stems encountered along a 3 m long by 1.8 m high transect facing north were counted unless they were noted to arise from a previously encountered rattan clump. In addition, each counted rattan stem was classified as ≤3 m or >3 m in height/length. This procedure was then repeated for identical transects facing the other three cardinal directions with all transects originating from a central point. Finally, this entire process was repeated in the remaining three corners of each plot and the 16 transect values summed to gain an overall representative value of rattan abundance per 20 m2 plot.
The second aim of our study was to identify the environmental and ecological predictors associated with rattan abundance and demography at both the landscape and local level. To identify these we collected information on known correlates of rattan and liana abundance e.g. those identified within the literature39, 40, 50, 73, 108 for incorporation in the individual generalized linear mixed models (GLMMs) listed below (see Data Analysis subheading for full description). Parameters examined included: liana abundance, tree abundance, tree DBH (cm), tree bark type, tree buttressing, canopy cover (%), number of fallen logs (≥10 cm diameter), plot elevation (m), plot slope (degrees), mean annual rainfall (mm), mean dry quarter (July-September) rainfall, plot distance to forest edge (m), and plot carbon storage (tonnes/ha).
Liana and tree measures
The abundance of lianas (≥1 cm diameter breast height: DBH) was determined for five 20 × 20 m plots at each of the 10 sites as per standard methodology47, 109, 110. Liana stems were counted as individuals unless clearly joined and were not excavated to determine vegetative propagation. Tree abundance and size (≥10 cm DBH) was also measured with tree size measured at 1.3 m above the ground or 10 cm above buttresses.
Forest disturbance and localized environmental parameters
Two measures of forest disturbance were determined for each plot: canopy cover and the number of fallen trees (≥10 cm diameter). Canopy cover was estimated at the four corners and the center of each plot, measured by averaging four spherical densitometer readings taken facing the cardinal directions (N, E, S, W) at each point. The number of fallen trees (≥10 cm diameter) was counted within each plot.
To determine physical traits of plots we examined their slope and elevation. The degree of slope of each plot was calculated using a clinometer, whilst elevation of all sites was assessed using climatic model interpolation data provided by the Wet Tropics Management Authority, Cairns, Australia111. These data were also accessed to determine the annual rainfall (mm) and dry quarter rainfall (July-September, mm) of sites.
Plot live carbon was used to compare the structural parameters of fragmented and intact forest sites. This was estimated by combining carbon from above ground estimates of all live trees (≥10 cm DBH) and lianas (≥1 cm DBH) within a 20 × 20 m plot. Liana above-ground biomass (AGB) was calculated using the liana specific allometric equation (1) developed by Schnitzer et al.109:
where D is the diameter at 130 cm from the roots47 expressed in centimetres, while AGB is the predicted above ground oven-dry weight of the liana in kilograms.
Tree above ground biomass (ABG) was calculated using the allometric equation developed by Chave et al.112 (see below) as Preece et al.113 compared the accuracy of multiple biomass estimation methods for forests within the Wet Tropics bioregion and concluded that the Chave et al.112 allometric provided the best and most reliable estimate for the region. To convert AGB into biomass carbon storage we used a conversion factor of 0.47 which is the recommended value from the Intergovernmental Panel for Climate Change for tropical forests114. In addition, AGB was calculated using wood density estimates at the reported default value for Australian tropical forests of 0.5 g cm−3 (500 kgm−3) Department of Climate Change and Energy115. Consequently, tree AGB estimates were calculated using the following equation (2):
Where AGB is measured in kg, DBH is measured in cm, and ρ is wood density measured in g cm−3.
Landscape variables
Data on forest fragment characteristics were collected from the aforementioned climatic model interpolations data and assessed using the program Fragstats116. Parameters assessed included: fragment area (m2), fragment perimeter (m), fragment isolation (m), fragment shape (perimeter/minimum possible perimeter for a fragment that size) and fragment proximity which is a measure of isolation which also includes the proportion of similar vegetation within distinct buffer zones (1000 m and 5000 m) surrounding individual fragments.
Data analyses
Rattan abundance and demography: intact vs fragmented forests
We evaluated the influence of landscape and environmental parameters on rattan abundance and demography using individual, negative binomial, generalized linear mixed models (GLMMs). Prior to model generation we checked for correlated predictor variables through examination of the variance inflation factor (VIF) and eliminated those that showed a VIF > 3 following the protocol of Zurr et al.117. This resulted in the removal of the mean dry quarter rainfall variable. Additionally, as there were five plots within each site (stratified by forest edge distance), plots were not fully independent. As such, we included site ID as a random effect. In each model-fitting exercise we selected a priori a global model in which the response variable (total rattan abundance, juvenile abundance, and adult abundance per plot) was examined as a function of the following nine environmental and ecological drivers: forest state (intact vs. fragmented), edge distance, liana abundance, tree abundance, number of fallen logs, canopy cover, mean annual rainfall, altitude and slope. We additionally included the interaction between forest state and edge distance. Model analysis was performed using the R package glmmADMB 118.
The most parsimonious model was determined using a multimodel inference approach119 where we ran all combinations of models using function dredge in package MuMIn 120 and selected the best model based on Akaike information criteria values (AIC). Whenever we had more than one plausible model (i.e., when ∆ AIC < 2 for more than one model119) we computed average estimates for each variable across all models. This procedure was followed for model fitting for each response variable.
Rattan abundance and demography: within forest fragments
We used the subset of forest fragment sites (i.e. excluded intact forest sites) to evaluate the effect of the fragment specific traits such as fragment area, fragment isolation, fragment shape and fragment proximity, on the response variables of total rattan abundances and the abundance of juvenile and adult rattans per plot. Again, these impacts were assessed in conjunction with the previously mentioned environmental and ecological drivers (listed below) known to influence rattan abundance. Analyses were preformed using individual GLMMs and followed the procedure mentioned above. Full models here included the following explanatory variables: fragment size, fragment shape, fragment isolation, fragment proximity, distance to the forest edge, liana abundance, tree abundance, number of fallen logs, canopy cover, mean annual rainfall, altitude and slope. We followed the same procedure outlined above for model fitting, selection and averaging.
Environmental traits of fragmented and intact forests
Disturbance and forest gap dynamics along with the availability and size of trees (as rattan supports) are known to be the major drivers of the distribution of rattans and lianas within forests48, 73, 74, 76, 77. To assess these traits within fragmented and intact forests, canopy cover and tree abundance were compared along with their relationships with the previously mentioned (see above) environmental and ecological drivers. Assessment was again determined using individual GLMMs. For full results see supplementary material.
Program R121 was used for all statistical analyses.
References
Laurance, W. F. & Bierregaard Jr, R. O. Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities. (The University of Chicago Press, 1997).
Wilcove, D. S., McLellan, C. H. & Dobson, A. P. Habitat fragmentation in the temperate zone. in Conservation biology: the science of scarcity and diversity (ed M. E. Soule) 237–256 (Sinauer Associates, 1986).
Bhagwat, S. The history of defoestation and forest fragmentation: a global perspective. in Global Forest Fragmentation (eds C. J. Kettle & L. P. Koh) Ch. 2, 5–19 (CABI, 2014).
Wade, T. G., Riitters, K. H., Wickham, J. D. & Jones, K. B. Distribution and causes of global forest fragmentation. Conservation Ecology 7, 7 (2003).
Riitters, K., Wickham, J., Costanza, J. K. & Vogt, P. A global evaluation of forest interior area dynamics using tree cover data from 2000 to 2012. Landscape Ecol 31, 137-148 (2016).
Mercer, B. Tropical forests: a review. (Mercer Environment Associates, 2015).
Haddad, N. M. et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Science Advances 1, e1500052 (2015).
Fahrig, L. Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution, and Systematics 34, 487–515 (2003).
Fischer, J. & Lindenmayer, D. B. Landscape modification and habitat fragmentation: a synthesis. Global Ecology and Biogeography 16, 265–280 (2007).
Laurance, W. F. et al. The fate of Amazonian forest fragments: a 32-year investigation. Biological Conservation 144, 56–67 (2011).
Laurance, W. F. et al. Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conservation Biology 16, 605–618 (2002).
Laurance, W. F. Edge effects in tropical forest fragments: application of a model for the design of nature-reserves. Biological Conservation 57, 205–219 (1991).
Williams-Linera, G. Vegetation structure and environmental conditions of forest edges in Panama. Journal of Ecology 78, 356–373 (1990).
Briant, G., Gond, V. & Laurance, S. G. W. Habitat fragmentation and the desiccation of forest canopies: a case study from eastern Amazonia. Biological Conservation 143, 2763–2769 (2010).
Laurance, W. F. Hyper-disturbed parks: edge effects and the ecology of isolated rainforest reserves in tropical Australia. in Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities (eds W. F. Laurance & R.O Bierregaard Jr) 71–83 (The University of Chicago Press, 1997).
Laurance, W. F. & Curran, T. J. Impacts of wind disturbance on fragmented tropical forests: a review and synthesis. Austral Ecology 33, 399–408 (2008).
Laurance, W. F., Delamonica, P., Laurance, S. G., Vasconcelos, H. L. & Lovejoy, T. E. Conservation: rainforest fragmentation kills big trees. Nature 404, 836–836 (2000).
Magrach, A., Laurance, W. F., Larrinaga, A. R. & Santamaria, L. Meta‐analysis of the effects of forest fragmentation on interspecific interactions. Conservation Biology 28, 1342–1348 (2014).
Campbell, M., Laurance, W. F. & Magrach, A. Ecological effects of lianas in fragmented forests. in Ecology of Lianas (eds S. A. Schnitzer, F. Bongers, R. Burnham, & F. E. Putz) Ch. 29, 447–454 (Wiley-Blackwell Publishing, 2015).
Peh, K. S. H., Lin, Y., Luke, S. H., Foster, W. A. & Turner, E. C. Forest fragmentation and ecosystem function. in Global Forest Fragmentation (eds C. J. Kettle & Lian Pin Koh) Ch. 8, 96–114 (CABI, 2014).
Terborgh, J. et al. Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926 (2001).
Guindon, C. F. The importance of forest fragments to the maintenance of regional biodiversity in Costa Rica. in Forest Patches in Tropical Landscapes (eds J. Schelhas & R. Greenberg) (Island Press, 1996).
Arroyo-Rodriguez, V., Pineda, E., Escobar, F. & Benitez-Malvido, J. Value of small patches in the conservation of plant-species diversity in highly fragmented rainforest. Conservation Biology 23, 729–739 (2009).
Arroyo-Rodriguez, V. & Mandujano, S. The importance of tropical rain forest fragments to the conservation of plant species diversity in Los Tuxtlas, Mexico. Biodiversity and Conservation 15, 4159–4179 (2006).
Laurance, W. F., Ferreira, L. V., Rankin-de Merona, J. M. & Laurance, S. G. Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology 79, 2032–2040 (1998).
Laurance, W. F. et al. Rapid decay of tree-community composition in Amazonian forest fragments. Proceedings of the National Academy of Sciences of the United States of America 103, 19010–19014 (2006).
Gentry, A. H. & Dodson, C. Contribution of non-trees to species richness of a tropical rain forest. Biotropica 19, 149–156 (1987).
Ros-Tonen, M. A. F. The role of non-timber forest products in sustainable tropical forest management. Holz als Roh- und Werkstoff 58, 196–201 (2000).
Sastry, C. B. Rattan in the twenty-first century: an outlook. in Rattan Current Research Issues and Prospects for Conservation and Sustainable Development (eds J. Dransfield, F. O. Tesoro & N. Manokaran) 237–244 (FAO, 2002).
Dransfield, J. The conservation status of rattans in 1987: a cause for great concern. in International Rattan Seminar. 12–14 (Chang Mai, 1987).
Hirschberger, P. Global rattan trade: pressure on forest resources, analysis and challenges. (Austria, 2011).
Dransfield, J. T. biology and ecology of rattan. UNASYLVA-FAO 52, 11–13 (2001).
Dransfield, J. et al. Genera Palmarum: the Evolution and Classification of Palms. (Kew Publishing, 2008).
Uhl, N. W. & Dransfield, J. Genera Palmarum. A Classification of Palms Based on the Work of Harold E. Moore, Jr. The LH Bailey Hortorium and the International Palm Society. (Allen Press, 1987).
Baker, W. J. A revised delimitation of the rattan genus Calamus (Arecaceae). Phytotaxa 197, 139–152 (2015).
Gentry, A. H. The distribution and evolution of climbing plants. in The Biology of Vines (eds F. E. Putz & H. A. Mooney) (Cambridge University Press, 1991).
De Beer, J. & McDermott, M. The economic value of non-timber forest products in South-East Asia. (Netherlands Committee for the World Conservation Union, 1989).
Dransfield, J. & Manokaran, N. Rattans. (PROSEA, Indonesia, 1994).
Siebert, S. F. The Nature and Culture of Rattan: Reflections on Vanishing Life in the Forests of Southeast Asia. (University of Hawaii Press, 2012).
Siebert, S. F. The abundance and site preferences of rattan (Calamus exilis and Calamus zollingeri) in two Indonesian national parks. Forest Ecology and Management 59, 105–113 (1993).
Watanabe, N. & Suzuki, E. Species diversity, abundance, and vertical size structure of rattans in Borneo and Java. Biodiversity and Conservation 17, 523–538 (2008).
Dransfield, J. The ecology and natural history of rattans. in A Guide to the Cultivation of Rattan (eds W. M. Wan Razali, J. Dransfield, & N. Manokaran) 27–33 (Forest Research Institute Malaysia, 1992).
Putz, F. E. & Chai, P. Ecological-studies of lianas in Lambir National-Park, Sarawak, Malaysia. Journal of Ecology 75, 523–531 (1987).
Siebert, S. F. The abundance and distribution of rattan over an elevation gradient in Sulawesi, Indonesia. Forest Ecology and Management 210, 143–158 (2005).
Stiegel, S., Kessler, M., Getto, D., Thonhofer, J. & Siebert, S. Elevational patterns of species richness and density of rattan palms (Arecaceae: Calamoideae) in Central Sulawesi, Indonesia. Biodiversity and Conservation 20, 1987–2005 (2011).
Thonhofer, J., Getto, D., van Straaten, O., Cicuzza, D. & Kessler, M. Influence of spatial and environmental variables on rattan palm (Arecaceae) assemblage composition in Central Sulawesi, Indonesia. Plant Ecology 216, 55–66 (2015).
Gerwing, J. J. et al. A standard protocol for liana censuses. Biotropica 38, 256–261 (2006).
Putz, F. E. Growth habits and trellis requirements of climbing palms (Calamus spp.) in north-eastern Queensland. Australian Journal of Botany 38, 603–608 (1990).
Bøgh, A. Abundance and growth of rattans in Khao Chong National Park, Thailand. Forest Ecology and Management 84, 71–80 (1996).
Laurance, W. F. et al. Rain forest fragmentation and the structure of Amazonian liana communities. Ecology 82, 105–116 (2001).
Tomlinson, P. B. & Huggett, B. A. Cell longevity and sustained primary growth in palm stems. American Journal of Botany 99, 1891–1902 (2012).
Dransfield, J. Growth forms of rain forest palms. In Tropical Trees as Living Systems Vol. 247 (eds P. B. Tomlinson & M. H. Zimmerman) 247–268 (Cambridge University Press, 1978).
Yorke, S. R., Schnitzer, S. A., Mascaro, J., Letcher, S. G. & Carson, W. P. Increasing liana abundance and basal area in a tropical forest: the contribution of long-distance clonal colonization. Biotropica 45, 317–324 (2013).
Putz, F. E. The natural history of lianas on Barro-Colorado island, Panama. Ecology 65, 1713–1724 (1984).
Isnard, S. & Rowe, N. P. The climbing habit in palms: biomechanics of the cirrus and flagellum. American Journal of Botany 95, 1538–1547 (2008).
Rowe, N. & Isnard, S. Biomechanics of climbing palms and how they climb. Plant Signaling & Behavior 4, 875–877 (2009).
Isnard, S. & Rowe, N. P. Mechanical role of the leaf sheath in rattans. New Phytologist 177, 643–652 (2008).
Magrach, A., Rodríguez-Pérez, J., Campbell, M. & Laurance, W. F. Edge effects shape the spatial distribution of lianas and epiphytic ferns in Australian tropical rain forest fragments. Applied Vegetation Science 17, 754–764 (2014).
Browne, L. & Karubian, J. Diversity of palm communities at different spatial scales in a recently fragmented tropical landscape. Botanical Journal of the Linnean Society 182, 451–464 (2016).
Laurance, W. F. et al. Apparent environmental synergism drives the dynamics of Amazonian forest fragments. Ecology 95, 3018–3026 (2014).
Tomlinson, P. B. Systematics and ecology of the Palmae. Annual Review of Ecology and Systematics 10, 85–107 (1979).
Camargo, J. L. C. & Kapos, V. Complex edge effects on soil moisture and microclimate in Central Amazonian forest. Journal of Tropical Ecology 11, 205–221 (1995).
Magnago, L., Rocha, M., Meyer, L., Martins, S. & Meira-Neto, J. Microclimatic conditions at forest edges have significant impacts on vegetation structure in large Atlantic forest fragments. Biodiversity and Conservation 24, 2305–2318 (2015).
Turton, S. & Freiburger, H. Edge and Aspect Effects on the microclimate of a small tropical forest remnant on the Atherton Tableland, northeastern Australia. In Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities (eds W. F. Laurance & R. O. Bierregaard Jr) 45–54 (The University of Chicago Press, 1997).
Harper, K. A. et al. Edge influence on forest structure and composition in fragmented landscapes. Conservation Biology 19, 768–782 (2005).
Murcia, C. Edge effects in fragmented forests: implications for conservation. Trends in Ecology & Evolution 10, 58–62 (1995).
Saunders, D. A., Hobbs, R. J. & Margules, C. R. Biological Consequences of Ecosystem Fragmentation: A Review. Conservation Biology 5, 18–32 (1991).
Laurance, W. F. Hyperdynamism in fragmented habitats. Journal of Vegetation Science 13, 595–602 (2002).
Hill, J. L. & Curran, P. J. Area, shape and isolation of tropical forest fragments: effects on tree species diversity and implications for conservation. Journal of Biogeography 30, 1391–1403 (2003).
Laurance, W. F. et al. Biomass collapse in Amazonian forest fragments. Science 278, 1117–1118 (1997).
Williams-Linera, G., Domínguez-Gastelú, V. & García-Zurita, M. E. Microenvironment and floristics of different edges in a fragmented tropical rainforest. Conservation Biology 12, 1091–1102 (1998).
Hill, J. L. & Curran, P. J. Fragment shape and tree species composition in tropical forests: a landscape level investigation. African Journal of Ecology 43, 35–43 (2005).
Ledo, A. & Schnitzer, S. A. Disturbance and clonal reproduction determine liana distribution and maintain liana diversity in a tropical forest. Ecology 95, 2169–2178 (2014).
Schnitzer, S. & Bongers, F. Lianas and gap phase regeneration: implications for forest dynamics and species diversity. in Forest Climbing Plants of West Africa: Diversity, Ecology and Management (eds F. Bongers, M. P. E. Parren & D. Traore) 59–72 (CABI Publishing, 2005).
Schnitzer, S. A. & Carson, W. P. Treefall gaps and the maintenance of species diversity in a tropical forest. Ecology 82, 913–919 (2001).
Schnitzer, S. A. & Carson, W. P. Lianas suppress tree regeneration and diversity in treefall gaps. Ecology Letters 13, 849–857 (2010).
Schnitzer, S. A., Dalling, J. W. & Carson, W. P. The impact of lianas on tree regeneration in tropical forest canopy gaps: evidence for an alternative pathway of gap-phase regeneration. Journal of Ecology 88, 655–666 (2000).
Hegarty, E. E. Vine host interactions. In The Biology of Vines (eds F. E. Putz & H. A. Mooney) 357–376 (Cambridge University Press, 1991).
Penalosa, J. Basal branching and vegetative spread in two tropical rain forest lianas. Biotropica 16, 1–9 (1984).
Viana, V. M., Tabanez, A. A. J. & Batista, J. L. Dynamics and restoration of forest fragments in the Brazilian Atlantic Moist Forest. In Tropical Forest Remnants (eds W. F. Laurance & R. O. Bierregaard Jr) (University of Chicago Press, 1997).
Oliveira, A. T., deMello, J. M. & Scolforo, J. R. S. Effects of past disturbance and edges on tree community structure and dynamics within a fragment of tropical semideciduous forest in south-eastern Brazil over a five-year period (1987–1992). Plant Ecology 131, 45–66 (1997).
Hegarty, E. E. & Caballe, G. Distribution and abundance of vines in forest communities. In The Biology of Vines (eds F. E. Putz & H. A. Mooney) (Cambridge University Press, 1991).
Arroyo-Rodriguez, V. & Toledo-Aceves, T. Impact of landscape spatial pattern on liana communities in tropical rainforests at Los Tuxtlas, Mexico. Applied Vegetation Science 12, 340–349 (2009).
Addo-Fordjour, P., Rahmad, Z. B. & Shahrul, A. M. S. Effects of human disturbance on liana community diversity and structure in a tropical rainforest, Malaysia: implication for conservation. Journal of Plant Ecology 5, 391–399 (2012).
Addo-Fordjour, P., El Duah, P. & Agbesi, D. K. K. Factors influencing liana species richness and structure following anthropogenic disturbance in a tropical forest, Ghana. ISRN Forestry 2013, 11 (2013).
Muthuramkumar, S. et al. Plant community structure in tropical rain forest fragments of the Western Ghats, India. Biotropica 38, 143–160 (2006).
Couvreur, T. L. P. et al. Global diversification of a tropical plant growth form: environmental correlates and historical contingencies in climbing palms. Frontiers in Genetics 5, 452 (2014).
Siebert, S. F. Biology, utilization, and silvicultural management of rattan palms. In The Biology of Vines (eds F. E. Putz & H. A. Mooney) Ch. 17, 477–492 (Cambridge University Press, 1991).
Dowe, J. Australian Palms: Biogeography, Ecology and Systematics. (CSIRO Publishing, 2010).
Wright, S. J., Hernandez, A. & Condit, R. The bushmeat harvest alters seedling banks by favoring lianas, large seeds, and seeds dispersed by bats, birds, and wind. Biotropica 39, 363–371 (2007).
Gibson, L. et al. Near-complete extinction of native small mammal fauna 25 years after forest fragmentation. Science 341, 1508–1510 (2013).
Laurance, W. F. Ecological correlates of extinction proneness in Australian tropical rain forest mammals. Conservation Biology 5, 79–89 (1991).
Laurance, W. F. Responses of mammals to rainforest fragmentation in tropical Queensland: a review and synthesis. Wildlife Research 24, 603–612 (1997).
Bureau of Meterology. Monthly rainfall: Malanda Alert (http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=139&p_display_type=dataFile&p_startYear=&p_c=-201758844&p_stn_num=031183). (Bureau of Meterology, 2016).
Turton, S. M. Securing landscape resilience to tropical cyclones in Australia’s Wet Tropics under a changing climate: lessons from cyclones Larry (and Yasi). Geographical Research 50, 15–30 (2012).
Turton, S. M. & Siegenthaler, D. T. Immediate impacts of a severe tropical cyclone on the microclimate of a rain forest canopy in north-east Australia. Journal of Tropical Ecology 20, 583–586 (2004).
Turton, S. M. & Stork, N. E. Impacts of tropical cyclones on forests in the Wet Tropics of Australia. in Living in a Dynamic Tropical Forest Landscape 47–58 (Blackwell Publishing, Ltd, 2009).
Tracey, J. G. The Vegetation of the Humid Tropical Region of North Queensland. (CSIRO, 1982).
Queensland Herbarium. Regional Ecosystem Description Database (REDD). Version 9.0 (April 2015). (Queensland Department of Science, Information Technology and Innovation: Brisbane, Brisbane, 2015).
Metcalfe, D. J. & Ford, A. J. Florisitcs and plant biodiversity of the rainforests of the Wet Tropics. In Living in a Dynamic Tropical Forest Landscape (eds N. E. Stork & S. M. Turton) 123–132 (Blackwell Publishing, Ltd, 2009).
Centre for Australian National Biodiversity Research. Australian tropical rainforest plants edition 6.1 [online version]. (Centre for Australian National Biodiversity Research, 2010).
Winter, J. W., Bell, F. C. & Pahl, L. I. Rainforest clearfelling in northeastern Australia. Proceedings of the Royal Society of Queensland 98, 41–57 (1987).
E Historical Society. Eacham Shire, Atherton Tableland, Nrth Queensland: Yesterday and Today. (Eacham Historical Society, 1979).
Eacham Historical Society. Malanda: in the Shadow of Bartle Frere. (Eacham Historical Society, 1995).
Smith, L. W. The Trees That Fell: a History and Description of the Timber Industry of North Queensland from 1898 to 1988, With Reminiscences and Factual Information From the North Queensland Logging Association. (Smith, L. W., 1991).
Pearson, L. The Log Trade in Far North Queensland. (Pearson, L. M., 2008).
Malcom, D. T., Nagel, B. K. A., Sinclair, I. & Heiner, I. J. Soils and agricultural land suitability of the Atherton Tablelands north Queensland. (Department of Natural Resources, 1999).
Schnitzer, S. A. & Bongers, F. The ecology of lianas and their role in forests. Trends in Ecology & Evolution 17, 223–230 (2002).
Schnitzer, S. A., DeWalt, S. J. & Chave, J. Censusing and measuring lianas: a quantitative comparison of the common methods. Biotropica 38, 581–591 (2006).
Schnitzer, S. A., Rutishauser, S. & Aguilar, S. Supplemental protocol for liana censuses. Forest Ecology and Management 255, 1044–1049 (2008).
Wet Tropics Management Authority. Vegetation of the Wet Tropics of Queensland. (Wet Tropics Management Authority, 2009).
Chave, J. et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145, 87–99 (2005).
Preece, N. D., Crowley, G. M., Lawes, M. J. & van Oosterzee, P. Comparing above-ground biomass among forest types in the Wet Tropics: small stems and plantation types matter in carbon accounting. Forest Ecology and Management 264, 228–237 (2012).
IPCC. IPCC guidelines for national greenhouse gas inventories, prepared by the national greenhouse gas inventories programme; institute for global environmental strategies. (Kanagawa, Japan, 2006).
Department of Climate Change and Energy Efficiency. Australian National Greenhouse Accounts National Inventory Report 2008, The Australian Government Submission to the UN Framework Convention on Climate Change May 2010. (Canberra, 2010).
FRAGSTATS v3: Spatial Pattern Analysis Program for Categorical Maps. v. v3 (University of Massachusetts, University of Massachusetts, Amherst, 2002).
Zuur, A. F., Ieno, E. N. & Elphick, C. S. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14 (2010).
Generalized Linear Mixed Models using AD Model Builder (GLMMADMB) v. R Package version 0.7.2.12 (2012).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: a Practical Information-Theoretic Approach. (Springer-Verlag New York, 2002).
MuMIn: multi-model inference v. R package version 1.9.13 (2013).
R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria, 2015).
Acknowledgements
This research was supported by an ARC Discovery Grant awarded to WL. AM was funded by an ETH fellowship and MC received funding from a Cowan Bursary and Australian Postgraduate Award.
Author information
Authors and Affiliations
Contributions
M.J.C. collected the data with some initial assistance from A.M., M.J.C. and A.M. analyzed the data with advice from W.E. and G.P., M.J.C. wrote the first draft of the chapter. The subsequent drafts were revised by M.J.C. with editorial input from W.E., A.M., S.G.L., M.A., G.P. and W.F.L. M.J.C. created the figures and tables.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Campbell, M.J., Edwards, W., Magrach, A. et al. Forest edge disturbance increases rattan abundance in tropical rain forest fragments. Sci Rep 7, 6071 (2017). https://doi.org/10.1038/s41598-017-06590-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06590-5
This article is cited by
-
Rattan composition and diversity assessment in tropical rainforests of Peninsular Malaysia for conservation
Biodiversity and Conservation (2021)
-
Reconciling Livelihoods and Conservation for Rattan Sustainable Harvesting in Lore Lindu National Park, Indonesia
Small-scale Forestry (2021)
-
High-risk infrastructure projects pose imminent threats to forests in Indonesian Borneo
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.