Abstract
Induced pluripotent stem cells (iPSCs) are similar to embryonic stem cells and can be generated from somatic cells. We have generated episomal plasmid-based and integration-free iPSCs (E-iPSCs) from human fetal foreskin fibroblast cells (HFF1). We used an E-iPSC-line to model hepatogenesis in vitro. The HLCs were characterized biochemically, i.e. glycogen storage, ICG uptake and release, UREA and bile acid production, as well as CYP3A4 activity. Ultra-structure analysis by electron microscopy revealed the presence of lipid and glycogen storage, tight junctions and bile canaliculi- all typical features of hepatocytes. Furthermore, the transcriptome of undifferentiated E-iPSC, DE, HE and HLCs were compared to that of fetal liver and primary human hepatocytes (PHH). K-means clustering identified 100 clusters which include developmental stage-specific groups of genes, e.g. OCT4 expression at the undifferentiated stage, SOX17 marking the DE stage, DLK and HNF6 the HE stage, HNF4α and Albumin is specific to HLCs, fetal liver and adult liver (PHH) stage. We use E-iPSCs for modeling gene regulatory networks associated with human hepatogenesis and gastrulation in general.
Similar content being viewed by others
Introduction
Human embryonic stem cells (hESCs) derived from inner cell mass cells of the blastocyst undergo symmetric self-renewal and are pluripotent i.e. can give rise to all cells within the three embryonic germ layers-endoderm, ectoderm, mesoderm and also germ cells1. Human Induced pluripotent stem cells (iPSCs) were initially derived from dermal fibroblasts by viral transduction mediated over-expression of four embryonic transcription factors OCT4, SOX2, KLF4 and c-MYC or OCT4, SOX2, NANOG and LIN282, 3. Human iPSCs share similar properties with hESCs, however, the integration of pro-viruses into the host genome of viral-derived iPSC is a risk factor for clinical applications in the future4, 5. To overcome these drawbacks, non-viral reprogramming methods have been described using non-integrating Sendai viruses, episomal-based plasmid vectors, in vitro-derived mRNA and miRNA5,6,7,8,9,10,11.
The liver is the largest internal organ and hepatocytes are the main functional cells in the liver. Hepatocytes perform a number of complex functions which are essential for life e.g. production of plasma proteins, synthesis of bile acids, the uptake and storage of glucose as well as drug detoxification. The use of primary human hepatocytes (PHH) is problematic, first, they cannot be expanded in vitro and second, they are difficult to obtain routinely or in sufficient quantities12, 13. Alternatives such as human hepatocarcinoma-derived and transformed, permanent cell lines, including HepG2, THLE and HepaRG, have phenotypes significantly diverged from normal primary hepatocytes14,15,16. A potential alternative could be the differentiation of iPSCs to hepatocyte-like cells. Hepatocyte-like cells (HLCs) generated from human iPSC have shown great promise as an inexhaustible source of cells that mirror the genotype of the donor to satisfy this need. Several groups have already shown how multifunctional applicable HLCs generated from iPSC can serve as cellular models for drug screening and toxicology studies, as a source for disease modeling17,18,19.
To date most studies use viral-derived iPSC to generate HLC, however these have drawbacks (e.g. genome integration). Additionally, most studies focus on one aspect of the multifunctional application of HLCs-derived from iPSCs such as the generation of HLCs from iPSCs, the maturation, modeling liver diseases, drug screening and toxicology17,18,19,20,21,22,23,24.
In this study we used an integration-free, episomal-derived induced pluripotent stem cell line (E-iPSC) from human neonatal foreskin fibroblast (HFF1)25, 26 to derive and characterize hepatocyte-like cells (HLCs) as well as untangle human hepatogenesis-associated gene regulatory networks.
Results
Differentiation of E-iPSCs to hepatocyte-like cells (HLCs)
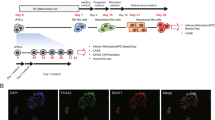
We derived an integration-free, episomal-based induced pluripotent stem cell line (E-iPSC) from human neonatal foreskin fibroblast (HFF1)25 to derive HLCs for this study. The derivation of HLCs was based on a slight modification of the protocol described by Sullivan et al.18. The differentiation to HLCs consists of three steps (Fig. 1A). First, the cells were differentiated towards definitive endoderm (DE) resulting in down-regulated expression of the pluripotent markers OCT4, SOX2 and NANOG and activation of DE specific markers such as SOX17. The second step in the protocol resulted in the emergence of hepatic endoderm cells (HE) as defined by the expression of hepatoblasts markers such as AFP, PROM1 and LGR5 (Fig. 1B,C). Finally, the HE cells were forced into maturation resulting in HLCs expressing mature liver markers such as ALB, A1AT, FOXA2, HNF4α TBX3, FAH and TDO2 but still maintaining AFP expression (Fig. 1B,C)20, 27,28,29,30,31. Quantitative real-time PCR confirmed the results of the immunofluorescence-based detection of protein expression (Fig. 1C).
Derivation of hepatocyte-like cells (HLC) from E-iPSCs. (A) First row phase contrast images of the differentiation stages, from undifferentiated stage the episomal induced pluripotent stem cells (E-iPSCs) to definitive endoderm (DE), then hepatic endoderm (HE) and finally hepatocyte-like cells (HLCs). Second row immunofluorescence-based staining of stage specific proteins overlapped with DAPI (staining of nucleus). Scale bar: 100 μm Alexa Flour 594 (red). (B) Immunofluorescence-based staining of HLC specific proteins AFP, ALB, HNF4α and A1AT. Scale bar: 100 μm Alexa Flour 594 (red). (C) Expression patterns of liver specific marker genes during HLC differentiation compared to fetal liver and primary human hepatocytes (PHH) are shown by quantitative real-time PCR (qPCR). Three biological replicates in technical triplicates of each sample were analyzed. The data were normalized to E-iPSCs. The standard deviation is depicted by the error bars.
Functional analyses of E-iPSC derived HLCs
The HLCs bear hallmarks of primary hepatocytes, i.e. (i) cobblestone-shaped epithelial cells expressing E-Cadherin (E-CAD), (ii) the ability to store glycogen as confirmed by Periodic-Acid-Schiff (PAS) staining (Fig. 2A). (iii) Uptake and release of ICG (data not shown) and CDFDA were measured (Fig. 2B). (iv) BSEP was detactable in HLCs by immunofluorescence-based protein staining (Fig. 2C). (v) Urea secretion was measured in all three stages of the differentiation protocol, as anticipated the highest level of production was in HLCs (Fig. 2D). (vi) Bile acids (CA, GCA, GCDCA) were also produced by HLCs (Fig. 2E and Supplementary Figure S1D). (vii) CYP3A4 activity was measured (Fig. 2F). Quantitative real-time PCR and heatmap-based analysis confirmed the expression of CYP3A4 as well as other members of the cytochrome P450 super family of enzymes (Fig. 2G and Supplementary Figure S1A). (viii) Electron microscopy revealed the ultra-structure typical of hepatocytes such as bile canaliculi with microvilli, lipid storage and tight junctions (Fig. 3A). (ix) Bi-nucleated cells could be shown by bright field microscopy (Fig. 3B). Finally, the efficiency of HLC differentiation was scored by HNF4α expression, as well as a double staining of ALBUMIN and HNF4α (Fig. 3C,D).
Functional analysis of hepatocyte-like cells (HLCs) derived from E-iPSCs. (A) E-Cadherin (E-CAD) antibody staining marking cell shape (left panel), Glycogen storage (right panel), Periodic Acid-Schiff (PAS) assay was used. Glycogen storage is indicated by pink or dark red-purple cytoplasm. (B) Visualization of 5 (and 6)-Carboxy-2′,7′-dichlorofluorescein diacetate (CDFDA), immunofluorescence image of HLCs direct after incubation with CDFDA (left panel), and immunofluorescence image of HLCs six hours later (right panel). (C) Immunofluorescence-based protein staining of bile salt export pump (BSEP). (D) Analysis of UREA production in E-iPSC-DE (DE), E-iPSC-HE (HE) and E-iPSC-HLCs (HLC). Three biological replicates in technical triplicates of each sample were analyzed. The levels of urea are presented as a percentage, considering measured levels of urea in mg/dL/24 h. The error bars indicate the standard errors of the mean. (E) Measurement of bile acid secretion of E-iPSC-HLCs (HLC). (F) Measurement of CYP3A4 secretion of HLC samples. Three biological replicates in technical triplicates were analyzed. The levels of CYP3A4 are presented as relative light units per milliliter (R.L.U./ml). The error bars indicate the standard errors of the mean. (G) Quantitative real-time PCR (qPCR) analysis of cytochrome P450 family member activity of all stages E-iPSC-DE (DE), E-iPSC-HE (HE) and E-iPSC-HLC (HLC) are shown. Three biological replicates in technical triplicates of each sample were analyzed. The data were normalized to E-iPSCs. The standard deviation is depicted by the error bars.
Ultra structure and maturation. (A) Electron microscopy image of E-iPSC-HLCs. BC = bile canaliculi; L = lipid; CCP = clathrin coated pits; M = mitochondrion; RER = rough endoplasmic reticulum; N = nucleus; arrow = tight junctions; arrowhead = microvilli. (B) Bright field microscopy of HLCs. Bi-nuleated cells are marked by arrow-heads. (C) Immunofluorescence-based staining of HNF4α in end-stage HLCs. 79.4% (+/−3.7%) of the cells counted positive for HNF4α. Scale bar: 200 μm Alexa Flour 594 (red). (D) Immunofluorescence-based double staining of Albumin (ALB) and HNF4α in end-stage HLCs. 91.7% (+/− 3.9) of HNF4α positive cells were also positive for ALB. Scale bar: 100 μm, Alexa Flour 594 (red) and Alexa Flour 488 (green).
Hepatogenesis associated transcriptional road map
A cluster dendrogram and accompanying correlation co-efficients demonstrates high similarities between replicates. Furthermore, fetal liver and PHH formed a cluster and iPSCs, HE and DE formed a cluster which is then extended by HLCs (Fig. 4A). K-means clustering identified 100 clusters which include developmental stage-specific groups of genes, e.g. OCT4 expression at the undifferentiated stage, SOX17 marking DE stage, HNF6 at the HE stage, PROX1 at the HLC stage, AFP marking the fetal liver stage and ALB marking the mature liver (PHH) stage (Fig. 4B and Supplementary Figure S1B). We further identified upstream regulators of genes within the six selected clusters shown in Fig. 4B by transcription factor over-representation analysis via the oPOSSUM data base32. These gene regulatory networks associated with these clusters are presented in Supplementary Figure 3. The network for iPSCs (Supplementary Figure S4A) shows the well known regulatory relations between OCT4 (POU5F1), SOX2, NANOG, KLF4. Most significant factors from the oPOSSUM analysis were STAT1, MZF1 and KLF4 (Z-Score > 10). In the network for DE (Supplementary Figure S4B) SP1, INSM1, MZF1, KLF4, REST are most significant (Z-Score >= 10), in HE (Supplementary Figure S4C) LHX3, MIZF, CTCF, in HLC (Supplementary Figure S4D), PLAG1, EWSR1-FLI1, IRF2, in fetal liver (Supplementary Figure S4E) TAL1::GATA1, HNF1A, ZFN143, GATA1, HNF1B and in primary human hepatocytes (Supplementary Figure S4F) HNF1A, CTCF, ZFX, HNF4A, FOXA2, FOXA1, CEBPA.
Transcriptional dynamic of hepatocyte-like cells (HLCs) derived from E-iPSCs. (A) A cluster dendrogram similarities between replicates and relationship of the samples E-iPSC (iPSC_B1), DE, HE, HLC, fetal liver and PHH. (B) K-means cluster 68 contained OCT4 marking undifferentiated stage (iPSC), cluster 81 (sub-cluster 1) contained SOX17 marking DE stage (DE), cluster 37 (sub-cluster 2) represents HE stage (HE), cluster 51 represents HLC stage (HLC), cluster 72 contained AFP marking fetal liver stage (fetal liver) and cluster 91 contained liver marker ALB (PHH). (C) Confirmation of microarray data by quantitative real-time (qPCR). On the left hand the array expression data and on the right hand the qPCR expression data of the following genes are shown: KRT17 and CXCR4 marking the definitive endoderm stage (DE), ANXA1, TTR and TBX3 represents the hepatocyte-like cell stage (HLC), AFP marking the fetal liver stage and ALB represents the mature liver (PHH) stage. Three biological replicates in technical triplicates of each sample were analyzed. The data were normalized to E-iPSCs. The standard deviation is depicted by the error bars. (D) Heatmap of the top 30 genes from each K-means clusters in Fig. 4B. (E) KeyGenes prediction for k-means Hepatocyte-like-cell (HLC) cluster9. Data sets for human liver, brain, intestine, kidney, lung and heart were downloaded from NCBI GEO and the KeyGenes tool was employed to generate a training set for these Illumina microarray platform data. As the test set genes from the HLC cluster 9 and HLC, fetal liver and primary human hepatocyte samples were used.
The microarray data and quantitative real-time PCR confirmed the expression profile of the listed genes. KRT17 and CXCR4 marking the definitive endoderm stage (DE), ANXA1, TTR and TBX3 represents the hepatocyte-like cell stage (HLC), AFP marking the fetal liver stage and ALB represents the mature liver (PHH) stage (Fig. 4C). Figure 4D shows a heatmap of the top 30 most abundantly expressed genes in each differentiation stage (k-means cluster). In order to assign a tissue-type to the HLCs we applied a tissue prediction tool - KeyGenes33 (Fig. 4E and Supplementary Table S1), which confirmed HLC (cluster 9) as liver, comparable to fetal liver and PHH. Furthermore, venn diagram analysis shows the numbers of significantly differentially expressed genes between HLCs, fetal liver and PHHs (Fig. 5A). The HLC-related genes ANXA1, TTR and TBX3 as well as the fetal liver-related gene AFP and ALB representing the matured liver stage PHH are located in the intersection of all three samples and are included in the 11506 genes (Fig. 5A and Supplementary Table S2). A closer look into the exclusive expressed genes in HLCs (1808 genes) uncovered tight junction-specific genes such as CLDN9, CLDN18, OCLN, PARD6A and PARD6B (Fig. 5B and Supplementary Table S3). One step further, a venn diagram was generated from HLC vs. fetal liver, HLC vs. PHH and fetal liver vs. PHH (Fig. 5C and Supplementary Table S4). DAVID analysis of Hippo signaling related genes from the intersection of HLC vs. PHH and HLC vs. fetal liver dedicated the activity of cell-cell contact related pathways, adherent and tight junction pathway. A chart of these Hippo pathway related genes underlines the predominant expression of cell-cell contact related genes in HLCs (Fig. 5D and Supplementary Table S5). The Hippo pathway, which is responsible for maturation and stabilization of the tight junctions in hepatocytes, and ABC transporters, which are accountable for the uptake and efflux of e.g. bile acids and metabolites, are over represented in HLC, fetal liver and PHH. Bile acid related transporter genes such as NTCP, MRP2, ASBT and MDR2/3 are highly expressed in HLC compared to the DE and HE stage (Fig. 5D and Supplementary Figure S1C–E).
Comparative transcriptome profile analyses. (A) Venn diagram of HLCs, fetal liver and PHH. (B) GO cellular components of HLC exclusively expressed genes (Fisher extract p < 0.01). (C) Venn diagram of fetal liver vs. PHH anova, HLC vs. fetal liver anova and HLC vs. PHH anova. (D) GO cellular components of HIPPO pathway genes exclusively expressed genes (Fisher extract p < 0.01) in the intersection of HLC vs. fetal liver anova and HLC vs. PHH anova (intersection with 1958 genes).
A venn diagram of genes which were expressed in DE, HE and HLC shows the relation between the stages of the HLC differentiation (Fig. 6A and Supplementary Table S6). DAVID analysis based on these genes uncover genes which are related to pathways that define the functionality of the liver such as drug metabolism, metabolism of xenobiotics and fatty acid metabolism (Supplementary Figure S2).
Cell fate decision. (A) Venn diagram of DE, HE and HLC. (B) Heatmap of bipotential progenitor-associated, hepatocyte-related and cholangiocyte-associated genes. (C) An induced network of transcription factors with a Z-score >10 (green, size of circle corresponds to Z-score) and associated genes (red).
Cell fate determination: hepatocytes or cholangiocytes?
To date most studies on liver cell fate decision making have been conducted in mice34, 35. This type of analyses can now be conducted in human using iPSCs. To demonstrate this we generated a heatmap consisting of key genes co- or differentially- expressed in undifferentiated iPSC, DE, HE and HLCs (Fig. 6B and Supplementary Figure S3 and Supplementary Table S7). Progenitor-related genes such as HNF1A and HNF1B are expressed in HE and HLCs whereas PROX1 is expressed exclusively in HLCs. Hepatocyte-specific genes such as ALB, AFP, ABCB4 and CYP3A7 are expressed in the HLC samples. Additionally, there exist a group of hepatocyte-related genes which are expressed in HLCs and HE, e.g. ABCC2, RARB and TRR. WNT3A as a marker for cholangiocytes as well as SOX9 and KRT7 are expressed in both HE and HLCs, whereas AQP1 and DLK1 are expressed exclusively in HLCs (Fig. 6A,B). To analyze the relation between these genes for cell fate decision a transcription factor network was created by the use of the data base oPOSSUM32 (Supplementary Figure S4). To make it more manageable transcription factors with a Z-score >10 (green circles) and downstream regulating transcription of the progenitor genes (red circles) are shown (Fig. 6C and Supplementary Figure S5). Myc, HNF1A, SP1, MZF1 5–13, HNF4A, Klf4 were the most significant upstream transcription factors (Z-score > 10, p < 1e-15). This demonstrates the dedicated functionalities of this core regulatory network: HNF1A and HNF4A determine the liver fate by regulating ALB and AFP while KLF4 regulates SOX17. Bipotential markers DLK1 and NOTCH3 are both regulated by MYC, Klf4, SP1 and MZF1 5–13. All cholangiocyte marker genes are regulated by KLF4, SP1 and MZF1. SOX9 is only regulated by these three, while ONECUT1, ONECUT2 and SALL4 are regulated by these three and additionally by MYC and HNF4A. Furthermore, MYC was the most prominent transcription factor in this network (Z-score of 12.54) and regulates PROX1 and the transcription factor HNF1A, which is also regulated by HNF4A, as well as the cholangiocyte-related gene WNT3A (Fig. 6C, Supplementary Table S8)28, 36, 37.
Discussion
In this study we used an episomal-derived and integration-free iPSC line to model hepatogenesis in vitro. Yu et al.6, 26 showed that iPSCs generated using episomal-based plasmids are free of vector and transgene sequences as we have also shown25. A whole-genome sequencing of iPSCs, which were generated by episomal vectors based on the EBNA1/OriP episomal replicon, showed (i) lack of integration of the episomal vector DNA in the host genome, (ii) loss of the episomal vectors in the iPSCs and (iii) no visible changes in the genomes of the iPSCs26, 38, 39. The episomal approach is a reliable method for iPSC derivation40.
Hepatocytes are the main cell type supporting the detoxification function of the liver and as such they are already extensively used for toxicology screens. Our episomal-derived, viral- and integration-free iPSC line is able to differentiate into hepatocyte-like cells (HLCs) which have similar functional properties as liver-biopsy derived primary human hepatocyes (Figs 1, 2, 3 and 4). These cells can be used for (i) toxicology and drug screening, (ii) future application in tissue replacement therapies, (iii) modeling human diseases in vitro.
An important pathway for hepatogenesis is the Hippo-signaling pathway which influences liver cell fate and size41, 42. This signal transduction pathway is crucial for early embryonic development, embryonic and adult stem cells, cell proliferation, differentiation, apoptosis, organ size, specific functions in adult organs and tumorgenesis41, 43,44,45,46. The Hippo pathway is responsible for maturation and stabilization of the tight junctions in hepatocytes47 and is over represented in HLCs, fetal liver and PHH. The existence of tight junctions in E-iPSC-derived HLCs is shown by electron microscopy (Figs 3A and 5D). ABC transporters, which are accountable for the uptake and efflux of e.g. bile acids, are also over represented in HLCs, fetal liver and PHH (Fig. 4D). Bile acid measurement shows the excretion of primary bile acids in HLCs. Primary bile acids are involved in drug metabolism and synthesis of cholesterol, steroids and other lipids48. Hepatocytes synthesize primary bile acids which carried out by the gut microbiota convert to secondary bile acids by several reactions including dehydroxylation, dehydrogenation and epimerization49. A heatmap of bile acid related transporter genes underlines the functionality of the HLCs and shows the highest expression of NTCP, MRP2, ASBT and MDR2/3 in the HLC samples (Fig. 4D).
A cluster dendrogram shows the highest similarities between replicates. Furthermore, fetal liver and PHH formed a cluster and iPSCs, HE and DE formed a cluster which is then extended by HLCs (Fig. 4A). Differences and commonalities between HLCs, fetal liver and PHH are assessed by statistical tests vs. iPSCs and pairwise statistical tests of the three experiments vs. each other. Most genes (4953) are in the intersection set common to all three experiments. Pairwise intersections fetal liver/PHH have 2823 genes, HLCs/PHH 1699 genes and HLCs/fetal liver 988 genes (Supplementary Table S9).
To assess the differences between HLCs, fetal liver and PHH an ANOVA followed by pairwise t-tests was performed. Venn diagram shows the numbers of significantly differentially expressed genes in these pairwise t-tests after filtering for the most variable genes found in the ANOVA (Fig. 5A).
Transcription factor binding site analysis revealed transcription factors are over-represented in these clusters. Top transcription factors by z-score were STAT1, MZF1, KLF4, SP1 and IRF1 for iPSCs, ELF5, FEV, INSM1, FOXI1 and STAT1 for DE, LHX3, MIZF, CTCF, NR3C1 and PAX6 for HE, PLAG1, EWSR1-FLI1, IRF2, MEF2A and ELF5 for HLCs, TAL1:GATA1, HNF1A, ZNF143, GATA1 and HNF1B for fetal liver, HNF1A, CTCF, HNF4A and FOXA2 for PHH (data not shown).
The cluster of iPSCs, definite endoderm (DE) and hepatic endoderm (HE) is successively extended by hepatocyte-like cells (HLCs), fetal liver and primary human hepatocytes (PHH). In most cases fetal liver and PHH are located in one cluster as well as iPSCs, DE and HE are formed another cluster. HLCs mostly are located in between the fetal liver/PHH and the early differentiation stage cluster (Fig. 4A).
Liver specific analysis of genes which are exclusively expressed in HLCs by venn diagram, PaGenBase and DAVID uncover the functionality of the HLC derived from E-iPSCs by showing the activity of cell-cell contact related pathways as well as liver-specific metabolism pathways such as drug metabolism, metabolism of xenobiotics and fatty acid metabolism (Figs 5 and 6 and Supplementary Figure S2).
We demonstrated the feasibility of using iPSCs as in vitro models for studying liver cell fate decision making. The heatmap presented in Fig. 6B shows differential expression of key cell fate regulating genes specific to the hepatic endoderm and in some cases also in the HLCs. This implies that the HLCs also harbor cell populations with bipotential properties similar to hepatoblasts. For example, the hepatic endoderm cells express DLK2 and also a set of transcription factors specific to this stage (Onecut1/HNF-6) which are not expressed in HLCs. These set of transcription factors are putative candidates for directing biliary epithelial cells/cholangiocytes cell fate. However, HLCs express DLK1 and progenitor-related genes such as PROX1 and LGR5 which are not expressed in HEs. Genes such as WNT3A, NOTCH3, HNF1A, HNF1B and SOX9 are expressed in both stages (Fig. 6B). Our overall findings based on gene expression patterns supports the notion that hepatic endoderm (HE) and HLCs in our hepatocyte differentiation protocol are equivalent to the DLK, HNF6 and SOX9-positive bipotential hepatoblasts present in fetal liver and are common progenitors for hepatocytes and biliary epithelial cells/cholangiocytes (Fig. 6B)34, 35.
The Notch signaling, hepatocyte nuclear factor-6 (HNF-6) and PROX1 are amongst factors known to regulate lineage commitment in the bipotential hepatoblast progenitor cell population35, 50, 51. It should be possible to change the fate of HE cells by manipulating the expression levels of e.g. PROX1, SOX9, and HNF6 or even by using small molecules targeting for instance Notch signaling.
Odom et al.52 described a core transcriptional regulatory circle in human hepatocytes consisting of six transcription factors (ONECUT1/HNF-6, FOXA2, HNF1A, HNF4A, CREB1 and USF1). These transcription factors bound promoters that are central for liver development and function. For this they used a mixture of human hepatocytes from multiple healthy donors to maximize the diversity of gender and age52. Our study shows the expression of the transcription factors ONECUT1/HNF-6, FOXA2, HNF1A, HNF4A in human HLCs derived from human E-iPSCs. Furthermore, we have demonstrated that amongst other transcription factors, HNF1A and HNF4A are involved in orchestrating cell fate decision of bipotential hepatoblast cells to become either hepatocytes or biliary epithelial cells/cholangiocytes (Fig. 6C).
The generation of a transcription factor network via the oPOSSUM data base32 uncovered transcription factors which are involved in cell fate decisions during hepatogenesis. The most prominent transcription factor in our network is MYC -transcription factors which regulates numerous biological processes such as glycolysis, cell proliferation and differentiation53, 54. MYC also regulates the expression of bipotential hepatoblast-related genes (e.g. DLK1, PROX1), cholangicyte-related genes (e.g. ONECUT1, WNT3A and SALL4) as well as hepatocyte-related genes (e.g. HNF1A). The second prominent transcription factor is HNF4A- a central regulator of hepatocyte differentiation and function55 and regulates hepatocyte-related genes (e.g. ALB, AFP), cholangiocyte-related genes (e.g. ONECUT1, SALL4) as well as the hepatoblast-related gene PROX1 (Fig. 6C, Supplementary Table S8)28, 36, 37. his transcription network underlines the bipotential progenitor-related characteristics of the HE and HLC stage in our differentiation procedure. This implies that there are bipotential progenitors within HE and HLC cell populations.
K-means clustering identified developmental stage-specific groups of genes, e.g. OCT4 expression at the undifferentiated stage, SOX17 marking the DE stage, DLK and HNF6 the HE stage, HNF4A and Albumin is specific to HLCs, fetal liver and adult liver (PHH) stage gain an insight into hepatogenesis. Furthermore, gene regulatory networks generated by oPOSSUM data base uncovered the presence of bipotential progenitor populations in both stages in HE and HLC. This analysis should lay the foundation for future efforts to generate long-term cultures of cholangiocytes and HLCs.
The bipotential progenitor population in iPSC-derived HLCs and the presence of AFP expression underline their fetal status. However, we uncovered human hepatogenesis-associated gene regulatory networks which are involved in cell fate decision making during hepatogenesis.
Conclusion
In summary, we have demonstrated the derivation of integration-free E-iPSCs from somatic cells and differentiated these to hepatocyte-like cells (HLCs) capable of storing glycogen, ICG uptake and release, UREA and bile acid production, as well as CYP3A4 activity. Ultra-structure analysis by electron microscopy revealed the presence of lipid and glycogen storage, tight junctions and bile canaliculi- all typical features of biopsy-derived primary hepatocytes. Model organisms such as zebrafish or mouse are used in order to analyze developmental processes of hepatogenesis34, 35, 56. HLCs derived from human E-iPSCs can be used to analyze the human hepatogenesis in details. We uncovered a gene regulatory network which uncovered the presence of bipotential progenitor populations in HE and HLC stage. Additionally, MYC was identified as a prominent regulator of bipotential hepatoblast-related genes expression.
Methods
Cell Culture and Differentiation
Human neonatal foreskin fibroblasts HFF1 were purchased from ATCC (HFF1 #SCRC-1041) and were maintained in Dulbecco’s modified Eagle medium (DMEMTM, Gibco) containing 10% fetal bovine serum (FBSTM, Invitrogen) and 0.5% penicillin and streptomycin (Invitrogen). Human ES and iPS cells were maintained on irradiated mouse embryonic fibroblast (MEF) cells in KnockOutTM DMEM (Invitrogen) supplemented with 20% KnockOutTM Serum Replacement (Invitrogen), 0.1 mM non-essential amino acids (Invitrogen), 0.1 mM L-glutamine (Invitrogen), 0.1 mM ß-Mercaptoethanol (Sigma), 0.5% penicillin and streptomycin and 8ng/ml basic fibroblast growth factor (bFGF, Invitrogen) as described by Wolfrum et al.57. The human ESC lines H1 and H9 were purchased from WiCell Research Institute (Madison, WI, USA, www.wicell.org, #WA01 and #WA09).
All used cells and cell lines were cultured at 37 °C and 5% CO2 in an incubator (INNOVA CO-170 Incubator, New Brunswick Scientific) under humidified atmosphere. All treatments and maintenance procedures were carried out using a clean bench type HeraSafe (Haereus Instruments).
For differentiation iPSC into HLC the protocol from Sullivan et al.18 was used. We modified the last step of HLC generation. During the last step of differentiation we used 25ng/ml dexamethasone instead of 10 μM hydrocortisone 21-hemisuccinate.
Functional Assays for HLC
PAS staining
Glycogen storage was identified by Periodic Acid-Schiff (PAS) Staining System (Sigma-Aldrich). Cells were fixed with 4% paraformaldehyde for 15 min and stained according to the manufacturer’s instructions.
Uptake and release
To detect the uptake and release of substances ICG (indocyanine green; Cardiogreen, ICG; Sigma) and CDFDA (5 (and 6)-Carboxy-2′,7′-dichlorofluorescein diacetate, CDFDA; Sigma) were used. The cells were incubated in culture medium with freshly diluted ICG (1 mg/ml) or CDFDA (1 μM) for 30 min. at 37 °C. The cells were washed with PBS, fresh culture medium was added and uptake of dye was documented. The release of ICG and CDFDA was examined after 6 h. The results of ICG and PAS assays were examined under an Olympus CK2 phase-contrast microscope and representative morphology was recorded at a magnification of ×50 using a Canon 300D digital camera. The fluorophore of CDFDA was visualized using a Zeiss, LSM 510 Meta confocal microscope with a connected camera for microscopy model AxioCam ICc3 and the software Axiovision 4.6 at a magnification of ×200.
Urea measurement
Urea secretion was quantified by a colorimetric assay QuantiChromTM Urea Assay Kit (DIUR-500 BioAssay Systems) following the manufacturer’s instructions. The assay detects urea directly by using substrates that specifically bind urea. Urea assays were carried out in 96-well plates, and concentrations were measured using a plate reader. Analysis of urea production in E-iPSC-DE (DE), E-iPSC-HE (HE) and E-iPSC-HLC (HLC) were performed. Three biological replicates in technical triplicates of each sample were analyzed. The levels of urea are presented as a percentage, considering measured levels of urea in mg/dL/24 h.
Bile acid measurement
Bile acids (cholic acid CA, chenodeoxycholic acid CDCA, deoxycholic acid DCA, ursodeoxycholic acid UDCA, lithocholic acid LCA) including their glycine- and taurine derivatives were analyzed by UPLC-MS/MS. The system consisted of an Acquity UPLC-H Class (Waters, UK) coupled to a Xevo-TQS tandem mass spectrometer (Waters, UK) which is equipped with an ESI source operating in the negative ion mode. Quantitative data were conducted in the multiple reaction monitoring (MRM) mode. The chromatographic separation was performed on Waters UPLC BEH C18 column (100 mm, 2.1 mm ID, 1.7 µm; Waters, UK) using acetonitrile and acidic water (0.1% formic acid) as mobile phases. Analytes were separated by a gradient elution. The injection volume was 5 µL and the column was maintained at 40 °C.
CYP3A4 measurement
CYP3A4 activity was measured by using the pGlo kit (Promega) according to manufacturer’s instruction for nonlytic CYP450 activity estimation. The CYP3A4 production was measured in E-iPSC-HLC (HLC). Three biological replicates in technical triplicates of each sample were analyzed. The levels of CYP3A4 are presented as relative light units per milliliter (R.L.U./ml). The error bars indicate the standard errors of the mean.
Electron microscopy
Cells grown on Thermanox® plastic coverslips (Nunc), were fixed in a modified Karnofsky solution, 2%PFA/2,5%GA in 50 mM Cacodylate buffer, pH7.4 at 4 °C. Cells were washed in 50 mM Cacodylatpuffer/50 mM NaCl and post-fixed for 90 min at room temperature with 0,5% OsO4 in the same buffer. After washing steps with water, cells were incubated for 40 min with 0,1% tannic acid in 250 mM Hepes pH7,4, washed with water and stained with 2% uranyl acetate, 90 min at room temperature. Cells were dehydrated in a graded series of ethanol and embedded in Spurr’s resin (Low Viscosity Spurr Kit, Ted Pella, CA, USA). Ultra-thin sections (70 nm) were prepared with an ultramicrotome (Reichert Ultracut E, Leica) and mounted on pioloform-coated slot grids from copper. Sections were counterstained with uranyl acetate and lead citrate.
Ultrathin-sections were first examined using a Philips CM100 transmission electron microscope operated at 100 kV and finally imaged using a FEI Tecnai Spirit transmission electron microscope operated at 120 kV, which was equipped with a 2 k × 2 k Eagle CCD camera (FEI). The MSI-Raster application within the Leginon Software package58 was used to automatically image selected regions of interest at a final nominal magnification of 15000× applying a defocus of −4 µm. Raw micrographs were stitched using the Trakem2 plugin implemented in the Fiji software platform59, 60.
Microarray -Based Gene Expression Analysis
Total RNA from iPSC, DE, HE, HLC, PHH and fetal liver in replicates were extracted using the MiniRNeasy Kit (Qiagen) according to the manufacturer’s instructions and quality checked by Nanodrop analysis (Nanodrop Technologies, Wilmington, DE, USA, http://www.nanodrop.com). Approximately 500 ng of DNase treated RNA was sent to ATLAS Biolabs (http://www.atlas-biolabs.de) for whole transcriptome analysis by using microarray. All basic expression data analysis was carried out using the BeadStudio software 3.0. Raw data were background-subtracted and normalized using the “rank invariant” algorithm and then filtered for significant expression on the basis of negative control beads. For correlation coefficient analysis and the generation of Venn diagrams, detected gene expression was defined by a Detection P Value < 0.01 as output by BeadStudio. For differential gene expression analyses, genes had to be at least 1.5 fold up- or down-regulated in a group-wise comparison, to be considered significantly differentially expressed.
For more detailed information see supplementary Materials and Methods.
Data access
GEO Submission (GSE66282).
References
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science. 282, 1145–1147 (1998).
Takahashi, K., Okita, K., Nakagawa, M. & Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2, 3081–3089 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 318, 1917–1920 (2007).
Chen, L. & Liu, L. Current progress and prospects of induced pluripotent stem cells. Sci China C Life Sci. 52, 622–636 (2009).
Drews, K., Jozefczuk, J., Prigione, A. & Adjaye, J. Human induced pluripotent stem cells–from mechanisms to clinical applications. J Mol Med (Berl). 90, 735–745 (2012).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 324, 797–801 (2009).
Zhou, H. et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 4, 381–384 (2009).
Kiskinis, E. & Eggan, K. Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest. 120, 51–59 (2010).
Plews, J. R. et al. Activation of pluripotency genes in human fibroblast cells by a novel mRNA based approach. PLoS One. 5, e14397 (2010).
Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 7, 618–630 (2010).
Miyoshi, N. et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 8, 633–638 (2011).
Elaut, G. et al. Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr Drug Metab. 7, 629–660 (2006).
Fraczek, J. et al. Primary hepatocyte cultures for pharmaco-toxicological studies: at the busy crossroad of various anti-dedifferentiation strategies. Arch Toxicol. 87, 577–610 (2013).
Hart, S. N. et al. A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug Metab Dispos. 38, 988–994 (2010).
Gerets, H. H. J. et al. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol. 28, 69–87 (2012).
Jetten, M. J. A. et al. Baseline and genotoxic compound induced gene expression profiles in HepG2 and HepaRG compared to primary human hepatocytes. Toxicol In Vitro. 27, 2031–2040 (2013).
Rashid, S. T. et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 120, 3127–3136 (2010).
Sullivan, G. J. et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 51, 329–335 (2010).
Yusa, K. et al. Targeted gene correction of 1-antitrypsin deficiency in induced pluripotent stem cells. Nature. 478, 391–394 (2011).
Jozefczuk, J., Prigione, A., Chavez, L. & Adjaye, J. Comparative analysis of human embryonic stem cell and induced pluripotent stem cell-derived hepatocyte-like cells reveals current drawbacks and possible strategies for improved differentiation. Stem Cells Dev. 20, 1259–1275 (2011).
Hannan, N. R. F., Segeritz, C. P., Touboul, T. & Vallier, L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc. 8, 430–437 (2013).
Takebe, T. et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 9, 396–409 (2014).
Greenhough, S., Medine, C. N. & Hay, D. C. Pluripotent stem cell derived hepatocyte like cells and their potential in toxicity screening. Toxicology. 278, 250–255 (2010).
Takayama, K. et al. Prediction of interindividual differences in hepatic functions and drug sensitivity by using human iPS-derived hepatocytes. Proc Natl Acad Sci USA 111, 16772–16777 (2014).
Matz, P. & Adjaye, J. Episomal-based generation of an iPS cell line from human fetal foreskin fibroblasts. Stem Cell Research. 16, 67–69 (2016).
Yu, J. et al. Efficient feeder-free episomal reprogramming with small molecules. PLoS One. 6, e17557 (2011).
Agarwal, S., Holton, K. L. & Lanza, R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 26, 1117–1127 (2008).
Hay, D. C. et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 26, 894–902 (2008).
Schmidt, S. K. et al. Antimicrobial and immunoregulatory properties of human tryptophan 2,3-dioxygenase. Eur J Immunol. 39, 2755–2764 (2009).
Lüdtke, T. H. W., Christoffels, V. M., Petry, M. & Kispert, A. Tbx3 promotes liver bud expansion during mouse development by suppression of cholangiocyte differentiation. Hepatology. 49, 969–978 (2009).
Teckman, J. H. & Jain, A. Advances in alpha-1-antitrypsin deficiency liver disease. Curr Gastroenterol Rep. 16, 367 (2014).
Kwon, A. T., Arenillas, D. J., Worsley Hunt, R. & Wasserman, W. W. oPOSSUM-3: advanced analysis of regulatory motif over-representation across genes or ChIP-Seq datasets. G3 (Bethesda). 2, 987–1002 (2012).
Roost, M. S. et al. KeyGenes, a Tool to Probe Tissue Differentiation Using a Human Fetal Transcriptional Atlas. Stem Cell Rep. 4, 1112–1124 (2015).
Tanimizu, N. et al. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 116, 1775–1786 (2003).
Vanderpool, C. et al. Genetic interactions between hepatocyte nuclear factor-6 and Notch signaling regulate mouse intrahepatic bile duct development in vivo. Hepatology. 55, 233–243 (2012).
Dianat, N. et al. Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology. 60, 700–714 (2014).
Seth, A. et al. Prox1 ablation in hepatic progenitors causes defective hepatocyte specification and increases biliary cell commitment. Development. 141, 538–547 (2014).
Mayshar, Y. et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 7, 521–531 (2010).
Cheng, X. et al. Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell. 10, 371–384 (2012).
Schlaeger, T. M. et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol. 33, 58–63 (2015).
Camargo, F. D. et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 17, 2054–2060 (2007).
Yimlamai, D. et al. Hippo pathway activity influences liver cell fate. Cell. 157, 1324–1338 (2014).
Pan, D. Hippo signaling in organ size control. Genes Dev. 21, 886–897 (2007).
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Tremblay, A. M. & Camargo, F. D. Hippo signaling in mammalian stem cells. Semin Cell Dev Biol. 23, 818–826 (2012).
Li, P. et al. Functional role of Mst1/Mst2 in embryonic stem cell differentiation. PLoS One. 8, e79867 (2013).
Varelas, X. et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell. 19, 831–844 (2010).
Lamba, V., Ghodke, Y., Guan, W. & Tracy, T. S. microRNA-34a is associated with expression of key hepatic transcription factors and cytochromes P450. Biochem Biophys Res Commun. Mar 445, 404–411 (2014).
Hofmann, A. F. & Hagey, L. R. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 55, 1553–1595 (2014).
Clotman, F. et al. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development. 129, 1819–1828 (2002).
Dudas, J. et al. The homeobox transcription factor Prox1 is highly conserved in embryonic hepatoblasts and in adult and transformed hepatocytes, but is absent from bile duct epithelium. Anat Embryol (Berl). 208, 359–366 (2004).
Odom, D. T. et al. Core transcriptional regulatory circuitry in human hepatocytes. Mol Syst Biol. 2, 2006–2017 (2006).
Cai, Q., Lin, T., Kamarajugadda, S. & Lu, J. Regulation of glycolysis and the Warburg effect by estrogen-related receptors. Oncogene. 32, 2079–2086 (2013).
Qu, A. et al. Role of Myc in hepatocellular proliferation and hepatocarcinogenesis. J Hepatol. 60, 331–338 (2014).
Watt, A. J., Garrison, W. D. & Duncan, S. A. HNF4: a central regulator of hepatocyte differentiation and function. Hepatology. 37, 1249–1253 (2003).
Gao, M. et al. Hepassocin is required for hepatic outgrowth during zebrafish hepatogenesis. Biochem Biophys Res Commun. 463, 466–471 (2015).
Wolfrum, K. et al. The LARGE principle of cellular reprogramming: lost, acquired and retained gene expression in foreskin and amniotic fluid-derived human iPS cells. PLoS One. 5, e13703 (2010).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J Struct Biol. 151, 41–60 (2005).
Saalfeld, S., Cardona, A., Hartenstein, V. & Tomančák, P. As-rigid-as-possible mosaicking and serial section registration of large ssTEM datasets. Bioinformatics. 26, i57–i63 (2010).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9, 676–682 (2012).
Acknowledgements
J.A. acknowledges support from the German Federal Ministry of Education and Research (BMBF) grants (01GN0807) and the medical faculty of Heinrich Heine University, Düsseldorf.
Author information
Authors and Affiliations
Contributions
Peggy Matz: Conception and design, Collecting and assembly of data, Data analysis and interpretation, Manuscript writing. Wasco Wruck: Bioinformatic analysis, interpretation and manuscript writing. Beatrix Fauler: Electron microscopy. Diran Herebian: Bile acid measurements. Thorsten Mielke: Electron microscopy. James Adjaye: Conception, interpretation, editing and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matz, P., Wruck, W., Fauler, B. et al. Footprint-free human fetal foreskin derived iPSCs: A tool for modeling hepatogenesis associated gene regulatory networks. Sci Rep 7, 6294 (2017). https://doi.org/10.1038/s41598-017-06546-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06546-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.