Abstract
Primary hepatocytes cultured in vitro are a powerful tool to study the functions of hepatocytes and to evaluate the metabolism and toxicity of new drugs. However, in vitro culture of hepatocytes has proven to be very difficult. Ordinary culture conditions lead to dedifferentiation of hepatocytes, resulting in rapid change in cell morphology and significant reduction in specific cell functions. In the current study, we show that hepatocyte dedifferentiation is a rapid process under 21% O2 conditions. Hepatocytes cultured in 21% O2 undergo epithelial-to-mesenchymal transition (EMT), obtain fibroblast-like morphology, and show decreased hepatic functions. In contrast, 5% O2 is very effective in maintaining the epithelial morphology and many functions of the primary hepatocytes cultured in vitro for up to five days. These functions include albumin production, glycogen storage, LDL-uptake and CYP450-mediated drug metabolism. Furthermore, we find that 5% O2 can relieve the production of reactive oxygen species (ROS) and decrease the level of DNA damage in primary cultured hepatocytes. In addition, we also show that blocking the ERK and GSK-3β pathways can inhibit the dedifferentiation of hepatocytes to a certain extent. Lowering the oxygen tension in cell culture is easily achievable, we believe it could be combined with other methods, such as the use of small molecule cocktails and 3D culture, to maintain proliferation and functions of primary hepatocytes in vitro.
Similar content being viewed by others
Introduction
The liver is one of the most important and complex organs in the body as it plays a central role in the metabolic homeostasis1. It is also the main detoxifying organ, which removes wastes and xenobiotics by metabolic conversion and biliary excretion2. The liver is comprised of a multitude of cell types. However, the hepatic parenchymal cell, also known as the hepatocyte, is the main cell type and executes most functions of the liver3. Primary cultured human or rodent hepatocytes are great tools to study the development and functions of the liver, such as the investigation of transcription factors involved in liver development4, 5, and the exploration of molecular mechanisms involved in the liver’s response to cytokines and growth factors during liver regeneration6, 7. Primary hepatocytes are also important tools for the study of drug metabolism, toxicity and transporter functions8, 9. When isolated and cultured correctly, they can reserve the metabolizing enzymes and transporter activity at a physiological level and be regulated via the cellular processes which occur in vivo 8.
However, many basic functions of hepatocytes, such as bile canaliculi formation, bile secretion, polarity and metabolic activities (including detoxification by Cyps and other drug-metabolizing enzymes), are rapidly reduced during in vitro culture10. The loss of hepatocytes’ polarity and functions is called hepatocyte dedifferentiation. A number of pathways, including transforming growth factor (TGF)-β, sonic hedgehog (Hh), Wnt, Notch, epithelial growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), hypoxia induced factor (HIF), and many others11, have been reported to play a role in hepatocyte dedifferentiation in in vitro culture. It has also been reported that hepatocytes dedifferentiation is a consequence of overactive Ras/MEK/ERK signaling12. To resolve the dedifferentiation problem and preserve the functions of primary hepatocytes in in vitro culture, scientists have optimized the culture conditions by modifying the media components, adding small molecule compounds13,14,15, culturing in bioreactors or 3D systems16, 17, or co-culturing the hepatocytes with non-parenchymal cells18. As previously reported, a multiwall co-culture system based on elastomeric stencils for human liver cells and mouse 3T3-J fibroblasts could be optimized to form proper microscale architecture and could maintain the phenotypic functions of hepatocytes for several weeks19.
The 3D culture of hepatocytes or co-culture of hepatocytes with other hepatic cell types intends to mimic the in vivo microenvironment of the liver. One important fact that should be considered is the oxygen tension in the in vivo environment. In the human liver, hepatocytes reside in a physiologically low oxygen environment; the partial pressure of oxygen (pO2) in liver is (5.4 ± 0.7)% (40.6 ± 5.4 mm Hg)20, which is much lower than the in vitro condition (~21%, 160 mm Hg). In cell culture, oxygen tension has a great influence on cell fate. Notably, low O2 tension promotes the survival of neural crest cells and hematopoietic stem cells, enhances the generation of induced mouse pluripotent stem cells, and prevents differentiation of human ESCs21, 22. Recently, low O2 tension has been reported to enhance the generation of lung progenitor cells, definitive endoderm cells, and distal lung cells from mouse pluripotent stem cells, and enhances the generation of retinal progenitor cells from human pluripotent stem cells23,24,25.
In this study, we sought to evaluate the function of physiological oxygen tension in in vitro culture of primary hepatocytes, and found that 5% O2 reduces the epithelial-mesenchymal transition (EMT) of the hepatocytes, and helps to preserve the functions of hepatocytes.
Result
Physiological oxygen level helps to maintain the properties of primary hepatocytes in culture
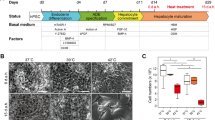
Mouse primary hepatocytes cultured in 21% O2 underwent rapid morphological changes. At day 1 in culture, these cells exhibited typical endothelial phenotype. However, at day 3 and 5, these hepatocytes spread out and started to lose cell-cell contacts and finally obtained the fibroblast morphology (Fig. 1A). In contrast, hepatocytes cultured in 5% O2 maintained the epithelial phenotype for 5 days (Fig. 1A). Staining of F-actin confirmed this observation. At day 1 in both culture conditions, actin fibers were mainly located on the inner side of the cell membrane and formed a belt like structure around the cells. However, when cultured in 21% O2, the actin cytoskeletons started to re-structure and form the stress fiber as typically seen in fibroblasts (Fig. 1B). In contrast, the actin structure remained unchanged in the cells cultured in 5% O2 (Fig. 1B).
Physiological oxygen level helps maintain the endothelial phenotype of primary hepatocytes. (A) Morphology of primary hepatocytes cultured in 21% or 5% O2. Scale bar: 50 μM. (B) Immunofluorescence staining of F-actin (red) in primary hepatocytes cultured in 21% or 5% O2. Nuclei were stained with Hoechst. Scale bar: 10 μm.
The ability to produce albumin is an important index of hepatocyte function. The intracellular staining of albumin decreased rapidly in hepatocytes cultured in 21% O2 (Fig. 2A,B), and the secreted albumin in hepatic culture was also significantly reduced (Fig. 2C). But in 5% O2, both the intracellular and secreted albumin remained unchanged (Fig. 2A–C). Glycogen storage and glucose output are also important functions of hepatocytes. Periodic acid-schiff (PAS) staining revealed that 21% O2 culture condition led to a rapid decrease of glycogen stores in primary hepatocytes (Fig. 2D). And gluconeogenesis from these cells was also greatly reduced (Fig. 2E). In contrast, hepatocytes cultured in 5% O2 remained PAS positive at day 5, and the glucose output ability was maintained (Fig. 2D,E). LDL-uptake ability was also evaluated in hepatocytes cultured in those two conditions. As expected, hepatocytes cultured in 5% O2 displayed a significantly higher ability to intake ac-LDL as compared to the cells cultured in 21% O2 (Fig. 2F,G).
Physiological oxygen level preserves primary hepatocyte functions. (A) Immunofluorescence staining of albumin (green) in hepatocytes cultured in 21% or 5% O2. Nuclei were stained with Hoechst. Scale bar: 10 μm. (B) Statistical data of the intensity of albumin staining in (A). Data are shown as fold change relative to the 21% O2 group in day 1. Data are Means ± SEM (six random fields) in a representative experiment. (C) ELISA analysis of the secreted albumin levels in culture media of primary hepatocytes cultured in 21% or 5% O2. Data are Means ± SEM (n = 3). (D) PAS staining of the glycogen storage of primary hepatocytes cultured in 21% or 5% O2. Scale bar: 50 μm. (E) Glucose production of primary hepatocytes cultured in 21% or 5% O2. (F) Representative immunofluorescence images of Dil-Ac-LDL uptake (red) in primary hepatocytes cultured in different conditions. Scale bar: 10 μm. (G) Statistical data of the mean intensity of LDL per cell as shown in (D). Data are shown as fold change relative to the 21% oxygen group in day 1. Data are Means ± SEM (seven random fields) in a representative experiment. *p < 0.05, **p < 0.01, ***p < 0.001.
Hepatocytes cultured in physiological oxygen level maintain the expression and function of CYP450
The liver is the most important organ for drug metabolism. Approximately 60% of the marketed drugs are cleared by hepatic CYP450-mediated metabolism. In vitro culture led to rapid reduction in mRNA levels of CYP1a2, 3a11 and 3a41 in hepatocytes in both the 21% O2 and 5% O2 conditions. However, the reduction was significantly faster in hepatocytes cultured in 21% O2. At day 3, the mRNAs of these enzymes were almost undetectable in cells cultured in 21% O2, but in cells cultured in 5% O2, the expression of these enzymes was still detectable at day 5 (Fig. 3A). Immunofluorescent staining also confirmed a significant decrease of Cyp1a2 protein in hepatocytes cultured in 21% O2 (Fig. 3B,C).
Physiological oxygen level maintains the expression and function of cytochrome P450 enzymes in primary hepatocytes. (A) Quantitative RT-PCR measurement of Cyp1a2, Cyp3a11 and Cyp3a41 in hepatocytes cultured in 21% or 5% O2. (B) Representative immunofluorescence images of Cyp1a2 in primary hepatocytes cultured in different conditions. Scale bar: 10 μm. (C) Statistical data of the mean intensity of Cyp1a2 per cell as shown in (B). Data are shown as fold change relative to the 21% oxygen group in day 1. Data are Means ± SEM (seven random fields) in a representative experiment. (D) Evaluation of the metabolic stability of phenacetin (200 nM) in primary hepatocytes cultured in 21% or 5% O2 for 3 days (n = 3). (E) The in vitro intrinsic clearance (CLint) of phenacetin was determined from the metabolic stability study presented in Fig. 3D. n = 3, *p < 0.05, **p < 0.01, ***p < 0.001.
Hepatocytes cultured in 21% O2 or 5% O2 for 3 days were then subjected to an in vitro drug metabolism assay. The in vitro clearance of phenacetin, a pain-relieving and fever-reducing drug and a substrate of CYP1a2, was tested. As demonstrated in Fig. 3D, hepatocytes cultured in 5% O2 displayed a higher clearance ability for phenacetin. After 480 min incubation, more than 90% phenacetin was cleared by hepatocytes cultured in 5% O2, but in the 21% O2 group, only about 60% of the phenacetin was cleared. The in vitro intrinsic clearance for phenacetin is significantly higher in hepatocytes cultured in 5% O2 than 21% O2 (13.33 vs 4.09 ul/min/million cells, respectively) (Fig. 3E).
Physiological oxygen level blocks EMT involved in dedifferentiation of primary hepatocytes
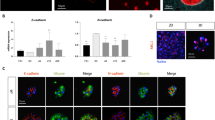
As we demonstrated above, mice primary hepatocytes cultured in 21% O2 quickly lost the typical endothelial phenotype and obtained the fibroblast morphology, while 5% O2 culture condition prevented such morphological changes (Fig. 1). The morphological changes involved in hepatocyte dedifferentiation were accompanied by the dramatic upregulation of mesenchymal markers, including Collage1α1, N-cadherin, FN, Vimentin, α-SMA, Snail, and Slug, further indicating the occurrence of epithelial-mesenchymal transition (EMT) during long-term culture of primary hepatocytes in 21% O2 condition. In contrast, 5% O2 significantly prevented the upregulation of all the mesenchymal-related genes (Fig. 4A). Immunofluorescent staining also confirmed the significant increase of α-SMA+ cells in hepatocytes cultured in 21% O2 for 3 and 5 days. However, in 5% O2 condition, very few α-SMA+ cells were detected (Fig. 4B and C). Western blot analysis also revealed the protein levels of N-cadherin and α-SMA were increased in 21% O2 but not in 5% O2 culture condition (Fig. 4D).
Physiological oxygen level suppresses EMT in the in vitro culture of primary hepatocytes. (A) Quantitative RT-PCR analysis of mesenchymal marker collage1α1, N-cadherin, FN, Vimentin, α-SMA, snail, and slug in hepatocytes cultured in 21% or 5% O2. Data are Means ± SEM (n = 3). (B) Immunofluorescence staining of α-SMA in hepatocytes cultured in 21% or 5% O2. Nuclei were stained with Hoechst. Scale bar: 20 μm. (C) Statistical data of the number of α-SMA+ clusters as shown in (B). Data are Means ± SEM (eight random fields) in a representative experiment. *p < 0.05, **p < 0.01, ***p < 0.001. (D) Western blot analysis of N-cadherin and α-SMA in hepatocytes cultured in 21% or 5% O2. GAPDH: loading control.
Low oxygen leads to less oxidative stress and DNA damage
Previous studies have shown that oxidative stress plays an important role in inducing cytotoxicity and genotoxicity in human hepatoma (HepG2)26 as well as promoting DNA damage and apoptosis in human hepatocyte cell line L0227. Furthermore, reactive oxygen species (ROS) are an important factor in inducing EMT in epithelial cells28. To better understand whether oxidative damage is involved in the different culture conditions, we measured the ROS generated by hepatocytes cultured in 21% or 5% O2. As demonstrated in Fig. 5A, the intracellular ROS continued to rise in cells cultured in 21% O2. In contrast, the ROS level in cells cultured in low oxygen remain unchanged. ROS generation has been shown to contribute to DNA damage29. To better evaluate the possible influence of ROS in the two culture conditions, we determined the DNA damage level by γH2A.X staining. Hepatocytes cultured in 21% O2 showed a significantly higher level of γH2A.X staining comparing to the low oxygen group (Fig. 5B and C). Western blot analysis revealed a similar result (Fig. 5D). These data demonstrate that hepatocytes cultured in low oxygen tension accumulate less ROS and less DNA damage.
Physiological oxygen level reduces the production of ROS and DNA damage in primary hepatocytes. (A) Measurement of the intracellular ROS levels of primary hepatocytes cultured in 21% or 5% O2 at day 1, day 3 and day 5. Data are Means ± SEM (n = 6). (B) Immunofluorescence staining of γH2A.X in hepatocytes cultured in 21% or 5% O2. Nuclei were stained with Hoechst. Scale bar: 100 μm. (C) Statistical data of the intensity of γH2A.X staining in (B). Data are Means ± SEM (seven random fields) in a representative experiment. (D) Western blot analysis of γH2A.X in hepatocytes culture in 21% or 5% O2. GAPDH: loading control.
ERK1/2 and GSK-3β pathways are involved in maintaining the epithelial phenotype of hepatocytes
Previous studies have shown that a vast majority of signaling pathways are involved in the regulation of EMT in various cell types. To better understand which signaling pathways may play important roles in the regulation of EMT in primary hepatocytes, we tested a number of pathway blockers, including TGF-α inhibitor Repsox and A83-01, GSK-3β inhibitor CHIR99021, notch inhibitor Compound E, ERK inhibitor PD0325901, TNF-α inhibitor Necrostatin-1, and HIF-1α inhibitor CAY10585, in primary hepatocytes cultured in 21% O2 condition. We found that the ERK inhibitor PD0325901 and the GSK-3β inhibitor CHIR99021 helped to maintain the epithelial phenotype of primary hepatocytes cultured in 21% O2 for 5 days. The combination of these two compounds provided even better results (Fig. 6). These experiments indicate that both the ERK1/2 and GSK-3β pathways are involved in the maintenance of the epithelial phenotype of hepatocytes. Thus, small molecule inhibitors targeting both pathways could be used to maintain the culture of primary hepatocytes.
Blocking ERK1/2 and GSK-3β pathways reduce EMT in primary culture of hepatocytes. (A) Morphology of primary hepatocytes cultured in 21% O2 in the presence of ERK1/2 inhibitor (PD0325901, 10 μM) and GSK-3β inhibitor (CHIR99021, 10 μM). (B) Immunofluorescence staining of F-actin (red) in primary hepatocytes cultured in 21% O2 in the presence of PD0325901 (10 μM) and CHIR99021 (10 μM). Nuclei were stained with Hoechst.
Discussion
The liver is the largest internal organ in the human body, and plays a central role in energy balance and drug metabolism. In the human body, the liver is the only organ which possesses the powerful ability to proliferate in response to partial excision, toxic injury, or infection2. The first liver regeneration experiment was described by Higgins and Anderson in 1931. Using a rat model, two thirds of the liver was surgically removed, and resulted in the remaining liver enlarging to original volume in approximately 1 week30. Unfortunately, this amazing regeneration ability is almost eliminated in in vitro conditions. The cultured primary hepatocytes can hardly proliferate31. Moreover, the current protocols for culturing of primary hepatocytes could only maintain metabolic functions for hours to a few days32,33,34. Recently, adding small molecules in culture enables 10 rounds of proliferation of human hepatocytes, but not serial passages or long-term storage of these cells35. Low-level expression of the human papilloma virus genes E6 and E7 could lead to up to 40 population doublings of hepatocytes cultured in viro, but cells with such genes modification may not be suitable for clinical-related uses31.
In the liver, hepatocytes reside in a microenvironment formed by extracellular matrix, blood vessels and various types of cells, making the conditions hard to mimic in culture. Hepatocytes in single layer culture rapidly lose their epithelial morphology accompanied by their specific functions such as protein synthesis (albumin, fibrinogen), bile acid production, glycogen storage, xenobiotic metabolism, etc36. These cells undergo a process named epithelial to mesenchymal transition (EMT)37 and start to accumulate actin stress fibers, display elongated “fibroblast-like” morphology, lose polarity, weaken cell-cell interaction and reduce typical hepatic functions.
In vitro culture for tissues and cells isolated from mammalian bodies started about a century ago. Many efforts have been put forward to maintain physiological temperature, pH, salt concentration, nutrient and necessary cell factors in the culture. Until now, in most routine cell cultures, the oxygen concentration in the medium was still simply the result of equilibration with the oxygen in air. However, in the body, many cells and tissues reside in environments with lower partial pressure of oxygen38. pO2 in arterial blood is 95 mm Hg, and in normal tissues it ranges from 50 to 5 mm Hg (7–0.7%), which are considerably lower than pO2 in air (150 mm Hg, 21%).
The effect of low oxygen tension in in vitro culture has been mostly studied in stem cell research. Low pO2 has been reported to be beneficial in maintaining the pluripotency of human embryonic stem cells (hESCs)39 and the generation of murine and human induced pluripotent stem cells (iPSCs)40, 41. EMT plays important roles in pluripotent stem cell differentiation. Low oxygen is believed to block the EMT process and therefore helps to maintain the pluripotency of ESCs39. Recently, a study showed that low oxygen might have direct effects on heart regeneration in adult mice in vivo. Adult mice maintained in 7% oxygen had less reactive oxygen species and oxidative DNA damage, and could reactivate cardiomyocyte mitosis. Exposure to low oxygen 1 week after the induction of myocardial infarction induced a robust regenerative response in adult mice, with reduced myocardial fibrosis and improved left ventricular systolic function42. Myocardial fibrosis after injury is also an EMT process42. Low oxygen may reduce myocardial fibrosis and enhance cardiomyocyte regeneration by blocking the EMT process.
Since EMT is also involved in dedifferentiation and loss of functions of hepatocytes cultured in vitro, it is reasonable to hypothesize that low oxygen may help to maintain hepatocyte functions in culture. Indeed, our study demonstrated that physiological oxygen tension (5% O2) was very effective in maintaining the epithelial morphology and many of the functions, including albumin production, glycogen storage, LDL-uptake and CYP450-mediated drug metabolism of primary hepatocytes cultured in vitro for up to 5 days. Lowering the oxygen tension in cell culture is easily achievable, it could be combined with other methods, such as the use of small molecule cocktails and 3D culture, to maintain the proliferation and functions of primary hepatocytes in vitro.
Materials and Methods
Animals
C57BL/6 mice were obtained from shanghai Laboratory Animal Center (Chinese Academy of Sciences) and were maintained in pathogen-free conditions with standard laboratory chow and water ad libitum. All experiments were approved and conducted in accordance with the guidelines of the Animal Care Committee of Shanghai Institute of Materia Medica.
Primary hepatocytes isolation and culture
Primary hepatocytes were isolated from 8-week old C57BL/6 mice by standard two-step collagenase perfusion method. Briefly, the liver was perfused through the inferior vena with 30 mL Perfusion buffer I (0.5 mM EGTA, 16 mM NaHCO3, HBSS without Ca2+ and Mg2+) and then perfused with 25 mL Perfusion buffer II (0.4 mg/mL collagenase type IV, Gibco), 10 mM HEPES, 16 mM NaHCO3, HBSS with 5 mM Ca2+ and 1.2 mM Mg2+). After perfusion, the liver was removed from the abdominal cavity and hepatocytes were released into the DMEM medium using sterile surgical scissors. Cell suspension was filtered through a 350 micrometer cell strainer. Hepatocytes were purified with Percoll buffer (50% Percoll (Sigma-Aldrich), 50% DMEM) at low-speed centrifugation (1,500 r.p.m, 15 min). Viability of isolated hepatocytes was around 90% as determined by Trypan blue. Primary hepatocytes were cultured in hepato-medium (DMEM/F12 (Gibco), supplemented with 10% FBS, 1 µg/mL insulin, 100 nM Dexamethasone, 10 mM nicotinamide, 2 mM L-glutamax, 0.1 mM nonessential amino acids (NEAA), 100 units/mL penicillin and 100 µg/mL streptomycin, and 10 ng/mL EGF (Sigma-Aldrich)). The primary hepatocytes were cultured at 37 °C, 5% CO2 and 21% O2 in a regular incubator, or cultured at 37 °C, 5% CO2 and 5% O2 in a Ruskinn hypoxia workstation.
Immunofluorescence staining
Cells were fixed with 4% PFA and incubated with primary antibodies against albumin (Bethyl A90-234A), α-SMA (Sigma A2547), Cyp1a2 (Abcam ab22717) or γH2A.X (Abcam ab26350) followed by the appropriate secondary antibodies conjugated to Alexa Fluor 555 (Invitrogen A31570) or Alexa Fluor 488 (Invitrogen A11078). Images were taken with an Olympus IX51 inverted fluorescent microscope. For F-actin staining, cells were fixed with 3.7% PFA and incubated with fluorescent phallotoxins (Gibco A34055). Nuclei were stained with Hoechst (Sigma 33342).
PCR
Total mRNA was isolated using Trizol (Invitrogen) and 1 μg RNA was used to synthesize cDNA using PrimeScript RT reagent kit (Takara) according to the manufacturer’s protocol. Real-time PCR was performed using FastStart Universal Probe Master Mix (Roche) and a Stratagene Mx3000 P thermal cycler. Primers sequences are: Cyp1a1, forward 5′-GACCCTTACAAGTATTTGGTCGT-3′, reverse 5′-GGTATCCAGAGCCAGTAACCT-3′; Cyp1a2, forward 5′AGTACATCTCCTTAGCCCCAG-3′, reverse 5′-GGTCCGGGTGGATTC TTCAG-3′; Cyp3a11, forward 5′-TGAGGCAGAAGGCAAAGAAA-3′, reverse 5′-GGTATTCCATCTCCATCACA-3′; Cyp3a41, forward 5′-AAAGCCGCCTCG ATTCTAAGC-3′, reverse 5′-ACTACATCCCGTGGTACAACC-3′; Collage1α1 forward 5′-GCTCCTCTTAGGGGCCACT-3′, reverse 5′-CCACGTCTCAC CATTGGGG-3′; N-cadherin, forward 5′-CATGGCTCCTTCCACATGAT-3′, reverse 5′-AGGGCACCAATCACATCTGC-3′; FN, forward 5′-GAAATATTT GCTGTGTCTCAGGG-3′, reverse 5′-TAAATTTGGCACTTGCATGG-3′; Vimentin, forward 5′-GCACCACCACCCACGGAATCG-3′, reverse 5′-CGGAAAGTGGAATCCTTGCA-3′; α-SMA, forward 5′-AAAATGGA GCCAGTCACATGTGG-3′, reverse 5′-CCACCGCAAATGCTTCTAAGT-3′; Snail, forward: 5′-CACACGCTGCCTTGTGTCT-3′, reverse 5′-GGTCAGCA AAAGCACGGTT-3′; Slug, forward 5′-GAACAGTTGAGGGGCTACAC-3′, reverse 5′-GGTGAGGATCTCTGGTTTTGGTA-3′; GAPDH, forward 5′-TGGTC AAGAAACATTTCAACGCC-3′, reverse 5′-GGTCATGAGCCCTTCCAC AATG-3′.
Western blot
Cells were lysed, sonicated and boiled at 95–100 °C for 5 min in sample buffer (50 mM Tris-HCl, 2% w/v SDS, 10% glycerol, 1% β-mercaptoethanol, 0.01% bromophenyl blue (pH 6.8)). Cell lysates were separated on SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were first incubated with blocking buffer (TBS with 0.05% Tween 20 and 5% non-fat milk) for 1 h at room temperature and then incubated overnight at 4 °C in buffer containing antibodies against GAPDH (Cell signaling 14C10), N-cadherin (Sigma ab76057), γH2A.X (Abcam ab26350) or α-SMA (Sigma A2547). The membranes were washed three times and then incubated with goat anti-rabbit IgG HRP (Abmart M21002) or goat anti-mouse IgG HRP (Abmart M21001) for 1 h. After washing, immunostaining was visualized using Western Lightning Ultra (Perkin Elmer) and ChemiDoc imaging system (Bio-Rad).
PAS stain, Dil-ac-LDL and Albumin ELISA assay
To analyze the glucose storage ability of hepatocytes, cells cultured in 21% O2 or 5% O2 conditions were fixed by 4% PFA and staining with periodic acid-Schiff kit (PAS, Sigma) according to the manufacturer’s instructions. To analyze LDL-uptake ability, hepatocytes cultured in 21% O2 or 5% O2 conditions were cultured in DMEM/F12 containing DiI-Ac-LDL (10 µg/mL) for 4 h at 37 °C.Nuclei were stained by Hoechst and images were captured with an Olympus IX71 inverted fluorescent microscope. To measure albumin secretion, primary hepatocytes cultured in 21% O2 or 5% O2 conditions for 1, 3, or 5 days were transferred into fresh medium. Culture supernatant was collected 24 hr. after medium change. The amount of albumin in supernatant was determined by the mouse albumin ELISA Kit (Sigma) according to manufacturer’s instructions.
CYP metabolism assay
For the measure of CYP enzyme activities, primary hepatocytes were cultured in 21% O2 or 5% O2 conditions and pretreated with 10 μM 3-methylcholanthrene for 48 h to induce the expression of Cyp1a1 and Cyp1a2. Cells were then dissociated and resuspended at a density of 1 million cells/mL in hepato-medium containing 200 nM phenacetin. At 0, 60, 120, 240 and 480 min time points, pipette-mix the incubate and 20 μL of the sample was transferred to a “Quenching” tubes containing 180 μL 100% acetonitrile followed by pipette-mixing. Then the sample was frozen at −80 °C until analyses. After collection of all samples, the supernatants were collected by centrifuged the mixture at 12000 g for 10 minutes and the concentration of the remained phenacetin in the supernatants were measured by LC-MS/MS system (Waters UPLC I-Class and Waters Xevo TQ-S mass spectrometer). All compounds were also purchased from Sigma-Aldrich and used as standard samples.
Measurement of intracellular ROS production
The production of intracellular ROS was measured using the ROS detection reagents (Invitrogen) according to the manufacture’s instructions. In brief, primary hepatocytes were plated into 96-well plate and cultured in 21% O2 or 5% O2 conditions. At day 1, day 3, and day 5, the cells were washed twice with PBS and supplied with phenol red-free DMEM containing 10 μM DCFH-DA dye and then incubate for 30 min at 37 °C in dark. Cells were then washed with PBS for three times and the DCF fluorescence intensity was measured using microplate fluorescence reader (excitation wavelength: 488 nm and emission wavelength: 530 nm).
Statistical Analyses
Values are reported as the means ± SEM. P-values were calculated by Student’s t-test, P < 0.05 was considered statistically significant. All graphs were plotted with GraphPad Prism software.
References
Iber, F. L. Hepatology - a Textbook of Liver-Disease - Zakim,D, Boyer,Td. Jama-J Am Med Assoc 250, 675–675 (1983).
Taub, R. Liver regeneration: from myth to mechanism. Nature reviews. Molecular cell biology 5, 836–847 (2004).
Godoy, P. et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Archives of toxicology 87, 1315–1530 (2013).
Costa, R. H., Kalinichenko, V. V., Holterman, A. X. & Wang, X. Transcription factors in liver development, differentiation, and regeneration. Hepatology 38, 1331–1347 (2003).
Darlington, G. J. Molecular mechanisms of liver development and differentiation. Current opinion in cell biology 11, 678–682 (1999).
Michalopoulos, G. K. & DeFrances, M. C. Liver regeneration. Science 276, 60–66 (1997).
Kinoshita, T. & Miyajima, A. Cytokine regulation of liver development. Biochimica et biophysica acta 1592, 303–312 (2002).
Hewitt, N. J. et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug metabolism reviews 39, 159–234 (2007).
Hengstler, J. G. et al. Cryopreserved primary hepatocytes as a constantly available in vitro model for the evaluation of human and animal drug metabolism and enzyme induction. Drug metabolism reviews 32, 81–118 (2000).
Mitaka, T. The current status of primary hepatocyte culture. International journal of experimental pathology 79, 393–409 (1998).
Wang, Y. & Zhou, B. P. Epithelial-mesenchymal Transition–A Hallmark of Breast Cancer Metastasis. Cancer hallmarks 1, 38–49 (2013).
Godoy, P. et al. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis. Hepatology 49, 2031–2043 (2009).
Laishes, B. A. & Williams, G. M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. II. Dexamethasone enhanced longevity and maintenance of morphology. In vitro 12, 821–832 (1976).
Marks, G. S., Stephens, J. K., Fischer, P. W. & Morgan, R. O. Hormonal effects on the regulation of hepatic heme biosynthesis. Molecular and cellular biochemistry 25, 111–123 (1979).
Lee, W. R. et al. Apamin inhibits hepatic fibrosis through suppression of transforming growth factor beta1-induced hepatocyte epithelial-mesenchymal transition. Biochemical and biophysical research communications 450, 195–201 (2014).
Guillouzo, A. & Guguen-Guillouzo, C. Evolving concepts in liver tissue modeling and implications for in vitro toxicology. Expert opinion on drug metabolism & toxicology 4, 1279–1294 (2008).
Kim, Y. & Rajagopalan, P. 3D hepatic cultures simultaneously maintain primary hepatocyte and liver sinusoidal endothelial cell phenotypes. PloS one 5, e15456 (2010).
Bhatia, S. N., Balis, U. J., Yarmush, M. L. & Toner, M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 13, 1883–1900 (1999).
Khetani, S. R. & Bhatia, S. N. Microscale culture of human liver cells for drug development. Nature biotechnology 26, 120–126 (2008).
Carreau, A., El Hafny-Rahbi, B., Matejuk, A., Grillon, C. & Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15, 1239–1253 (2011).
Yoshida, Y., Takahashi, K., Okita, K., Ichisaka, T. & Yamanaka, S. Hypoxia Enhances the Generation of Induced Pluripotent Stem Cells. Cell stem cell 5, 237–241 (2009).
Ezashi, T., Das, P. & Roberts, R. M. Low O-2 tensions and the prevention of differentiation of hES cells. Proceedings of the National Academy of Sciences of the United States of America 102, 4783–4788 (2005).
Garreta, E., Melo, E., Navajas, D. & Farre, R. Low oxygen tension enhances the generation of lung progenitor cells from mouse embryonic and induced pluripotent stem cells. Physiological reports 2 (2014).
Pimton, P. et al. Hypoxia Enhances Differentiation of Mouse Embryonic Stem Cells into Definitive Endoderm and Distal Lung Cells. Stem cells and development 24, 663–676 (2015).
Bae, D. et al. Hypoxia Enhances the Generation of Retinal Progenitor Cells from Human Induced Pluripotent and Embryonic Stem Cells. Stem cells and development 21, 1344–1355 (2012).
Jin, X. et al. Furazolidone induced oxidative DNA damage via up-regulating ROS that caused cell cycle arrest in human hepatoma G2 cells. Toxicology letters 201, 205–212 (2011).
Dai, C., Li, D., Gong, L., Xiao, X. & Tang, S. Curcumin Ameliorates Furazolidone-Induced DNA Damage and Apoptosis in Human Hepatocyte L02 Cells by Inhibiting ROS Production and Mitochondrial Pathway. Molecules 21 (2016).
Cichon, M. A. & Radisky, D. C. ROS-induced epithelial-mesenchymal transition in mammary epithelial cells is mediated by NF-kB-dependent activation of Snail. Oncotarget 5, 2827–2838 (2014).
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 (2000).
Higgins, G. M. & Anderson, R. M. Experimental pathology of the liver I Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 12, 186–202 (1931).
Levy, G. et al. Long-term culture and expansion of primary human hepatocytes. Nature biotechnology 33, 1264 (2015).
Kidambi, S. et al. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proceedings of the National Academy of Sciences of the United States of America 106, 15714–15719 (2009).
Hewitt, N. J. et al. Primary hepatocytes: Current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug metabolism reviews 39, 159–234 (2007).
Guillen, M. I. et al. Oncostatin M down-regulates basal and induced cytochromes P450 in human hepatocytes. Journal of Pharmacology and Experimental Therapeutics 285, 127–134 (1998).
Shan, J. et al. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nature chemical biology 9, 514–U577 (2013).
Shulman, M. & Nahmias, Y. Long-term culture and coculture of primary rat and human hepatocytes. Methods in molecular biology 945, 287–302 (2013).
Wang, J. et al. Engineering EMT using 3D micro-scaffold to promote hepatic functions for drug hepatotoxicity evaluation. Biomaterials 91, 11–22 (2016).
Wion, D., Christen, T., Barbier, E. L. & Coles, J. A. PO(2) matters in stem cell culture. Cell stem cell 5, 242–243 (2009).
Ji, L. et al. Self-renewal and pluripotency is maintained in human embryonic stem cells by co-culture with human fetal liver stromal cells expressing hypoxia inducible factor 1alpha. Journal of cellular physiology 221, 54–66 (2009).
Shimada, H., Hashimoto, Y., Nakada, A., Shigeno, K. & Nakamura, T. Accelerated generation of human induced pluripotent stem cells with retroviral transduction and chemical inhibitors under physiological hypoxia. Biochem Biophys Res Commun 417, 659–664 (2012).
Yoshida, Y., Takahashi, K., Okita, K., Ichisaka, T. & Yamanaka, S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell stem cell 5, 237–241 (2009).
Nakada, Y. et al. Hypoxia induces heart regeneration in adult mice. Nature 541, 222–227 (2017).
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (2015CB964503), the Chinese Academy of Sciences (XDA01040301), the National Natural Science Foundation of China (81425024, 31371511, 81472862 and 31501189) and Shanghai Science and Technology Committee (14ZR1409500 and 15YF1402400).
Author information
Authors and Affiliations
Contributions
R.G. conducted most of the experiments, analyzed the results, and wrote the paper; X.X. and Y.L. conducted part of the experiments; X.X. conceived the idea for the project, analyzed the results and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, R., Xu, X., Lu, Y. et al. Physiological oxygen tension reduces hepatocyte dedifferentiation in in vitro culture. Sci Rep 7, 5923 (2017). https://doi.org/10.1038/s41598-017-06433-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06433-3
This article is cited by
-
Biomimetic hydrogel blanket for conserving and recovering intrinsic cell properties
Biomaterials Research (2022)
-
Investigating oxygen transport efficiencies in precision-cut liver slice-based organ-on-a-chip devices
Microfluidics and Nanofluidics (2021)
-
Human multi-organ chip co-culture of bronchial lung culture and liver spheroids for substance exposure studies
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.