Abstract
The present study investigated the effects of exogenous thymosin β4 (TB4) on carbon tetrachloride (CCl4)-induced acute liver injury and fibrosis in rodent animals. Results showed that both in mice and rats CCl4 rendered significant increases in serum alanine aminotransferase and aspartate aminotransferase, hepatic malondialdehyde formation, decreases in antioxidants including superoxide dismutase and glutathione, and up-regulated expressions of transforming growth factor-β1, α-smooth muscle actin, tumor necrosis factor-α and interleukin-1β in the liver tissues. Hydroxyproline contents in the rat livers were increased by CCl4. Histopathological examinations indicated that CCl4 induced extensive necrosis in mice livers and pseudo-lobule formations, collagen deposition in rats livers. However, all these changes in mice and rats were significantly attenuated by exogenous TB4 treatment. Furthermore, up-regulations of nuclear factor-κB p65 protein expression by CCl4 treatment in mice and rats livers were also remarkably reduced by exogenous TB4 administration. Taken together, findings in this study suggested that exogenous TB4 might prevent CCl4-induced acute liver injury and subsequent fibrosis through alleviating oxidative stress and inflammation.
Similar content being viewed by others
Introduction
Thymosin β4 (TB4) is a small 5 kD acidic peptide, originally isolated from calf thymus and identified as the main intracellular G-actin sequestering peptide in cell1, 2. It harbors multiple functions and is involved in many important pathophysiological processes, including angiogenesis3, 4, wound healing and repair5, 6, inflammation7,8,9 and cancer progression10,11,12.
In the past several decades many investigations have been done to study the physiological benefits of exogenous TB4 in the body. For example, exogenous TB4 administration enhances skin6 and corneal13 wound healing in mice by promoting keratinocyte migration and inhibiting inflammation6, 13. Exogenous TB4 treatment also provides cardio-protection against ischemic injury in mice through promoting cardiac cell migration, survival and reprogramming epicardial cells into cardiomyocytes14,15,16,17,18,19. However, since TB4 is ubiquitously expressed throughout the body20, the potential physiological functions of TB4 might be far more than current findings and needs further explorations. Recently, TB4 was investigated in the liver21. Clinical study showed that in patients with liver diseases the serum TB4 levels were negatively correlated with the liver function22. In vitro cell culture experiments demonstrated that TB4 treatment increased hepatic growth factor production and decreased PDGF-β receptor expression in hepatic stellate cells (HSCs)23; in vitro proliferation of human hepatocytes was promoted by exogenous TB4 treatment24. Exogenous TB4 administration also ameliorated ischemia reperfusion-induced hepatic injury in mice through activation of AKT-Bad signaling pathway25. In the liver tissues of carbon tetrachloride (CCl4)-treated rats TB4 mRNA levels were increased26. Reyes-Gordillo and his colleagues reported that exogenous TB4 treatment prevented the histological appearances of necrosis, inflammatory infiltration, up-regulations of α1(and 2) collagen, α-SMA, PDGF-β receptor and fibronectin mRNA expressions, and maintained quiescent phenotypic state of hepatic stellate cells in the liver tissues of CCl4-treated rats27. All these findings suggested that TB4 harbored hepatoprotective effects and might exert antifibrotic activities in vivo. Therefore, the main purposes of our present study were to confirm the hepatoprotective effects of exogenous TB4 against CCl4-induced acute mouse liver injury and investigate the in vivo antifibrotic activities of exogenous TB4 in the CCl4-induced rat liver fibrosis models.
Results
Acute liver injury
TB4 protected against CCl4-induced acute hepatic dysfunction
Activities of serum ALT and AST were measured to determine the effects of TB4 on the liver damage in CCl4-treated mice (Fig. 1). In CCl4-treated mice the activities of serum ALT and AST were markedly increased as compared with those in mice treated with saline (control group) or TB4 (TB4 group) (P < 0.001 for both group). Interestingly, treatment with TB4 significantly reduced the serum ALT and AST activities in CCl4-treated mice (P < 0.05).
TB4 alleviated CCl4-induced histological changes in the livers
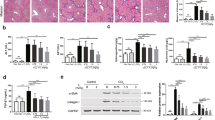
Effects of TB4 on CCl4-induced liver injury in mice were investigated through histology studies. As shown in Fig. 2, livers from control group and TB4 group exhibited normal histological morphologies (Fig. 2A and B), while livers from CCl4-treated mice presented extensive hepatocellular necrosis (Fig. 2C). However, TB4 treatment markedly ameliorated CCl4-induced damages in the mouse livers (Fig. 2D). Semi-quantitative analysis of the histopathological changes using Ishak scoring system also indicated the significant protection of TB4 on CCl4-induced acute liver injury (Fig. 2E). These histopathological analysis results were consistent with the serum diagnostic test reports.
Effect of exogenous thymosin β4 (TB4) on liver histology in CCl4-treated mice. Representative microphotographs of liver histology staining of hematoxylin-eosin (H.E) were shown (original magnification, × 100). (A) Control group; (B) TB4 group; (C) CCl4 group; Arrow heads indicated the necrotic area. (D) CCl4 + TB4 group. (E) Quantitative analysis of the liver injury using Ishak scoring system. Data were expressed as mean ± standard deviation (SD). Differences were compared using Student t-test. ###P < 0.05 vs Control group and TB4 group; ***P < 0.05 vs CCl4 group. (n = 10 for each group).
TB4 inhibited CCl4-induced oxidative stress and inflammation in the livers
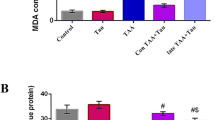
Oxidative stress was assessed by determining the MDA levels, protein tyrosine nitration (Nitro-Tyrosine, N-Tyr) and the anti-oxidation activities in the liver tissues. As shown in Fig. 3A, MDA levels were significantly increased in CCl4-treated mice as compared with those in control and TB4-treated mice (P < 0.01). What’s more, immunohistochemistry analyses indicated that N-Tyr levels in the mouse liver tissues were also markedly up-regulated by CCl4 treatment (Fig. 4). However, TB4 administration significantly lowered the MDA and N-Tyr levels in the livers of CCl4-treated mice (Figs 3A and 4).
Immunohistochemistry of α-SMA, TGF-β1, nitrative tyrosine, TNF-α and IL-1β in liver sections from different groups with treatment as indicated in the figures. Original magnification of the microphotographs was showed in the figure. Four liver sections were randomly selected from each group and used for immunohistochemistry analysis. Here showed the representative results of immunohistochemistry.
Anti-oxidation activities were evaluated by measuring SOD activities and GSH levels in the liver tissues. As demonstrated in Fig. 3B and C, CCl4 treatment significantly decreased SOD activities and GSH levels as compared with control group and TB4 group (P < 0.01 for both). However, CCl4-reduced SOD activities and GSH levels in the liver tissues were markedly reversed by TB4 administration (P < 0.05).
CCl4-induced inflammation in the liver tissue was assessed by determining the levels of pro-inflammatory cytokines including TNF-α and IL-1β. As shown in Fig. 3D and E, TNF-α and IL-1β levels in the livers were markedly increased in CCl4-treated mice as compared with control mice and TB4 alone treated mice (P < 0.01). However, TB4 treatment significantly decreased the TNF-α and IL-1β levels in the liver tissues of CCl4-treated mice (P < 0.05). Effects of TB4 on TNF-α and IL-1β expression in the liver tissues of CCl4-treated mice were also reconfirmed by immunohistochemistry assays (Fig. 4). Immunohistochemistry results showed that both TNF-α and IL-1β expressions in the livers were markedly increased by CCl4 treatment, which was greatly reduced by TB4 administration (Fig. 4).
TB4 inhibited CCl4-induced HSCs activation and reduced TGF-β1 expression in the livers
Immunohistochemistry assays demonstrated that more α-SMA-positive cells in the liver tissues were seen in CCl4-treated mice as compared with control mice and TB4 alone treated mice (Fig. 4), which indicated that CCl4 activated HSCs in vivo. However, up-regulation of α-SMA-positive cells in the liver tissues by CCl4 treatment was greatly reduced by TB4 administration. TGF-β1, a pro-fibrotic cytokine, was also up-regulated in the liver tissues of CCl4-treated mice, which was also reduced by TB4 administration (Fig. 4). These results suggested that exogenous TB4 administration might exhibit anti-fibrotic activities in the mouse livers.
TB4 suppressed CCl4-increased nuclear factor-κB (NF-κB) p65 protein expression in the livers
As shown in Fig. 5, CCl4 treatment significantly increased NF-κB p65 protein expression in the mouse livers (P < 0.01). However, the increase of p65 protein expression in the liver tissues was markedly suppressed by TB4 administration in CCl4-intoxicated mice (P < 0.05).
Western blot analysis of nuclear factor-κB (NF-κB) p65 protein in response to CCl4 and thymosin β4 (TB4) treatments. Six mice liver samples per group were analyzed by Western blot assays. Here showed the representative Western blot results and the semi-quantification results from Image J analysis. Data were expressed as mean ± standard error (n = 6). ###P < 0.01 vs. both control group and TB4 group; ***P < 0.05 vs. CCl4 group. β-actin was used as an internal control.
Liver fibrosis
TB4 reduced CCl4-induced hepatotoxicity
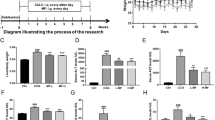
The SD rats were divided into three groups: control group, fibrosis model group, TB4 treatment group. Rats received eight-weeks of treatments as demonstrated in Fig. 6A. The body weights of all the rats were measured weekly and prior to scarification. As indicated in Fig. 6B, rats from the control group presented normal body weight gains from 220 g to 370 g over eight weeks. Whereas, the body weights of rats from fibrosis model group were significantly lower than those in control group. However, TB4 administration significantly prevented CCl4-induced body weight loss when comparing control group with fibrosis model group (Fig. 6B). In addition, CCl4-induced remarkable increases in serum ALT and AST activities were also significantly reduced by TB4 (Fig. 6C).
Experimental design and biochemical analysis. (A) Experimental design of the schedule for CCl4 and thymosin β4 (TB4). (B) Effect of TB4 on rat body weights; the body weight gains in CCl4-treated rats were significantly lower than those in control and CCl4 plus TB4 treated rats. #P < 0.05 vs. control group; *P < 0.05 vs. CCl4 group. (C) Effect of TB4 on serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in rats. ###P < 0.05 vs. control group; ***P < 0.05 vs. CCl4 group. (D) Effects of TB4 on hydroxyproline contents in rat livers. n = 6, control group; n = 10, CCl4 group; n = 10, CCl4 + TB4 group. ###P < 0.05 vs. control group; ***P < 0.05 vs. CCl4 group.
TB4 attenuated CCl4-induced hepatic fibrosis
Collagen deposition, one hepatic fibrosis marker, was determined and represented by hepatic hydroxyproline content. As shown in Fig. 6D, hydroxyproline contents in the liver tissues were significantly increased in CCl4-treated rats from fibrosis model group as compared with those from control group. However, TB4 treatment markedly decreased the hydroxyproline contents in CCl4-treated rats. Checks of macroscopic appearances of the livers showed that in comparison to normal livers in control group with a regular and smooth surface, the livers in CCl4-treated fibrosis model group were puffy, stiff, and acquired an irregular and granular surface. However, treatment with TB4 remarkably promoted the recovery of CCl4-damaged liver structure as shown in Fig. 7A.
Effects of TB4 on liver fibrosis in rats. (A) Representative results of macroscopic appearance and liver histology staining of hematoxylin-eosin (H.E), Masson and Sirius Red were shown. Original magnification was indicated on the figure. (B) Semi-quantitative analysis of Masson trichrome staining results. (C) Semi-quantitative analysis of Sirius Red staining results. (D) Semi-quantitative analysis of liver fibrosis and inflammation using Ishak scoring system. n = 6, control group; n = 10, CCl4 group; n = 10, CCl4 + TB4 group. Two liver sections were analyzed for each animals. ###P < 0.05 vs. control group; ***P < 0.05 vs. CCl4 group.
H.E staining results showed that livers from the control group exhibited a normal lobular architectures, whereas livers from fibrosis model group exhibited damaged lobular architectures, severe vacuolar degeneration of hepatocytes, large fibrous septa, pseudo-lobule formations and inflammatory cells infiltration which were dramatically ameliorated by TB4 treatment (Fig. 7A). These results were further confirmed by Masson and Sirius Red stainings (Fig. 7A,B and C). Masson and Sirius Red stainings demonstrated that liver tissues from control group showed few collagen deposition, whereas those from CCl4-treated fibrosis model group presented dense fibrous septa and increased deposition of collagen fibers. Semi-quantitative analysis of the liver injury, fibrosis and inflammation using Ishak scoring system also showed that TB4 treatment significantly reduced CCl4-induced liver inflammation, injury and fibrosis (Fig. 7D). All these results provided evidences supporting the protective activities of TB4 against CCl4-induced hepatic fibrosis.
TB4 inhibited CCl4-induced oxidative stress and inflammation in the livers
As shown in Fig. 8A, MDA, a marker of lipid peroxidation, was remarkably increased in the livers from fibrosis model group as compared with control group (P < 0.01). On the contrary, the antioxidants SOD activities and GSH levels were markedly decreased in the livers from fibrosis model group in comparison to control group (Fig. 8B and C) (P < 0.01 for both). However, all these changes induced by CCl4 were significantly suppressed by TB4 treatment (P < 0.05). Pro-inflammatory cytokine, TNF-α, was up-regulated by CCl4 in the liver tissues as compared with control group (P < 0.01), which was greatly reversed by TB4 (Fig. 8D, P < 0.05). Furthermore, Western blots showed that expression of nuclear factor-κB p65 protein, a key player in inflammation, was remarkably increased in CCl4-treated fibrosis model group and was greatly reduced in TB4 treatment group (Fig. 9A and D).
Western blot analysis of α-SMA, TGF-β1 and p65 in rat livers. Representative Western blot results (A) and semi-quantifications of the Western blot bands by Image J software were shown (B–D). Data were expressed as mean ± standard error of 6 rats per group. ###P < 0.01 vs. control group; ***P < 0.05 vs. CCl4 group; $$$P > 0.05 vs. control group. β-actin was used as an internal control. (Some of the gels/blots in this figure were cropped from original full-length films which were illustrated in the supplemental information file).
Influence of TB4 on expressions of fibrosis markers of α-SMA and TGF-β1 after CCl4 administration
Expression of α-SMA and TGF-β1 in the liver tissues were determined by Western blots (Fig. 9). As indicated in Fig. 9, both α-SMA and TGF-β1 expressions were significantly up-regulated by CCl4 treatment in fibrosis model group compared with control group, and were significantly reduced by TB4 in TB4 treatment group in comparison to fibrosis model group.
Discussion
Recent studies have report that TB4 is associated with fibrosis in several organs28,29,30,31, and more eyeballs of researchers are attracted by the role of TB4 in liver fibrosis32. Several studies illustrate that hepatocytes and hepatic stellate cells (HSCs) in the livers all express TB4 endogenously33, 34. The endogenous TB4 expression in HSCs is up-regulated in carbon tetrachloride (CCl4)-induced liver fibrosis34. In bile duct ligation (BDL)-induced liver fibrosis model endogenous TB4 expression is down-regulated in the fibrotic liver tissues35. In cultured human HSCs exogenous TB4 treatment inactivates HSCs through down-regulating PDGF-beta receptor expression and inhibiting PDGF-dependent phosphorylation and binding of AKT to actin23, 36. In LX2 (HSCs cell line) cell exogenous TB4 treatment inhibits its proliferation35. All these findings lead to a speculation that TB4 might be involved in the process of liver fibrogenesis32 and might be a promising target to treat liver fibrosis as exogenous TB4 has been proved to exhibit anti-fibrotic activities in kidney28 and lung29,30,31. However, so far, most of the key studies about TB4 in liver fibrosis are in vitro investigations23, 34,35,36 using primary cultures or cell lines of HSCs, a vital player in liver fibrogenesis37. Even so, conflicting evidences still exist at present. For example, Chen et al. reported that depletion of endogenous TB4 by siRNA activated HSCs in vitro 35; while Jung et al. proved that down-regulation of endogenous TB4 by siRNA inactivated HSCs in vitro 34. Moreover, even in some in vivo experiments conflicting results are also got. Results from Chen and his colleagues suggested that endogenous TB4 expression levels decreased during liver fibrogenesis35; however, reports from Jung et al. indicated that endogenous TB4 expression levels in livers increased during liver fibrogenesis34. So, at present, findings from those previous studies still can not clearly answer the question that whether exogenous TB4 exhibits inhibitory effects on liver fibrosis in vivo. In order to answer this question further in vivo experiments should be performed. In present study effects of exogenous TB4 were investigated in CCl4-induced acute mouse liver injury model and rat liver fibrosis model. Results showed that exogenous TB4 treatment markedly attenuated CCl4-induced acute mouse liver injury and chronic rat liver fibrosis. The mechanisms involved antioxidant and anti-inflammatory effects of TB4.
CCl4, a potent hepatotoxic agent, has been widely used to establish animal model to study liver injury which was characterized by typical centrilobular necrosis and was similar to the hepatotoxicity in human38, 39. The present study showed that in the acute mouse liver injury model a single intraperitoneal injection of CCl4 remarkably increased serum ALT and AST activities, which suggested the presence of acute hepatotoxicity since under normal condition AST and ALT only existed in both cytoplasm and mitochondria of hepatocytes. Histopathological examinations also reflected the severity of acute liver injury induced by CCl4. However, all these changes were significantly attenuated by TB4, which agreed with the previous conclusions reported by other researchers27. Immunohistochemistry results in current study demonstrated that CCl4 treatment increased the expressions of α-SMA, a marker of activated HSCs, and pro-fibrotic cytokine TGF-β both of which were reduced by TB4 treatment. Besides, the current study also showed that TB4 inhibited CCl4-induced oxidative stress and inflammation in acute mouse liver injury model, which was identified by TB4-induced suppression on CCl4-induced increases in MDA and nitro-tyrosine levels, decreases in SOD activities and GSH levels, up-regulations of TNF-α, IL-1β and nuclear factor-κB p65.
Although TB4 is investigated in previous animal studies of acute liver injury, so far it has not been studied in animal models of liver fibrosis. The current study aimed to explore the action of exogenous TB4 in CCl4-induced rat liver fibrosis. CCl4-induced rat liver fibrosis resembled human liver fibrosis as regards to the pathological processes and characteristics such as fiber formation, inflammation, regeneration and spontaneous recovery from fibrosis after removal of the toxic factor40. In current study rats were repeatedly exposed to CCl4 injection twice per week for eight weeks to induce liver fibrosis which were identified by increases in hepatic hydroxyproline contents and serum ALT and AST activities. Macroscopic appearance examinations showed that CCl4 injection rendered irregular and granular surface in rat livers. H.E staining results showed that extensive fibrotic lesions were present in rat liver tissues after 8-weeks of CCl4 treatment. Masson and Sirius Red staining results demonstrated that CCl4 injection caused pseudo-lobule formations, dense fibrous septa and increased collagen deposition in rat liver tissues. However, all these changes induced by chronic exposure to CCl4 were remarkably attenuated by TB4 treatment.
Fibrosis is the main pathophysiological consequences of chronic liver injuries which is caused by many factors including virus, autoimmune diseases, drug/toxin, alcohol and nonalcoholic fatty liver diseases41. Hepatic stellate cells (HSCs) activation plays a central role in the process of liver fibrogenesis42. During the liver injury trans-differentiation occurs in quiescent HSCs and makes quiescent HSCs activated. After activation HSCs acquire myofibroblast-like phenotypes with long processes and lose cytoplasmic lipid droplets. Furthermore, activated HSCs are pro-fibrogenic and promote fibrous extracellular matrix (ECM) proteins productions and deposition leading to liver fibrosis43. Previous studies have indicated that TGF-β played an important role in liver fibrosis by inducing myofibroblast-like cells formation. Through binding the transmembrane receptor TGF-β activates Smad signaling pathway and up-regulates expressions of ECM proteins44. As aforementioned above, single CCl4 injection increased the expressions of pro-fibrotic cytokine TGF-β1 and α-SMA, a fibrosis marker mainly expressed in activated HSCs. However, immunohistochemistry assays showed that TB4 treatment significantly suppressed the increases in TGF-β1 and α-SMA induced by single CCl4 injection. Similar to those results from acute liver injury study, Western blots results from chronic CCl4 exposure-induced rat liver fibrosis study showed that repeated CCl4 injection-induced up-regulations of TGF-β1 and α-SMA were also reduced by exogenous TB4 administration.
Oxidative stress mediated by free radicals derived from CCl4 is one of the main factors leading to hepatic damages. It causes cell membrane damage and consequent leakage of hepatotoxic marker enzymes39. Cytochrome P450 enzyme is involved in the process of CCl4-induced liver damages45. In hepatocytes cytochrome P450 catabolizes CCl4 to produce highly reactive trichloromethyl radical (·CCl3) and peroxyl radical (OOCCl3) which subsequently lead to cellular damages by initiating lipid peroxidation and covalently binding to macromolecules45. In present study oxidative stress was monitored by detecting oxidative stress parameters including MDA, SOD, GSH and protein tyrosine nitration. MDA is a product of lipid peroxidation and is used as a marker for lipid peroxidation, a key feature of CCl4-induced liver injury46, 47. SOD is an antioxidant enzyme that scavenged the superoxide anions48. GSH is the most important reducing substance in the body and collaborated with GSH-dependent enzymes to eliminate reactive intermediaries by reacting with hydroperoxides. It acts as free radical scavenger and plays an important role in maintaining protein sulfhydryl groups49. Previous study showed that GSH was depleted in hepatotoxicity50. Protein tyrosine nitration is considered as another marker of oxidative stress51. In CCl4-treated animals protein tyrosine nitration is increased in the liver tissues27. The current study showed that in both CCl4-induced acute liver injury model and fibrosis model MDA levels were markedly increased, and GSH levels and SOD activities were significantly decreased. Tyrosine nitration was increased in acute liver injury and was not detected in liver fibrosis at present. Administration of TB4 to CCl4-intoxicated animals significantly decreased MDA and nitro-tyrosine levels, but increased GSH levels and SOD activities in the liver tissues. These results indicate that TB4 harbors activities against oxidative stress induced by CCl4.
Besides oxidative stress, inflammation is also another important factor propagating CCl4-induced hepatotoxicity52. Numerous studies report that oxidative stress induced by CCl4 activate Kupffer cells which produce pro-inflammatory cytokines including TNF-α and IL-153,54,55,56. TNF-α is considered as the main endogenous deleterious player in experimental liver injury model for its direct cytotoxicity and capacity to initiate inflammation cascades57, 58. IL-1 is another important inflammatory mediator and takes part in the progression from liver injury to fibrosis59. Blocking IL-1 through IL-1 receptor antagonist protects mice from CCl4-induced liver damage60. Many previous studies reported that pro-inflammatory cytokines production were regulated by nuclear factor-κB (NF-κB) signaling pathway61, 62. What’s more, previous study also demonstrated that in CCl-4 induced liver injury model inflammatory cytokines production were strongly correlated with the activity of NF-κB signaling pathway63. In our present study results indicated that CCl4 insults up-regulated the expressions of TNF-α, IL-1β and NF-κB p65 protein in the liver tissues. However, exogenous TB4 administration significantly suppressed the increases induced by CCl4. All these findings suggest that TB4 might exert hepatoprotection against CCl4 insults through inhibiting inflammation.
Taken together, findings in current study suggest that TB4 might prevent CCl4-induced acute liver injury and subsequent fibrosis through alleviating oxidative stress and inflammation. However, protective effects of TB4 on organ injury and fibrosis have been investigated by many other previous studies28, 30, 31, 64,65,66,67. Conte E. et al. reported that TB4 treatment attenuated bleomycin-induced lung injury and early fibrosis in mice29, 30, 67. TB4 also provides protection against renal injury and promotes renal repair during fibrosis28, 64,65,66. Reyes-Gordillo K. et al. reported that TB4 inhibited CCl4-induced acute liver injury in rats27, and our present results confirmed their findings. Previous researches and our present study all showed that inhibition of oxidative stress and inflammation were involved in the hepatoprotective activity of TB465, 67. Nonetheless, more works should still be done to further elucidate the molecular mechanisms underlying TB4 actions. For example, since ac-SDKP, a degradation product of TB4, is anti-fibrotic in many organs including liver and kidney68,69,70,71,72, whether it is through ac-SDKP up-regulation that TB4 exerts anti-fibrotic activity in the liver? As TB4 could also modulate matrix metalloproteinases (MMPs) expressions in other organs or tissues73, 74 and MMPs play an important role in liver fibrosis75, then how does TB4 regulate MMPs expressions or activities in the liver tissues, and what are the significances of its regulation on MMPs in protection against liver injury and fibrosis? Clear answers for these questions warrant further investigations.
Materials and Methods
Ethics statement
This study was approved by the Institutional Ethics Committee for Animal Care and Use at Tianjin Medical University (Ethic No. TMUaMEC2015003). All animals received human care according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resource, 1996, Nat. Acad. Press).
Chemicals and reagents
Thymosin β4 (HPLC > 98%) was purchased from GL Biochem. (Shanghai) Ltd and dissolved in 0.9% saline. α-SMA (Cat: CBL171) and β-actin (Cat: MAB1501), nitro-tyrosine (N-Tyr) (Cat: 05–233) antibodies were bought from Millipore (Darmstadt, Germany). P65 (Cat: 8242) antibody was purchased from Cell Signaling Technology (Denver, Colorado, USA). TGF-β1 (Cat: 18978-1-AP) antibody was bought from Proteintech (Wuhan, China). TNF-α (Cat: ab6671) and IL-1β (Cat: ab2105) antibodies were purchased from Abcam (Shanghai, China). Secondary antibodies against rabbit (Cat: 111-035-003) and mouse (Cat: 115-035-003) were obtained from Jackson ImmunoResearch (Baltimore, Maryland, USA). Immobilon enhanced chemiluminescence (ECL) detection reagent (Cat: WBKLS0500) was purchased from Millipore (Darmstadt, Germany). All other reagents were of analytic grade.
Animals, treatments and groupings
Male Balb/c mice (6 weeks old, weighing ~20 g) and Sprague-Dawley (SD) rats (6–8 weeks old, weighing ~220 g) were bought from the Branch of National Breeder Center of Rodents (Beijing). All animals were maintained in specific-pathogen-free (SPF) environment (Experimental Animal Center at Tianjin Medical University) with a 12-h light/dark cycle and offered ad libitum access to food and water. Before experiments animals were acclimatized for a week under the SPF environment. At the end of the experiments animals were euthanized under pentobarbital anesthesia.
Acute liver injury was induced by a single intraperitoneal injection of 0.5 ml/kg.bw (bw, body weight) CCl4 in olive oil in Balb/c mice. Twenty-four hours later mice were sacrificed to collect blood and liver tissues for further analysis.
Rat liver fibrosis model was established through CCl4-indueced persistent chronic liver injury for eight weeks. CCl4 in olive oil was intraperitoneally administrated twice a week for 8 weeks. The dosage of CCl4 was 0.5 ml/kg.bw (bw, body weight).
Mice for acute liver injury induction were divided into four groups (n = 10/group) as followings: (1) Control group, mice in this group received nothing but equal volume of olive oil intraperitoneally; (2) TB4 group, mice in this group received intraperitoneal administration of both olive oil and 100 μg TB4/mouse/time; TB4 was administrated at 0 hour, 2 hours, 4 hours, 6 hours after olive oil injection; (3) CCl4 treatment group, mice in this group received only CCl4 in olive oil intraperitoneally to induce acute liver injury; (4) CCl4 + TB4 treatment group, mice in this group were treated with CCl4 and TB4 intraperitoneally; TB4 were administrated at 0 hour, 2 hours, 4 hours, 6 hours after CCl4 injection. The dose and time of TB4 were determined according to previous report76.
Rats for liver fibrosis induction were divided into three groups as followings: (1) Control group (n = 6), rats in this group received intraperitoneal injection of olive oil and saline; (2) Fibrosis model group (n = 10), rats in this group received intraperitoneal injection of CCl4 in olive oil and saline; (3) TB4 treatment group (n = 10), rats in this group were treated with CCl4 plus TB4; CCl4 was administrated as aforementioned; TB4 were intraperitoneally administrated once every three days at 1 mg/kg body weight. The dose and time of TB4 were determined according to previous study27.
Biochemical assays
Serum ALT and AST activities, SOD activities and MDA, GSH levels in the liver tissues, were determined by commercially available detection kits (Nanjing Jiancheng Institute of Biotechnology, Nanjing, China) according to the manufacturer’s instructions. Hepatic hydroxyproline content was also measured using a detection kit from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China) according to the manufacturer’s manual. The results were reported as microgram of hydroxyproline per gram of wet liver tissue. TNF-α was measured using a commercial ELISA kit (Cat: ab46070 and ab100747, Abcam, Shanghai, China). Mouse IL-1β ELISA kit were purchase from ThermoFisher Scientific (Cat: BMS6002, Shanghai, China).
Histopathology
Liver tissues were fixed in 10% neutral buffered formalin, processed routinely, embedded in paraffin and then were cut into 4-μm thick sections. Liver sections were stained with hematoxylin-eosin (H.E) according to standard procedure for routine histological examination. Liver sections were stained with Masson’s trichrome and Sirius Red stains to estimate liver fibrosis. Liver fibrosis was semi-quantitatively analyzed using Image J free software (http://rsb.info.nih.gov/ij/).Stained liver slices were examined under a Nikon light microscope by an experienced pathologist. Liver injury and fibrosis were evaluated and scored through Ishak scoring system77.
Immunohistochemistry
For immunohistochemistry, liver sections were deparaffinized and rehydrated. Endogenous peroxidase activities were blocked in 3% H2O2 for 10 min. Antigen was retrieved in citrate buffer (pH = 6.0) in a microwave oven for 15 min. Non-specific protein binding was blocked by BSA (5%). Then the sections were probed with specific primary antibodies against α-SMA (1:200), TGF-β1 (1:200), nitro-tyrosine (1:200), TNF-α (1:200) and IL-1β (1:200) overnight in a humidified chamber at 4 °C. After washing with PBS twice, the liver sections were incubated with a biotinylated secondary antibody. Then the immunoreaction was amplified with streptavidin–avidin–peroxidase complex. Liver sections were stained with diaminobenzidine (DAB) Chromogen for color development. At last, sections were lightly counter-stained with hematoxylin, mounted with mounting medium and examined under a light microscope by an experienced pathologist. Positive antigen stained brown against a blue hematoxylin background.
Western blot analysis
Total protein samples were extracted from snap-frozen liver tissues (80–100 mg) of different groups using 1 ml RIPA lysis buffer (Pierce, Rockford, Illinois, USA) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich, St. Louis, Missouri, USA), a protease inhibitor cocktail (Amresco, Solon, Ohio, USA) and phosphostop (Roche Diagnostics, Indianapolis, Indiana, USA). Total extracts were collected by centrifugation at 14000× g for 10 min at 4 °C. The concentration was determined using a BCA protein assay kit (Pierce, Rockford, Illinois, USA). Then protein samples were subjected to SDS-PAGE (15%) and transferred onto a 0.2-μm PVDF membrane (Millipore, Darmstadt, Germany). Membranes were blocked with 5% skimmed milk and incubated with specific primary antibodies against p65 (1:1000), α-SMA (1:1000), TGF-β1 (1:1000) and β-actin (1:10000) overnight at 4 °C. Membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit or goat anti-mouse secondary antibody (1:20000) at room temperature for 1 hour. At last, protein bands were visualized using Immobilon enhanced chemiluminescence (ECL) reagents and imaged using a GelDoc XR System (Bio-Rad, Shanghai, China). Bands densities were analyzed using Image J free software (http://rsb.info.nih.gov/ij/).
Statistical analysis
Statistical analyses were performed using SPSS software for Windows, version 21.0 (SPSS Inc., Chicago, Illinois, USA). Student t-test was employed to compare the differences between groups. P < 0.05 was considered as statistically significant.
References
Crockford, D., Turjman, N., Allan, C. & Angel, J. Thymosin β4: structure, function, and biological properties supporting current and future clinical applications. Annals of the New York Academy of Sciences 1194, 179–189, doi:10.1111/j.1749-6632.2010.05492.x (2010).
Goldstein, A. L., Hannappel, E. & Kleinman, H. K. Thymosin beta4: actin-sequestering protein moonlights to repair injured tissues. Trends in molecular medicine 11, 421–429, doi:10.1016/j.molmed.2005.07.004 (2005).
Philp, D., Huff, T., Gho, Y. S., Hannappel, E. & Kleinman, H. K. The actin binding site on thymosin beta4 promotes angiogenesis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 17, 2103–2105, doi:10.1096/fj.03-0121fje (2003).
Shelton, E. L. & Bader, D. M. Thymosin beta4 mobilizes mesothelial cells for blood vessel repair. Annals of the New York Academy of Sciences 1269, 125–130, doi:10.1111/j.1749-6632.2012.06713.x (2012).
Treadwell, T. et al. The regenerative peptide thymosin beta4 accelerates the rate of dermal healing in preclinical animal models and in patients. Annals of the New York Academy of Sciences 1270, 37–44, doi:10.1111/j.1749-6632.2012.06717.x (2012).
Kleinman, H. K. & Sosne, G. Thymosin beta4 Promotes Dermal Healing. Vitamins and hormones 102, 251–275, doi:10.1016/bs.vh.2016.04.005 (2016).
Conte, E. et al. Thymosin beta4 reduces IL-17-producing cells and IL-17 expression, and protects lungs from damage in bleomycin-treated mice. Immunobiology 219, 425–431, doi:10.1016/j.imbio.2014.02.001 (2014).
Evans, M. A. et al. Thymosin beta4-sulfoxide attenuates inflammatory cell infiltration and promotes cardiac wound healing. Nature communications 4, 2081, doi:10.1038/ncomms3081 (2013).
Young, J. D. et al. Thymosin beta 4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nature medicine 5, 1424–1427, doi:10.1038/71002 (1999).
Kang, Y. J. et al. Thymosin beta4 was upregulated in recurred colorectal cancers. Journal of clinical pathology 67, 188–190, doi:10.1136/jclinpath-2013-201940 (2014).
Nemolato, S. et al. Thymosin beta 4 in colorectal cancer is localized predominantly at the invasion front in tumor cells undergoing epithelial mesenchymal transition. Cancer biology & therapy 13, 191–197, doi:10.4161/cbt.13.4.18691 (2012).
Wirsching, H. G. et al. Thymosin beta 4 gene silencing decreases stemness and invasiveness in glioblastoma. Brain: a journal of neurology 137, 433–448, doi:10.1093/brain/awt333 (2014).
Sosne, G., Qiu, P., Kurpakus-Wheater, M. & Matthew, H. Thymosin beta4 and corneal wound healing: visions of the future. Annals of the New York Academy of Sciences 1194, 190–198, doi:10.1111/j.1749-6632.2010.05472.x (2010).
Bock-Marquette, I., Saxena, A., White, M. D., Dimaio, J. M. & Srivastava, D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 432, 466–472, doi:10.1038/nature03000 (2004).
Marx, J. Biomedicine. Thymosins: clinical promise after a decades-long search. Science 316, 682–683, doi:10.1126/science.316.5825.682 (2007).
Smart, N. et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445, 177–182, doi:10.1038/nature05383 (2007).
Wei, C., Kumar, S., Kim, I. K. & Gupta, S. Thymosin beta 4 protects cardiomyocytes from oxidative stress by targeting anti-oxidative enzymes and anti-apoptotic genes. PloS one 7, e42586, doi:10.1371/journal.pone.0042586 (2012).
Bao, W. et al. Cardioprotection by systemic dosing of thymosin beta four following ischemic myocardial injury. Frontiers in pharmacology 4, 149, doi:10.3389/fphar.2013.00149 (2013).
Rui, L. et al. Extending the time window of mammalian heart regeneration by thymosin beta 4. Journal of cellular and molecular medicine 18, 2417–2424, doi:10.1111/jcmm.12421 (2014).
Huff, T., Muller, C. S., Otto, A. M., Netzker, R. & Hannappel, E. beta-Thymosins, small acidic peptides with multiple functions. The international journal of biochemistry & cell biology 33, 205–220 (2001).
Kim, J. & Jung, Y. Thymosin Beta 4 Is a Potential Regulator of Hepatic Stellate Cells. Vitamins and hormones 102, 121–149, doi:10.1016/bs.vh.2016.04.011 (2016).
Xing, J. et al. Serum thymosin β4 level in patients with hepatocellular carcinoma. Journal of clinical hepatology 27, 387–390 (2011).
Barnaeva, E., Nadezhda, A., Hannappel, E., Sjogren, M. H. & Rojkind, M. Thymosin beta4 upregulates the expression of hepatocyte growth factor and downregulates the expression of PDGF-beta receptor in human hepatic stellate cells. Annals of the New York Academy of Sciences 1112, 154–160, doi:10.1196/annals.1415.035 (2007).
Xing, J. The effect of thymosin beta 4 on the proliferation of normal human hepatocytes in vitro and the detection of serum thymosin beta 4 in patients with hepatocellular carcinoma. Master thesis, Tianjin Medical University (2012).
Feng, B. Thymosin beta 4 alleviated hepatic ischemia reperfusion injury by activating AKT-Bad signaling pathway in mice Master thesis, Nanjing Medical University (2012).
CHEN, Y. et al. Expression of endogenous AcSDKP and its precursor thymosin beta 4 in early stage of liver fibrosis induced by carbon tetrachloride in rats. Chinese journal of gastroenterology and hepatology 19, 6–9 (2010).
Reyes-Gordillo, K., Shah, R., Arellanes-Robledo, J., Rojkind, M. & Lakshman, M. R. Protective effects of thymosin beta4 on carbon tetrachloride-induced acute hepatotoxicity in rats. Annals of the New York Academy of Sciences 1269, 61–68, doi:10.1111/j.1749-6632.2012.06728.x (2012).
Zuo, Y. et al. Thymosin beta4 and its degradation product, Ac-SDKP, are novel reparative factors in renal fibrosis. Kidney international 84, 1166–1175, doi:10.1038/ki.2013.209 (2013).
Conte, E. et al. Protective effects of thymosin beta4 in a mouse model of lung fibrosis. Annals of the New York Academy of Sciences 1269, 69–73, doi:10.1111/j.1749-6632.2012.06694.x (2012).
Conte, E. et al. Thymosin beta4 protects C57BL/6 mice from bleomycin-induced damage in the lung. European journal of clinical investigation 43, 309–315, doi:10.1111/eci.12048 (2013).
Li, X.-K., Chen, C., Wang, L., Li, Z.-J. & Ren, C.-Z. Thymosin β4 alleviates bleomycin-induced lung damage through inhibiting nitrative thioredoxin-1 inactivation. Int J Clin Exp Med 9, 6138–6142 (2016).
Kim, J. & Jung, Y. Potential role of thymosin Beta 4 in liver fibrosis. Int J Mol Sci 16, 10624–10635, doi:10.3390/ijms160510624 (2015).
Nemolato, S. et al. Expression pattern of thymosin beta 4 in the adult human liver. European journal of histochemistry: EJH 55, e25, doi:10.4081/ejh.2011.e25 (2011).
Kim, J. et al. Hepatic stellate cells express thymosin Beta 4 in chronically damaged liver. PLoS One 10, e0122758, doi:10.1371/journal.pone.0122758 (2015).
Xiao, Y. et al. Depletion of thymosin beta4 promotes the proliferation, migration, and activation of human hepatic stellate cells. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 34, 356–367, doi:10.1159/000363005 (2014).
Reyes-Gordillo, K. et al. Thymosin-beta4 (Tbeta4) blunts PDGF-dependent phosphorylation and binding of AKT to actin in hepatic stellate cells. The American journal of pathology 178, 2100–2108, doi:10.1016/j.ajpath.2011.01.025 (2011).
Elpek, G. O. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World journal of gastroenterology: WJG 20, 7260–7276, doi:10.3748/wjg.v20.i23.7260 (2014).
Basu, S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 189, 113–127 (2003).
Weber, L. W., Boll, M. & Stampfl, A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33, 105–136, doi:10.1080/713611034 (2003).
Iredale, J. P. et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. The Journal of clinical investigation 102, 538–549, doi:10.1172/JCI1018 (1998).
Pinzani, M. & Rombouts, K. Liver fibrosis: from the bench to clinical targets. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 36, 231–242, doi:10.1016/j.dld.2004.01.003 (2004).
Bataller, R. & Brenner, D. A. Liver fibrosis. J Clin Invest 115, 209–218, doi:10.1172/jci24282 (2005).
Friedman, S. L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiological reviews 88, 125–172, doi:10.1152/physrev.00013.2007 (2008).
Marcolin, E. et al. Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis. The Journal of nutrition 142, 1821–1828, doi:10.3945/jn.112.165274 (2012).
Recknagel, R. O., Glende, E. A. Jr., Dolak, J. A. & Waller, R. L. Mechanisms of carbon tetrachloride toxicity. Pharmacology & therapeutics 43, 139–154 (1989).
Nielsen, F., Mikkelsen, B. B., Nielsen, J. B., Andersen, H. R. & Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clinical chemistry 43, 1209–1214 (1997).
Mateos, R., Lecumberri, E., Ramos, S., Goya, L. & Bravo, L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 827, 76–82, doi:10.1016/j.jchromb.2005.06.035 (2005).
Bowling, A. C., Schulz, J. B., Brown, R. H. Jr. & Beal, M. F. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. Journal of neurochemistry 61, 2322–2325 (1993).
Meister, A. New aspects of glutathione biochemistry and transport–selective alteration of glutathione metabolism. Nutrition reviews 42, 397–410 (1984).
Campos, R., Garrido, A., Guerra, R. & Valenzuela, A. Silybin dihemisuccinate protects against glutathione depletion and lipid peroxidation induced by acetaminophen on rat liver. Planta medica 55, 417–419, doi:10.1055/s-2006-962055 (1989).
Ischiropoulos, H. Protein tyrosine nitration—An update. Archives of Biochemistry and Biophysics 484, 117–121, doi:10.1016/j.abb.2008.10.034 (2009).
Ebaid, H., Bashandy, S. A., Alhazza, I. M., Rady, A. & El-Shehry, S. Folic acid and melatonin ameliorate carbon tetrachloride-induced hepatic injury, oxidative stress and inflammation in rats. Nutrition & metabolism 10, 20, doi:10.1186/1743-7075-10-20 (2013).
Kiso, K. et al. The role of Kupffer cells in carbon tetrachloride intoxication in mice. Biological & pharmaceutical bulletin 35, 980–983 (2012).
Edwards, M. J., Keller, B. J., Kauffman, F. C. & Thurman, R. G. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicology and applied pharmacology 119, 275–279, doi:10.1006/taap.1993.1069 (1993).
Badger, D. A., Sauer, J. M., Hoglen, N. C., Jolley, C. S. & Sipes, I. G. The role of inflammatory cells and cytochrome P450 in the potentiation of CCl4-induced liver injury by a single dose of retinol. Toxicology and applied pharmacology 141, 507–519, doi:10.1006/taap.1996.0316 (1996).
Alric, L. et al. Reactive oxygen intermediates and eicosanoid production by Kupffer cells and infiltrated macrophages in acute and chronic liver injury induced in rats by CCl4. Inflammation Research 49, 700–707, doi:10.1007/s000110050649 (2000).
Czaja, M. J., Xu, J. & Alt, E. Prevention of carbon tetrachloride-induced rat liver injury by soluble tumor necrosis factor receptor. Gastroenterology 108, 1849–1854 (1995).
Morio, L. A. et al. Distinct roles of tumor necrosis factor-alpha and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicology and applied pharmacology 172, 44–51, doi:10.1006/taap.2000.9133 (2001).
Gieling, R. G., Wallace, K. & Han, Y. P. Interleukin-1 participates in the progression from liver injury to fibrosis. American journal of physiology. Gastrointestinal and liver physiology 296, G1324–1331, doi:10.1152/ajpgi.90564.2008 (2009).
Zhu, R. Z. et al. Protective effect of recombinant human IL-1Ra on CCl4-induced acute liver injury in mice. World journal of gastroenterology 16, 2771–2779 (2010).
Tak, P. P. & Firestein, G. S. NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107, 7–11, doi:10.1172/jci11830 (2001).
Barnes, P. J. & Karin, M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. The New England journal of medicine 336, 1066–1071, doi:10.1056/nejm199704103361506 (1997).
Xiao, J. et al. S-allylmercaptocysteine reduces carbon tetrachloride-induced hepatic oxidative stress and necroinflammation via nuclear factor kappa B-dependent pathways in mice. European journal of nutrition 51, 323–333, doi:10.1007/s00394-011-0217-0 (2012).
Zhu, J. et al. Thymosin β4 Attenuates Early Diabetic Nephropathy in a Mouse Model of Type 2 Diabetes Mellitus. American Journal of Therapeutics 22, 141–146, doi:10.1097/MJT.0b013e3182785ecc (2015).
Vasilopoulou, E., Winyard, P. J., Riley, P. R. & Long, D. A. The role of thymosin-beta4 in kidney disease. Expert opinion on biological therapy 15(Suppl 1), S187–190, doi:10.1517/14712598.2015.1009891 (2015).
Vasilopoulou, E. et al. Loss of endogenous thymosin beta4 accelerates glomerular disease. Kidney international 90, 1056–1070, doi:10.1016/j.kint.2016.06.032 (2016).
Conte, E. et al. Effects of thymosin β4 and its N-terminal fragment Ac-SDKP on TGF-β-treated human lung fibroblasts and in the mouse model of bleomycin-induced lung fibrosis. Expert opinion on biological therapy 15, 211–221, doi:10.1517/14712598.2015.1026804 (2015).
Cavasin, M. A., Liao, T. D., Yang, X. P., Yang, J. J. & Carretero, O. A. Decreased endogenous levels of Ac-SDKP promote organ fibrosis. Hypertension 50, 130–136, doi:10.1161/HYPERTENSIONAHA.106.084103 (2007).
Peng, H. et al. Antifibrotic effects of N-acetyl-seryl-aspartyl-Lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension 37, 794–800 (2001).
Chen, Y. W. et al. Preservation of basal AcSDKP attenuates carbon tetrachloride-induced fibrosis in the rat liver. Journal of hepatology 53, 528–536, doi:10.1016/j.jhep.2010.03.027 (2010).
Zhang, L. et al. Antifibrotic effect of N-acetyl-seryl-aspartyl-lysyl-proline on bile duct ligation induced liver fibrosis in rats. World journal of gastroenterology: WJG 18, 5283–5288, doi:10.3748/wjg.v18.i37.5283 (2012).
Kanasaki, K., Nagai, T., Nitta, K., Kitada, M. & Koya, D. N-acetyl-seryl-aspartyl-lysyl-proline: a valuable endogenous anti-fibrotic peptide for combating kidney fibrosis in diabetes. Frontiers in pharmacology 5, 70, doi:10.3389/fphar.2014.00070 (2014).
Philp, D. et al. Thymosin beta4 promotes matrix metalloproteinase expression during wound repair. Journal of cellular physiology 208, 195–200, doi:10.1002/jcp.20650 (2006).
Qiu, P., Kurpakus-Wheater, M. & Sosne, G. Matrix metalloproteinase activity is necessary for thymosin beta 4 promotion of epithelial cell migration. Journal of cellular physiology 212, 165–173, doi:10.1002/jcp.21012 (2007).
Okazaki, I., Watanabe, T., Hozawa, S., Arai, M. & Maruyama, K. Molecular mechanism of the reversibility of hepatic fibrosis: with special reference to the role of matrix metalloproteinases. Journal of gastroenterology and hepatology 15(Suppl), D26–32 (2000).
Philp, D. & Kleinman, H. K. Animal studies with thymosin beta, a multifunctional tissue repair and regeneration peptide. Annals of the New York Academy of Sciences 1194, 81–86, doi:10.1111/j.1749-6632.2010.05479.x (2010).
Ishak, K. et al. Histological grading and staging of chronic hepatitis. Journal of hepatology 22, 696–699 (1995).
Acknowledgements
This work was supported by Tianjin Research Program of Application Foundation and Advanced Technology (No. 15JCQNJC12900), Henan Research Program of Medical Science and Technology (No. 201403057) and Natural Science Foundation of China (No. U1504801).
Author information
Authors and Affiliations
Contributions
X.K.L. conceived the project. X.K.L., L.W. and C.C. were responsible for conception and design of the study and drafted the manuscript. X.K.L. performed the animal experiments and biochemical assays. C.C. contributed to some technical supports. L.W. and C.C. contributed to figure preparation and manuscript writing. X.K.L. supervised the entire research.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Wang, L. & Chen, C. Effects of exogenous thymosin β4 on carbon tetrachloride-induced liver injury and fibrosis. Sci Rep 7, 5872 (2017). https://doi.org/10.1038/s41598-017-06318-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06318-5
This article is cited by
-
Phillygenin Ameliorates Carbon Tetrachloride-Induced Liver Fibrosis: Suppression of Inflammation and Wnt/β-Catenin Signaling Pathway
Inflammation (2023)
-
Mucosal-associated invariant T cells restrict reactive oxidative damage and preserve meningeal barrier integrity and cognitive function
Nature Immunology (2022)
-
Mesenchymal stem cells ameliorate oxidative stress, inflammation, and hepatic fibrosis via Nrf2/HO-1 signaling pathway in rats
Environmental Science and Pollution Research (2021)
-
Locostatin Alleviates Liver Fibrosis Induced by Carbon Tetrachloride in Mice
Digestive Diseases and Sciences (2019)
-
Umbelliferone Ameliorates CCl4-Induced Liver Fibrosis in Rats by Upregulating PPARγ and Attenuating Oxidative Stress, Inflammation, and TGF-β1/Smad3 Signaling
Inflammation (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.