Abstract
Infectious hematopoietic necrosis virus (IHNV) and infectious pancreatic necrosis virus (IPNV) are important pathogens of salmon and trout. An active bivalent DNA vaccine was constructed with the glycoprotein gene of Chinese IHNV isolate Sn1203 and VP2–VP3 gene of Chinese IPNV isolate ChRtm213. Rainbow trout (5 g) were vaccinated by intramuscular injection with 1.0 µg of the bivalent DNA vaccine and then challenged with an intraperitoneal injection of IHNV, IPNV, or both, at 30 and 60 days post-vaccination (d.p.v.). High protection rates against IHNV were observed, with 6% and 10% cumulative mortality, respectively, compared with 90–94% in the mock-vaccinated groups. IPNV loads (531-fold and 135-fold, respectively) were significantly reduced in the anterior kidneys of the vaccinated trout. Significant protection against co-infection with IHNV and IPNV was observed, with cumulative mortality rates of 6.67% and 3.33%, respectively, compared with 50.0% and 43.3%, respectively, in the mock-vaccinated groups. No detectable infective IHNV or IPNV was recovered from vaccinated trout co-infected with IHNV and IPNV. The bivalent DNA vaccine increased the expression of Mx-1 and IFN-γ at 4, 7, and 15 d.p.v, and IgM at 21 d.p.v., and induced high titres (≥160) of IHNV and IPNV neutralizing antibodies at 30 and 60 d.p.v.
Similar content being viewed by others
Introduction
Infectious hematopoietic necrosis virus (IHNV) and infectious pancreatic necrosis virus (IPNV) are the causative agents of infectious hematopoietic necrosis (IHN) and infectious pancreatic necrosis (IPN), respectively. IHNV is an enveloped non-segmented single-stranded negative RNA virus in the genus Novirhabdovirus within the family Rhabdoviridae, which is responsible for major losses in salmonid production. The IHNV genome contains six genes in the order 3′-N–P–M–G–NV–L-5′, encoding the nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), non-virion protein (NV), and polymerase protein (L), respectively1. The virus infects several salmonid species2, with mortality rates of 80–90%. IPNV belongs to the family Birnaviridae and has a bisegmented genome of double-stranded RNA (segments A and B). Segment A encodes VP2 and VP3, the two major structural proteins of the virus. VP2 contains the determinants of antigenicity and virulence, and major neutralizing epitopes, and is important for IPNV immunogenicity3. VP3 is an internal structural protein in which some neutralizing epitopes have been identified4. Segment B contain a single open reading frame encoding VP1.
IHNV and IPNV are widespread in salmonid hatcheries from the Americas to Europe, Asia, and Australia5, 6. Fish that survive an IHNV or IPNV infection may become carriers of the virus for long periods and consequently transmit the virus to other susceptible fish or shellfish species7,8,9. Vaccination is one of the best methods for controlling these diseases. Various candidate IHNV vaccines have been designed, including attenuated vaccines10, 11, killed virus12, and DNA vaccines2, 13. Although the IHN DNA vaccine provided almost full protection to rainbow trout against IHNV infection, only one DNA vaccine has been commercialized, by the Canadian Food Inspection Agency14. Different kinds of IPNV vaccines have been reported for fish, including inactivated vaccines15, attenuated vaccines16, DNA vaccines17,18,19,20,21,22, and subunit vaccines23,24,25,26,27, but protection is not always complete5, 17, 28.
Although vaccines against IHNV and IPNV have been commercialized in several countries, outbreaks of IPNV and IHNV are still a major problem in modern aquaculture around the world. This may be because fish in the field can be exposed to several pathogens simultaneously. Therefore, multivalent vaccines against two or more pathogens are valuable tools in aquaculture29. Previous studies have demonstrated the co-infection of rainbow trout with IHNV and IPNV under natural conditions30, 31. Therefore, in this study, a bivalent DNA vaccine was constructed with the G gene of Chinese IHNV isolate Sn120332 and the VP2–VP3 genes of Chinese IPNV isolate ChRtm21333. Here, we report the successful design and construction of this bivalent DNA vaccine, designated pCh-IHN/IPN, which induced protective immune responses against IHNV infection, IPNV infection, and co-infection with IHNV and IPNV in the rainbow trout. This is the first study to construct a bivalent DNA vaccine targeting diverse viral pathogens in salmon and trout. This may be a feasible strategy for controlling IHN and IPN worldwide.
Results
Expression of antigen genes
Epithelioma papulosum cyprini (EPC) cells were transfected with the bivalent DNA vaccine pCh-IHN/IPN with a routine procedure (see Supplementary Figure S1 for a map of the bivalent DNA vaccine). The expression of both antigen genes was confirmed in vitro and in vivo with an immunofluorescence antibody test (IFAT) and western blotting, respectively. In the IFAT, specific green and red fluorescence was observed simultaneously in the same cells, which had been successfully transfected with pCh-IHN/IPN. Specific yellow fluorescence was observed in the merged images, whereas no specific fluorescent signal was observed in cells transfected with pcDNA3.1 (Fig. 1a). On a western blot, clear and specific bands were observed at 3, 7, and 15 days post-vaccination (d.p.v.) in muscle tissues from the sites of vaccine delivery in rainbow trout immunized with pCh-IHN/IPN, whereas no bands were observed in the lanes containing muscle tissues from empty-vector-immunized rainbow trout. The reference β-actin protein was observed in each lane (Fig. 1b) (full-length gels with markers are shown in Supplementary Figure S2). These results indicate that the G and VP2–VP3 genes were efficiently expressed by the pCh-IHN/IPN DNA vaccine in fish cells.
In vitro and in vivo expression of both antigen genes from the pCh-IHN/IPN DNA vaccine. An immunofluorescence antibody test confirmed the expression of both antigen genes in vitro (a). EPC cells transfected with pCh-IHN/IPN were incubated with an IHNV-glycoprotein-specific rabbit polyclonal antibody and a Cy3-conjugated goat anti-rabbit-IgG secondary antibody or a mouse anti-IPNV-VP2 polyclonal antibody and a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse-IgG antibody. EPC cells transfected with the pcDNA3.1 vector and treated identically were used as the negative control. Western blotting of muscle samples from vaccinated rainbow trout (n = 5), collected at 3, 7, and 15 days post-vaccination, detected the expression of both antigen genes in vivo (b). Muscle samples from pcDNA3.1-mock-vaccinated trout were used as the negative controls. β-Actin was used as the reference protein.
Protection against IHNV afforded by the DNA vaccine
Replicate groups of 50 rainbow trout fry (5 g), intramuscularly (i.m.) injected with 1.0 µg of pCh-IHN/IPN or treated with the various controls, were challenged at 30 or 60 d.p.v. The rainbow trout were significantly protected at 30 and 60 days compared with the pcDNA3.1-mock-vaccinated group (P < 0.05), and showed 6–10% cumulative mortality compared with 90–94% cumulative mortality in the pcDNA3.1-mock-vaccinated group. No mortality was observed in the sham-infected group (Fig. 2a), and no significant difference in mortality was observed between any replicates within any of the treatment groups in either experiment (data not shown). There was no significant difference in the relative percentage survival (RPS) at 30 and 60 d.p.v. (Fig. 2b; P > 0.05).
Cumulative percentage mortality (CPM) curves for pCh-IHN/IPN-vaccinated rainbow trout challenged with IHNV strain Sn1203 at 30 or 60 d.p.v. Rainbow trout injected with plasmid pcDNA3.1 (vector) were used as the negative controls. Duplicate groups of 30 fish were challenged with an intraperitoneal injection of 102 plaque-forming units of IHNV Sn-1203 per fish. No mortality was observed in the sham-infected control group, and no significant differences were observed in mortality between any replicates within any treatment group.
IPNV load
Duplicate groups of 50 rainbow trout that were vaccinated i.m. with 1.0 µg of pCh-IHN/IPN or treated with the various controls were challenged with an intraperitoneal (i.p.) injection of IPNV ChRtm213 at 30 and 60 d.p.v. We evaluated the protection afforded by the bivalent DNA vaccine against IPNV by measuring the viral load in the anterior kidney in terms of VP1 gene expression 15 days after challenge (Fig. 3). The fold changes in the viral load in the bivalent-DNA-vaccine-treated group were calculated relative to those in the pcDNA3.1-mock-vaccinated group. The EF-α gene was used to normalize VP1 gene expression, and the individual VP1 gene expression levels and average expression levels were described separately. The levels of virus varied greatly in the five phosphate-buffered saline (PBS)-injected fish and empty-vector-injected fish (Fig. 3a,b). The average fold changes in the viral load in fish challenged at 30 and 60 d.p.v. were 531-fold and 135-fold, respectively, and IPNV was only detected in one of the five fish sampled in both cases. These results indicate that the average viral load was significantly reduced in fish vaccinated with pCh-IHN/IPN compared with that in the empty-vector-treated group (Fig. 3b,d; P < 0.05).
Quantitative reverse transcription–PCR determination of IPNV load using VP1 gene expression in the anterior kidneys of vaccinated rainbow trout (n = 5) challenged with IPNV strain ChRtm213 at 30 or 60 d.p.v. IPNV loads were measured at 15 days post challenge. Rainbow trout injected with plasmid pcDNA3.1 alone or with PBS were used as the negative controls. EF-α was used to normalize the expression of the IPNV VP1 gene. Individual VP1 gene expression levels (a,c) and average expression levels (b,d) are shown separately. Differences were analysed, and different symbols above the bars indicate significant differences (P < 0.05).
Protection conferred by the vaccine against co-infection
Replicate groups of 30 rainbow trout fry (5 g), injected i.m. with 1.0 µg of pCh-IHN/IPN or treated with the various controls, were challenged with a mixture of IHNV sn1203 and IPNV ChRtm213 at 30 or 60 d.p.v. In this experiment, the pCh-IHN/IPN-vaccinated rainbow trout challenged at 30 and 60 d.p.v. were protected significantly better than the pcDNA3.1-vaccinated fish (P < 0.05), with 3.33–6.67% cumulative mortality compared with 43.3–50.0% in the pcDNA3.1-mock-vaccinated groups. There was no significant difference in RPS at 30 and 60 d.p.v. (Fig. 4a; P > 0.05). There was no cross-reaction between IHNV neutralizing antibodies (NAbs) and IPNV or between IPNV NAbs and IHNV (see Supplementary Figure S3 for cross-reaction tests). Viruses were recovered at 15 days post-challenge from the tissue pools from the challenged trout in Chinook salmon embryo (CHSE-214) cells, analysed with an IFAT, and quantified with flow cytometry. The proportions of IHNV-infected cells and IPNV-infected cells were 1.8% and 0%, respectively (challenged at 30 d.p.v.) and 0.42% and 0.056%, respectively (challenged at 60 d.p.v.) in cells inoculated with tissues from pCh-IHN/IPN-vaccinated trout. However, they were 8.1% and 60.5%, respectively (30 d.p.v.) and 6.0% and 66.2%, respectively (60 d.p.v.) in cells inoculated with tissues from pcDNA3.1-mock-vaccinated trout (Fig. 4b,c). Thus, significantly fewer IHNV and IPNV were detected in the CHSE-214 cells inoculated with tissues from the pCh-IHN/IPN-vaccinated trout than in cells inoculated with tissues from the pcDNA3.1-mock-vaccinated trout (Fig. 4d; P < 0.05).
Evaluation of the protection afforded by the bivalent DNA vaccine against co-infection with IHNV and IPNV. Cumulative percentage mortality curves (a) and flow-cytometric quantification of IHNV and IPNV in the tissues of rainbow trout (n = 5) challenged with IHNV and IPNV at 30 and 60 d.p.v. (b,c) Dual protection afforded by the vaccine against co-infection. Q1: IPNV-infected cells; Q2: dual-infected cells; Q3: uninfected cells; Q4: IHNV-infected cells. The average proportions of virus-infected cells are shown in the histograms (d). Rainbow trout injected with plasmid pcDNA3.1 were used as the negative controls. *Significantly different (P < 0.05).
Gene expression
The expression levels of Mx-1 and interferon γ (IFN-γ) in the anterior kidneys of the vaccinated fish were determined at 1, 4, 7, 15, and 21 d.p.v., and those of IgM, CD4, and CD8 were assessed at 15 and 21 d.p.v. The fold changes in expression were calculated relative to the levels in the pcDNA3.1-mock-vaccinated groups. The expression of the Mx-1 and IFN-γ genes was significantly upregulated in the pCh-IHN/IPN-treated trout at 4, 7, and 15 d.p.v. (Fig. 5; P < 0.05). The highest fold changes in Mx-1 and IFN-γ were 53-fold and 60-fold, respectively, which were observed at 15 and 7 d.p.v., respectively (Fig. 5). These results indicate that the bivalent DNA vaccine induced nonspecific immune responses in the rainbow trout as early as 4 d.p.v., which lasted until 15 d.p.v. The IgM expression detected in the kidneys at 15 d.p.v. increased significantly at 21 d.p.v (around 12-fold; P < 0.05). The expression levels of CD4 and CD8 were not as high as those of IgM, but significant changes were still observed at 21 d.p.v. (Fig. 5; P < 0.05).
Fold changes in the expression of immune-related genes induced by the combined DNA vaccine in rainbow trout (n = 5). β-Actin was used to normalize the transcription of each gene in anterior kidney samples from rainbow trout at 1, 4, 7, 15, and 21 days post-vaccination (d.p.v.). The fold changes in their expression were calculated relative to their expression in the pcDNA3.1-vaccinated group. The differences were analysed, and different symbols above the bars indicate significant differences (P < 0.05).
Neutralizing antibodies
Serum samples from vaccinated fish (n = 10) that had not been challenged with either virus were tested for seroconversion at 30 and 60 d.p.v. IHNV NAbs and IPNV NAbs were detected in all the serum samples. Most pCh-IHN/IPN-vaccinated fish had high titres (≥160) of both IHNV NAbs and IPNV NAbs (Table 1), whereas no NAbs were detected in the fish injected with the pcDNA3.1 vector (not shown). Positive control sera used in this study had titres >160 in all assays. The half-maximal inhibitory concentrations (IC50) of IHNV NAbs and IPNV NAbs were calculated as fold dilutions of serum. The IC50 of IHNV NAbs and IPNV NAbs were around 133 and 135, respectively, at 30 d.p.v, and 140 and 157 respectively, at 60 d.p.v. For IPNV, IC50 was significantly higher at 60 d.p.v. than at 30 d.p.v. (Table 1; P < 0.05). These results indicated that the bivalent DNA vaccine induced specific immune responses in the rainbow trout as early as 30 d.p.v., which persisted until 60 d.p.v.
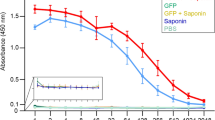
To determine the specificity of the antibodies elicited against IHNV and IPNV, a serum pool was used as the first antibody in an IFAT. Specific red and green fluorescence was observed in virally infected cells incubated with serum extracted from the pCh-IHN/IPN-vaccinated trout, whereas no specific fluorescent signal was observed in the virally infected cells incubated with serum extracted from the pcDNA3.1-vaccinated trout (Fig. 6).
Specificity of NAb serum for IHNV and IPNV. Virus-infected cells were incubated with NAb-containing serum (from vaccinated trout), a rabbit polyclonal antibody directed against rainbow trout IgM Fc, and a fluorescently labelled secondary antibody. Sera from trout vaccinated with pcDNA3.1 were used as the negative controls. NC: negative control.
Discussion
The first outbreaks of IHN and IPN were recorded in juvenile fish in rainbow trout hatcheries in northeast China in 1985 and 198634, respectively. The IHNV and IPNV strains have evolved in the decades since they were first introduced into China. The world’s IHNV isolates have been divided into five genogroups, M, L, U, E, and J, based on the glycoprotein gene sequence. All Chinese IHNV isolates belong to genogroup J, together with the other Asian IHNV strains35, 36. Although there is only one IHN viral serotype37, mutations in the G gene can cause low virulence or produce neutralization-resistant variant IHNV strains38. Previous IHNV vaccines have been generated with genogroup M and U IHNV isolates13, 37, 39. The worldwide IPNV isolates consist of nine serotypes33, and IPNV vaccines have been developed with the Spanish IPNV strain Sp5, 22, 24, 40, the Norwegian strain NVI01541, and the American VR299 strain16. There is no report of a vaccine developed from a Chinese IHNV or IPNV strain. IHN and IPN remain the most important diseases threatening salmonid aquaculture in China. Therefore, in this study, a bivalent DNA vaccine was produced from Chinese IHNV and IPNV strains. In this bivalent DNA vaccine, the IHNV G gene was inserted downstream from the Cytomegalovirus (CMV) promoter. The IPNV VP2 and VP3 genes were linked by a sequence encoding a flexible linker peptide (Gly4Ser)3 and inserted downstream from the internal ribosome entry site (IRES), which is responsible for expressing the VP2–VP3 genes. The bivalent DNA vaccine provided robust protection against Chinese IHNV and IPNV and against co-infection with the two viruses.
Previous studies have shown that IHNV DNA vaccines delivered by i.m. injection are safe, stable, and quickly produced, and induce robust nonspecific and specific immune responses42. A single nanogram dose provided almost complete protection to rainbow trout (3 g)37. In this study, we did not test the protection efficacy of lower doses. In a previous study43, the minimal dose of 1 μg of an IHNV DNA vaccine was determined and used throughout that study, and the bivalent DNA vaccine in the present study was constructed based on that IHNV DNA vaccine. Therefore, we used a single dose of 1.0 µg to vaccinate the rainbow trout (5 g). This dose is far lower than that used in mammals37, 44, 45. Fortunately, high protective efficacy conferred by the bivalent DNA vaccine was obtained at this dose. Protection as strong as that conferred by a DNA vaccine was also conferred by an attenuated IHNV vaccine delivered by nasal immunization. However, the attenuated vaccine was virulent and caused a low rate of mortality in the vaccinated trout39. Therefore, the IHNV DNA vaccine was safer than the attenuated IHNV vaccine. Some progress has been made in developing oral IHNV vaccines, an oral DNA vaccine46 and an oral yeast-surface-displayed vaccine47, which provide significant protection to trout. However, their protective efficacy was not as high as that of a DNA vaccine delivered by injection. These findings indicate that although the injection protocol is labour-intensive and time-consuming, an IHNV DNA vaccine delivered by i.m. injection is very safe and highly efficient.
Most vaccine efficacy tests determine the post-challenge RPS as a measure of protection. However, no mortality was detected in our IPNV challenge experiments, so the IPNV load or IPNV NAb titre was measured in the vaccinated trout as an indicator of protective efficacy, as described in previous studies9, 27, 40. In this study, both the IPNV load and IPNV NAb titre were determined. The IPNV load decreased dramatically and a high IPNV NAb titre was generated in the bivalent-DNA-vaccine-treated trout, indicating that good protection against IPNV infection was afforded by the bivalent DNA vaccine. The structural protein(s) of many viruses form specific aggregates through self-assembly in a variety of different expression systems48. These virally derived particles generally imitate the native viruses in size and morphology, and are referred to as ‘virus-like particles’ (VLPs)27. When viral proteins aggregate to form particles that do not mimic the viral capsid in size, but still form predictable complex(es), they are referred to as ‘subviral particles’ (SVPs). In previous studies, VLPs26 and SVPs27 against IPNV were designed with the VP2–VP3 or VP2 genes alone, and their protective efficacy was confirmed with IPNV NAb titration or by measuring the IPNV load. In our study, VP2–VP3 expressed by the bivalent DNA vaccine vector induced specific immune responses and reduced the IPNV load in the vaccinated rainbow trout, indicating that the VP2–VP3 protein linked by (Gly4Ser)3 was efficiently expressed and correctly folded. We did not determine whether VP2–VP3 was assembled into VLPs in this study. Since VLPs assemble in a variety of different expression systems, we speculate that the VP2–VP3 probably assembles into VLPs in fish cells.
Previous studies have reported that interactions between IHNV and IPNV can cause a loss of infectivity and reduce the infective titre49, 50. In the present study, the cumulative percentage mortality (CPM) of the mock-vaccinated rainbow trout co-infected with IHNV and IPNV was significantly lower than that of fish challenged with IHNV alone, which is consistent with previous results. In addition to the measurement of CPM, the viruses were recovered from the vaccinated trout and quantified with flow cytometry. CPM and viral quantification both showed that the pCh-IHN/IPN-vaccinated rainbow trout were significantly protected from co-infection, and almost no IHNV or IPNV was recovered from them. However, significantly high levels of IHNV and IPNV were recovered from the pcDNA3.1-mock-vaccinated rainbow trout, confirming that the bivalent DNA vaccine protected trout from co-infection by IHNV and IPNV.
Farmed fish are susceptible to different infectious disease agents, including viruses and bacteria. In a previous study, rainbow trout were doubly nasally vaccinated with an attenuated IHNV vaccine and a formalin-killed enteric red mouth bacterium29. The authors reported that dual vaccination against two different pathogens via the nasal route is a very effective vaccination strategy in aquaculture, particularly when the two vaccines are introduced separately into different nares, although this makes dual vaccination more complex than single vaccination. Therefore, multivalent vaccines against two or more pathogens are more practical when vaccinating cultured animals. The bivalent DNA vaccine produced in this study contained the two antigen genes in one vector. The vaccine did not require premixing before vaccination, and a single injection of the vaccine protected the rainbow trout from attack by both viruses. Although the injection protocol is labour-intensive and time-consuming, the fact that a single injection can provide protection against two acute viral pathogens is very attractive. Therefore, the bivalent DNA vaccine should play an important role in the control of IHN and IPN in China and inspire more strategies for controlling two or more pathogens with one vaccine.
Methods
Ethics statement
In this study, all animal experiments strictly followed protocols approved by the Animal Welfare Committee of China Agricultural University (permit number: XK662) and the study was carried out in strict accordance with the guidelines and regulations established by this committee.
Fish, viral strains, and cell lines
Specific-pathogen-free rainbow trout (mean weight, 5 g) were maintained in 50 L tanks with circulating water at 15 °C and fed a dry pelleted diet ad libitum. The Ja serotype of IPNV ChRtm21333 and the J genotype IHNV Sn120332 are laboratory stocks. IPNV ChRtm213 was propagated in CHSE-214 cells and IHNV Sn1203 was propagated in EPC cells, as described previously16, 51. The EPC cell line was originally deposited in the American Type Culture Collection (ATCC) as a carp (Cyprinus carpio) cell line, but has since been identified as derived from fathead minnow (Pimephales promelas; ATCC CRL-2872). It has also been used as a cell line for plasmid transfection because of its high transfection efficiency20.
Constructing the bivalent DNA vaccine
The G gene of IHNV Sn1203 was cloned into the multiple cloning site of the pcDNA3.1 vector using BamH I and Not I, to construct the recombinant plasmid pcDNA-IHN. The VP2 and VP3 genes of IPNV ChRtm213 were linked to a sequence encoding a (Gly4Ser)3 linker52 (Xu et al., 2014a) with overlapping PCR, to create the fused VP2–VP3 genes. The VP2–VP3 genes were cloned downstream from the IRES in pT-IRES with Sal I and Xho I, to construct the fused IRES–VP2–VP3 genes. The IRES–VP2–VP3 genes were cloned downstream from the G gene in the pcDNA-IHN vector with Not I and Xho I, to construct the bivalent DNA vaccine pCh-IHN/IPN. The bivalent DNA vaccine pCh-IHN/IPN and pcDNA3.1 were prepared with a plasmid extraction kit (Tiangen, Shanghai, China). A map of the recombinant plasmid pCh-IHN/IPN is given in Supplementary Figure S1 with the SnapGene Viewer software.
In vitro expression of antigen genes
The in vitro expression of the G and VP2–VP3 genes from the bivalent DNA vaccine in fish cells was confirmed by the transfection of EPC cells with the vaccine, followed by an IFAT, as described previously6. A rabbit polyclonal antibody directed against IHNV glycoprotein53 and a Cy3-conjugated goat anti-rabbit-IgG secondary antibody (cat. no. ab97075; Abcam, Cambridge, England) were used to determine the expression of the G protein, and a mouse anti-IPNV-VP3 polyclonal antibody (prepared with routine procedures) and a FITC-conjugated goat anti-mouse-IgG secondary antibody (cat. no. ab6785; Abcam) were used to detect the expression of the VP2–VP3 fusion protein.
In vivo expression of antigen genes
A dose of 1.0 µg of pCh-IHN/IPN was used in all vaccination experiments. Rainbow trout were anesthetized by immersion in tricaine methane sulfonate (MS-222; Sigma, St. Louis, MO, USA), and the bivalent DNA vaccine was delivered by i.m. injection at the base of the dorsal fin. Rainbow trout injected with the empty pcDNA3.1 vector were used as the negative control. The in vivo expression of the G and VP2–VP3 proteins was confirmed with the western blotting of muscle samples from rainbow trout (n = 5) vaccinated with pCh-IHN/IPN or pcDNA3.1, collected 3, 7, and 15 d.p.v. β-Actin was used as the reference protein. Western blotting was performed as described previously54, and the expression of the G protein was visualized with a rabbit anti-IHNV-glycoprotein polyclonal antibody and a horseradish peroxidase (HRP)-conjugated goat anti-rabbit-IgG secondary antibody (cat. no. sc-2004; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The expression of the VP2–VP3 protein was visualized with a mouse anti-IPNV-VP3 polyclonal antibody and an HRP-conjugated goat anti-mouse-IgG secondary antibody (cat. no. sc-2005; Santa Cruz Biotechnology).
Challenge with IHNV and CPM
Rainbow trout vaccinated with pCh-IHN/IPN or empty pcDNA3.1 were challenged with IHNV Sn1203 at 30 and 60 d.p.v. Duplicate groups of 30 fish were anesthetized and injected i.p. with 102 plaque-forming units (PFU) of IHNV Sn1203 in 100 µl of phosphate-buffered saline (PBS). Mock infections were performed by replacing the viral suspension with PBS. CPM was recorded daily in parallel experiments for 21 days. RPS was then calculated with the formula: RPS = [1 − (% mortality of fish given vaccine/% mortality of fish given pcDNA3.1)] × 10022.
Challenge with IPNV and IPNV load
Duplicate groups of 50 rainbow trout vaccinated with pCh-IHN/IPN or pcDNA3.1 were injected i.p. with IPNV ChRtm213 at a dose of 106 PFU in 100 µl of PBS at 30 or 60 d.p.v. Trout vaccinated with the pcDNA3.1 vector alone were challenged and used as the negative control. The anterior kidneys were collected from the rainbow trout (n = 5) vaccinated with pCh-IHN/IPN or empty pcDNA3.1 at 15 days after challenge to evaluate the effect of the vaccine on viral clearance and the viral load20, 27. RNA was extracted from the individual samples with TRIzol Reagent (cat. no. 15596-018; Invitrogen, CA, USA). IPNV VP1 gene expression was determined with quantitative reverse transcription–PCR (qRT–PCR) using the One Step SYBR PrimeScript PLUS RT–PCR Kit (Perfect Real Time) (cat. no. RR096A; Takara, Shiga, Japan) and previously published primers55. EF1-α was used as the reference gene against which the expression of the IPNV VP1 gene was normalized27.
Simultaneous challenge with IHNV and IPNV
To determine the protection afforded by the bivalent DNA vaccine against co-infection by IHNV and IPNV, rainbow trout vaccinated with pCh-IHN/IPN or empty pcDNA3.1 were challenged with an i.p. injection of a mixture of 102 PFU of IHNV Sn1203 and 106 PFU of IPNV ChRtm213 in 100 µl of PBS at 30 or 60 d.p.v. CPM was recorded daily for 21 days in parallel experiments.
Fish (n = 5) that survived for 15 days after challenge (when mortality ceased) were sampled. Their liver, spleen, and anterior kidney tissues were removed and pooled, and the virus was propagated in CHSE-214 cells. When subtle cytopathic effects were detected, the CHSE-214 cells were incubated with primary and secondary antibodies, as described for the IFAT in this study. The CHSE-214 cultures were then digested with 0.25% trypsin, and the IHNV and IPNV antigens in the CHSE-214 cells were quantified with a FACSAria™ Cell Sorter (BD Biosciences, San Jose, CA, USA).
Gene expression
The expression levels of IFN-γ and Mx-1, markers of the nonspecific innate immune response to viruses56, were measured at 1, 4, 7, 15, and 21 d.p.v. to assess the nonspecific immune response induced by the bivalent vaccine. On days 15 and 21 post-vaccination, the expression of IgM57 was also determined to evaluate the adaptive immune response. The transcription of CD4 and CD8 Th-cell markers was also assessed17. The RNA from the anterior kidney samples (n = 5) was prepared with TRIzol Reagent. qRT–PCR was performed with the One Step SYBR PrimeScript PLUS RT–PCR Kit (Perfect Real Time; Takara). The β-actin gene was used as the reference gene against which the gene expression levels were normalized58. The fold changes in gene expression were calculated relative to their expression in the mock-vaccinated control group (treated with pcDNA3.1 in PBS).
Characterization and titration of NAbs
Blood samples were collected by caudal transection from rainbow trout (n = 10) vaccinated with pCh-IHN/IPN or pcDNA 3.1 at 30 or 60 d.p.v., and the sera were prepared with a routine procedure59. The NAb titre of each serum sample was determined with a complement-dependent neutralization assay. Titres ≥20 were considered positive and titres <20 were considered negative60. The IC50 values of the pooled sera were measured with a routine procedure and calculated with the software GraphPad Prism 5. Serum samples were also used as the first antibody in an IFAT to characterize their specificity for IHNV and IPNV. The IFAT was performed as described in a previous study6, and a rabbit polyclonal antibody directed against rainbow trout IgM Fc61 was used to link the first antibody in the serum to fluorescently labelled goat anti-rabbit antibodies. EPC cells were used for the titration of IHNV NAbs, and CHSE-214 cells were used for the titration of IPNV NAbs. Serum samples from trout treated with pcDNA3.1 were used as the negative control.
Statistical analysis
Analysis of variance was used to assess the differences in gene expression levels. Student’s t test was used to compare some paired samples. P < 0.05 was considered significant.
References
Abbadi, M. et al. Molecular Evolution and Phylogeography of Co-circulating IHNV and VHSV in Italy. Frontiers in microbiology 7, 1306, doi:10.3389/fmicb.2016.01306 (2016).
LaPatra, S. E. et al. Protection of rainbow trout against infectious hematopoietic necrosis virus four days after specific or semi-specific DNA vaccination. Vaccine 19, 4011–4019 (2001).
Tarrab, E. et al. Evidence of a major neutralizable conformational epitope region on VP2 of infectious pancreatic necrosis virus. The Journal of general virology 76(Pt 3), 551–558, doi:10.1099/0022-1317-76-3-551 (1995).
Ye, C. et al. Inhibition of antiviral innate immunity by birnavirus VP3 protein via blockage of viral double-stranded RNA binding to the host cytoplasmic RNA detector MDA5. Journal of virology 88, 11154–11165, doi:10.1128/JVI.01115-14 (2014).
Dadar, M. et al. Protective and immunogenic effects of Escherichia coli-expressed infectious pancreatic necrosis virus (IPNV) VP2-VP3 fusion protein in rainbow trout. Fish & shellfish immunology 47, 390–396, doi:10.1016/j.fsi.2015.09.007 (2015).
Xu, L. M. et al. High throughput screening of recombinant antibodies against infectious hematopoietic necrosis virus from a combinatorial antibody library. Aquaculture 460, 32–36 (2016).
Julin, K., Johansen, L. H., Sommer, A. I. & Jorgensen, J. B. Persistent infections with infectious pancreatic necrosis virus (IPNV) of different virulence in Atlantic salmon, Salmo salar L. Journal of fish diseases 38, 1005–1019, doi:10.1111/jfd.12317 (2015).
Wargo, A. R., Scott, R. J., Kerr, B. & Kurath, G. Replication and shedding kinetics of infectious hematopoietic necrosis virus in juvenile rainbow trout. Virus research 227, 200–211, doi:10.1016/j.virusres.2016.10.011 (2017).
de Las Heras, A. I., Perez Prieto, S. I. & Rodriguez Saint-Jean, S. In vitro and in vivo immune responses induced by a DNA vaccine encoding the VP2 gene of the infectious pancreatic necrosis virus. Fish & shellfish immunology 27, 120–129, doi:10.1016/j.fsi.2008.11.021 (2009).
Fryer, J. L., Rohovec, J. S., Tebbit, G. L., Mcmichael, J. S. & Pilcher, K. S. Vaccination for control of infectious diseases in Pacific Salmon. Fish Pathology 10, 155–164 (1976).
Ristow, S. S. et al. Responses of cloned rainbow trout Oncorhynchus mykiss to an attenuated strain of infectious hematopoietic necrosis virus. Diseases of aquatic organisms 42, 163–172, doi:10.3354/dao042163 (2000).
Anderson, E., Clouthier, S., Shewmaker, W., Weighall, A. & LaPatra, S. Inactivated infectious haematopoietic necrosis virus (IHNV) vaccines. Journal of fish diseases 31, 729–745, doi:10.1111/j.1365-2761.2008.00960.x (2008).
Anderson, E. D. et al. Genetic immunization of rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis virus. Molecular marine biology and biotechnology 5, 114–122 (1996).
Tonheim, T. C., Bøgwald, J. & Dalmo, R. A. What happens to the DNA vaccine in fish? A review of current knowledge. Fish & shellfish immunology 25, 1–18 (2008).
Dixon, P. F. & Hill, B. J. Inactivation of infectious pancreatic necrosis virus for vaccine use. Journal of fish diseases 6, 399–409 (2010).
Rivas-Aravena, A. et al. Evaluation of the immune response against immature viral particles of infectious pancreatic necrosis virus (IPNV): a new model to develop an attenuated vaccine. Vaccine 30, 5110–5117, doi:10.1016/j.vaccine.2012.05.062 (2012).
Ballesteros, N. A., Rodriguez Saint-Jean, S. & Perez-Prieto, S. I. Food pellets as an effective delivery method for a DNA vaccine against infectious pancreatic necrosis virus in rainbow trout (Oncorhynchus mykiss, Walbaum). Fish & shellfish immunology 37, 220–228, doi:10.1016/j.fsi.2014.02.003 (2014).
Ballesteros, N. A., Saint-Jean, S. S., Perez-Prieto, S. I. & Coll, J. M. Trout oral VP2 DNA vaccination mimics transcriptional responses occurring after infection with infectious pancreatic necrosis virus (IPNV). Fish & shellfish immunology 33, 1249–1257, doi:10.1016/j.fsi.2012.09.004 (2012).
Mikalsen, A. B. et al. Protection of atlantic salmon Salmo salar against infectious pancreatic necrosis after DNA vaccination. Diseases of aquatic organisms 60, 11–20, doi:10.3354/dao060011 (2004).
Cuesta, A. et al. An active DNA vaccine against infectious pancreatic necrosis virus (IPNV) with a different mode of action than fish rhabdovirus DNA vaccines. Vaccine 28, 3291–3300, doi:10.1016/j.vaccine.2010.02.106 (2010).
de las Heras, A. I., Rodriguez Saint-Jean, S. & Perez-Prieto, S. I. Immunogenic and protective effects of an oral DNA vaccine against infectious pancreatic necrosis virus in fish. Fish & shellfish immunology 28, 562–570, doi:10.1016/j.fsi.2009.12.006 (2010).
Ballesteros, N. A., Rodriguez Saint-Jean, S. & Perez-Prieto, S. I. Immune responses to oral pcDNA-VP2 vaccine in relation to infectious pancreatic necrosis virus carrier state in rainbow trout Oncorhynchus mykiss. Veterinary immunology and immunopathology 165, 127–137, doi:10.1016/j.vetimm.2015.04.001 (2015).
Valdenegro-Vega, V. A., Cook, M., Crosbie, P., Bridle, A. R. & Nowak, B. F. Vaccination with recombinant protein (r22C03), a putative attachment factor of Neoparamoeba perurans, against AGD in Atlantic salmon (Salmo salar) and implications of a co-infection with Yersinia ruckeri. Fish & shellfish immunology 44, 592–602, doi:10.1016/j.fsi.2015.03.016 (2015).
Min, L. et al. Immunogenicity of Lactobacillus-expressing VP2 and VP3 of the infectious pancreatic necrosis virus (IPNV) in rainbow trout. Fish & shellfish immunology 32, 196–203, doi:10.1016/j.fsi.2011.11.015 (2012).
Moon, C. H. et al. Comparison of the immunogenicity of recombinant VP2 and VP3 of infectious pancreatic necrosis virus and marine birnavirus. Archives of virology 149, 2059–2068 (2004).
Shivappa, R. B. et al. Development of a subunit vaccine for infectious pancreatic necrosis virus using a baculovirus insect/larvae system. Developments in biologicals 121, 165–174 (2005).
Dhar, A. K., Bowers, R. M., Rowe, C. G. & Allnutt, F. C. Expression of a foreign epitope on infectious pancreatic necrosis virus VP2 capsid protein subviral particle (SVP) and immunogenicity in rainbow trout. Antiviral research 85, 525–531, doi:10.1016/j.antiviral.2009.12.009 (2010).
Dhar, A. K., Manna, S. K. & Thomas Allnutt, F. C. Viral vaccines for farmed finfish. Virusdisease 25, 1–17, doi:10.1007/s13337-013-0186-4 (2014).
LaPatra, S., Kao, S., Erhardt, E. B. & Salinas, I. Evaluation of dual nasal delivery of infectious hematopoietic necrosis virus and enteric red mouth vaccines in rainbow trout (Oncorhynchus mykiss). Vaccine 33, 771–776, doi:10.1016/j.vaccine.2014.12.055 (2015).
Alonso, M., Rodriguez Saint-Jean, S. & Perez-Prieto, S. I. Virulence of infectious hematopoietic necrosis virus and Infectious pancreatic necrosis virus coinfection in rainbow trout (Oncorhynchus mykiss) and nucleotide sequence analysis of the IHNV glycoprotein gene. Archives of virology 148, 1507–1521, doi:10.1007/s00705-003-0116-7 (2003).
Byrne, N., Castric, J., Lamour, F., Cabon, J. & Quentel, C. Study of the viral interference between infectious pancreatic necrosis virus (IPNV) and infectious haematopoietic necrosis virus (IHNV) in rainbow trout (Oncorhynchus mykiss). Fish & shellfish immunology 24, 489–497, doi:10.1016/j.fsi.2007.08.010 (2008).
Xu, L. M. et al. Epitope mapping of the infectious hematopoietic necrosis virus glycoprotein by flow cytometry. Biotechnology letters 36, 2109–2116, doi:10.1007/s10529-014-1586-2 (2014).
Ji, F. et al. Complete genomic sequence of an infectious pancreatic necrosis virus isolated from rainbow trout (Oncorhynchus mykiss) in China. Virus genes. doi:10.1007/s11262-016-1408-9 (2016).
Niu, L. Q. & Zhao, Z. Z. The Epidemiological IHN and IPN of Rainbow Trout in Northeast China. Journal of Fishery Sciences of China 12, 327–332 (1988).
Jia, P. et al. Determination of the complete genome sequence of infectious hematopoietic necrosis virus (IHNV) Ch20101008 and viral molecular evolution in China. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases 27, 418–431, doi:10.1016/j.meegid.2014.08.013 (2014).
Kurath, G. et al. Phylogeography of infectious haematopoietic necrosis virus in North America. The Journal of general virology 84, 803–814, doi:10.1099/vir.0.18771-0 (2003).
Corbeil, S., LaPatra, S. E., Anderson, E. D. & Kurath, G. Nanogram quantities of a DNA vaccine protect rainbow trout fry against heterologous strains of infectious hematopoietic necrosis virus. Vaccine 18, 2817–2824 (2000).
Kim, C. H., Winton, J. R. & Leong, J. C. Neutralization-resistant variants of infectious hematopoietic necrosis virus have altered virulence and tissue tropism. Journal of virology 68, 8447–8453 (1994).
Salinas, I., LaPatra, S. E. & Erhardt, E. B. Nasal vaccination of young rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis and enteric red mouth disease. Developmental and comparative immunology 53, 105–111, doi:10.1016/j.dci.2015.05.015 (2015).
Martinez-Alonso, S., Vakharia, V. N., Saint-Jean, S. R., Perez-Prieto, S. & Tafalla, C. Immune responses elicited in rainbow trout through the administration of infectious pancreatic necrosis virus-like particles. Developmental and comparative immunology 36, 378–384, doi:10.1016/j.dci.2011.07.010 (2012).
Munang’andu, H. M. et al. Comparison of vaccine efficacy for different antigen delivery systems for infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L.) in a cohabitation challenge model. Vaccine 30, 4007–4016, doi:10.1016/j.vaccine.2012.04.039 (2012).
Guerrero-Rodriguez, J. et al. Virus-like particles from Escherichia Coli-derived untagged papaya ringspot virus capsid protein purified by immobilized metal affinity chromatography enhance the antibody response against a soluble antigen. Molecular biotechnology 56, 1110–1120, doi:10.1007/s12033-014-9791-8 (2014).
Xu, L. et al. A effective DNA vaccine against diverse genotype J infectious hematopoietic necrosis virus strains prevalent in China. Vaccine 35, 2420–2426, doi:10.1016/j.vaccine.2017.03.047 (2017).
Lee, H. et al. Preclinical evaluation of multi antigenic HCV DNA vaccine for the prevention of Hepatitis C virus infection. Scientific reports 7, 43531, doi:10.1038/srep43531 (2017).
Larocca, R. A. et al. Vaccine protection against Zika virus from Brazil. Nature 536, 474–478, doi:10.1038/nature18952 (2016).
Ballesteros, N. A., Alonso, M., Saint-Jean, S. R. & Perez-Prieto, S. I. An oral DNA vaccine against infectious haematopoietic necrosis virus (IHNV) encapsulated in alginate microspheres induces dose-dependent immune responses and significant protection in rainbow trout (Oncorrhynchus mykiss). Fish & shellfish immunology 45, 877–888, doi:10.1016/j.fsi.2015.05.045 (2015).
Zhao, J. Z. et al. Preliminary study of an oral vaccine against infectious hematopoietic necrosis virus using improved yeast surface display technology. Molecular immunology 85, 196–204, doi:10.1016/j.molimm.2017.03.001 (2017).
Ludwig, C. & Wagner, R. Virus-like particles-universal molecular toolboxes. Current opinion in biotechnology 18, 537–545, doi:10.1016/j.copbio.2007.10.013 (2007).
Alonso, M., Rodriguez, S. & Perez Prieto, S. I. Nested PCR improves detection of infectious hematopoietic necrosis virus in cells coinfected with infectious pancreatic necrosis virus. Journal of virological methods 81, 1–9 (1999).
Rodriguez, S., Alonso, M. & Perez-Prietol, S. I. Comparison of two birnavirus-rhabdovirus coinfections in fish cell lines. Diseases of aquatic organisms 67, 183–190, doi:10.3354/dao067183 (2005).
LaPatra, S. E., Lauda, K. A. & Jones, G. R. Antigenic variants of infectious hematopoietic necrosis virus and implications for vaccine development 20, 119–126 (1994).
Xu, L. M. et al. scFv antibodies against infectious bursal disease virus isolated from a combinatorial antibody library by flow cytometry. Biotechnology letters 36, 1029–1035, doi:10.1007/s10529-014-1463-z (2014).
Zhao, J. et al. Expression and immunogenicity analysis of truncated glycoprotein of infectious hematopoietic necrosis virus in fish. Xi bao yu fen zi mian yi xue za zhi = Chinese journal of cellular and molecular immunology 30, 1238–1242 (2014).
Xu, L. M. et al. Recombinant scFv antibodies against infectious pancreatic necrosis virus isolated by flow cytometry. Journal of virological methods 237, 204–209, doi:10.1016/j.jviromet.2016.07.029 (2016).
Joh, S. J. et al. Molecular characterization and genogrouping of VP1 of aquatic birnavirus GC1 isolated from rockfish Sebastes schlegeli in Korea. Journal of veterinary science 9, 85–90 (2008).
Whyte, S. K. The innate immune response of finfish–a review of current knowledge. Fish & shellfish immunology 23, 1127–1151, doi:10.1016/j.fsi.2007.06.005 (2007).
Lorenzen, N., Olesen, N. J. & Jorgensen, P. E. V. Antibody-response to VHS virus proteins in rainbow trout. Fish & shellfish immunology 3, 461–473 (1993).
Cao, Y. et al. The kinetics and protection of the antiviral state induced by recombinant iIFN1a in rainbow trout against infectious hematopoietic necrosis virus. Molecular immunology 76, 55–61, doi:10.1016/j.molimm.2016.06.002 (2016).
Penaranda, M. M., Lapatra, S. E. & Kurath, G. Specificity of DNA vaccines against the U and M genogroups of infectious hematopoietic necrosis virus (IHNV) in rainbow trout (Oncorhynchus mykiss). Fish & shellfish immunology 31, 43–51, doi:10.1016/j.fsi.2011.03.003 (2011).
LaPatra, S. E., Turner, T., Lauda, K. A., Walker, S. C. & Jones, G. R. Characterization of the humoral response of rainbow trout to infectious hematopoietic necrosis virus. Journal of aquatic animal health 5, 165–171 (1993).
Zhao, J. Z. et al. Expression and rabbit antisera preparation of igm heavy chain gene in rainbow trout(oncorhynchus mykiss). Journal of Fisheries of China 8, 1175–1181 (2014).
Acknowledgements
This study was supported by grants from the Central-Level Non-profit Scientific Research Institutes Special Funds (grant numbers HSY201514 and 2016HY-ZD0504) and Heilongjiang Province Research and Development of Applied Technology (grant number GA13B401).
Author information
Authors and Affiliations
Contributions
L.X. and T.L. designed the experiments, analysed the data, and wrote the manuscript; L.X. constructed the bivalent DNA vaccine and determined the in vivo and in vitro expression of the antigen genes; J.Z. and M.L. performed the protective efficacy experiments; G.M. determined the gene expression levels and characterized the neutralizing antibodies; F.J. and J.Y. provided and maintained the experimental fish; H.L. was responsible for the statistical analyses; F.J and T.L. supervised the project; and T.L. edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, L., Zhao, J., Liu, M. et al. Bivalent DNA vaccine induces significant immune responses against infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus in rainbow trout. Sci Rep 7, 5700 (2017). https://doi.org/10.1038/s41598-017-06143-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06143-w
This article is cited by
-
A review on the recent advances and application of vaccines against fish pathogens in aquaculture
Aquaculture International (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.