Abstract
There is no information on the association between oral exposure to arsenic (As) and hearing loss in humans or mice. In this combined epidemiological study and experimental study, the association of oral exposure to As with hearing loss in people aged 12–29 years and young mice was examined. Subjects in the exposure group (n = 48), who were drinking tube well water contaminated with As, showed significantly higher risks of hearing loss at 4 kHz [odds ratio (OR) = 7.60; 95% confidence interval (CI): 1.56, 57.88], 8 kHz (OR = 5.00; 95% CI: 1.48, 18.90) and 12 kHz (OR = 8.72; 95% CI: 2.09, 47.77) than did subjects in the control group (n = 29). We next performed an experiment in which young mice were exposed to As via drinking water at 22.5 mg/L, which is a much greater concentration than that in human studies. The exposure group showed hearing loss and accumulation of As in inner ears. Ex vivo exposure of the organ of Corti from mice exposed to As significantly decreased the number of auditory neurons and fibers. Thus, our combined study showed that oral exposure to As caused hearing loss in young people and young mice.
Similar content being viewed by others
Introduction
Contamination of arsenic (As) in drinking well water has been reported worldwide1,2,3. About 20 million people in Bangladesh are exposed to As by drinking well water in which As levels are above the national standard of 50 µg/L4. A previous study using univariate analysis showed that 10-year-old children in an As-polluted area had hearing losses at frequencies of 125, 250 and 8,000 Hz5. Thus, exposure to As is a potential risk for hearing loss in children. However, there has been no epidemiological study with multivariate analysis showing an association between oral exposure to As and hearing loss in young people aged 10–30 years.
Inner ears, which are important organs for hearing, contain the organ of Corti with inner hair cells (IHCs) and outer hair cells (OHCs) and spiral ganglion neurons (SGNs)6. A previous study showed that intraperitoneal injection of As at 200 mg/L into guniea pigs for 2 months resulted in morphological impairments of the inner ears7. Thus, it is possible that exposure to As induces hearing loss in experimental animals. However, there is no direct evidence about whether oral exposure to As via drinking water causes hearing loss in mice determined by auditory brainstem response (ABR). It is also unclear whether oral exposure to As via drinking water modulates the As level in inner ears of mice.
In this study, we examined the association of oral exposure to As with hearing loss in young people and young mice. For this purpose, we compared hearing levels of young people aged 12–29 years in a control group drinking tap water and an exposure group drinking tube well water contaminated with As in Bangladesh using a multivariate analysis adjusted for age, sex, body mass index (BMI) and smoking. We compared hearing levels by ABR and accumulation levels of As in inner ears of wild-type mice exposed to As via drinking water. We then tried to clarify the etiology of As-mediated hearing loss.
Results
Consumption of tube well water contaminated with As by people aged 12–29 years is significantly associated with hearing loss
The basic characteristics of the subjects are shown in Table 1. The subjects were divided into a control group (n = 29) drinking tap water with 0.6 ± 0.7 µg/L of As and an exposure group (n = 48) drinking tube well water contaminated with As. Subjects in the exposure group shared four tube wells with As levels of 20.6, 53.8, 221.0 and 22.2 µg/L. The ages (mean ± SD) of subjects in the control and exposure groups were 21.52 ± 2.34 years and 20.38 ± 5.17 years, respectively. Levels of As in toenails, hair and urine in the exposure group were significantly higher than those in the control group (p < 0.0001) (Fig. 1). Hearing levels in the exposure group were significantly worse than those in the control group at 4 kHz (p = 0.0063), 8 kHz (p < 0.0001) and 12 kHz (p < 0.0001) (Fig. 2).
As levels in biological samples in from people aged 12–29 years drinking As-contaminated water and control group. As levels (means ± SD) in the control group (control; n = 29) and exposure group (exposure; n = 48) in toenails (A), hair (B) and urine (C) were measured. Significant differences (***p < 0.0001) were determined by the Mann-Whitney U test.
Hearing loss in people aged 12–29 years drinking As-contaminated water. Hearing thresholds (means ± SD) in the control group (control; n = 29) and the exposure group (exposure; n = 48) at 1, 4, 8 and 12 kHz were measured. Significant differences (**p < 0.01; ***p < 0.0001) were determined by the Mann-Whitney U test.
Multivariate analysis of As-mediated hearing loss in people aged 12–29 years
We next performed binary logistic regression analysis for hearing loss with adjusted models for age, sex, smoking history and BMI8,9,10,11,12. We categorized hearing levels with the mean values (6 dB at 1 kHz, 7 dB at 4 kHz, 19 dB at 8 kHz and 35 dB at 12 kHz) of hearing thresholds in young people in this study. The multivariate analysis showed significantly higher risks of hearing loss at 4 kHz [odds ratio (OR) = 7.60; 95% confidence interval (CI): 1.56, 57.88], 8 kHz (OR = 5.00; 95% CI: 1.48, 18.90) and 12 kHz (OR = 8.72; 95% CI: 2.09, 47.77) in the exposure group than those in the control group (Table 2). To verify the validity of our model, we changed the cut-off values of the dependent variable dichotomizing hearing levels from 7 to 15 dB at 4 kHz, from 19 to 25 dB at 8 kHz and from 35 to 45 dB at 12 kHz. We found that significant correlations of hearing loss in the exposure group remained.
Oral exposure to As caused accumulation of As in inner ears and hearing loss in young mice aged 3 months
Based on the results obtained by our epidemiological study, we next performed an experimental study to verify hearing loss in mice orally exposed to As at 22.5 mg/L via drinking water for 2 months. After oral exposure of mice to As, we determined hearing levels by ABR and As levels in the inner ears by inductively coupled plasma mass spectrometry (ICP-MS). Before exposure to As, hearing levels in the control group (ctrl; n = 9) and exposure group (As; n = 8) were comparable (Fig. 3A). After exposure to As, hearing levels in the exposure group were significantly worse than those in the control group at 4 kHz (p = 0.0216), 12 kHz (p = 0.0167), 20 kHz (p = 0.0055) and 32 kHz (p = 0.0250) (Fig. 3B). Arsenic in the inner ears of the exposure group (As; n = 7) was detected at 0.3 µg/g of wet tissue weight and was significantly higher than that in the control group (ctrl; n = 5) (Fig. 4). We finally performed ex vivo exposure of the organ of Corti to As at 0.3 µg/mL in order to clarify the etiology of hearing loss by exposure to As at 0.3 µg/mL, which was the average level of As in inner ears of mice orally exposed to As. Ex vivo exposure to As at 0.3 µg/mL for 48 and 72 hours significantly decreased the number of neurofilament 200-positive auditory neurons but not the number of phalloidin-positive hair cells (Fig. 5).
Oral exposure to As causes hearing loss in young mice aged 3 months. ABR thresholds (means ± SD) at 4, 12, 20 and 32 kHz in the control group (ctrl, closed squares, n = 9) and exposure group (As, open trianglers, n = 8) before (A) and after exposure to As (B) are presented. Significant differences (*p < 0.05; **p < 0.01) were determined by the Mann-Whitney U test.
Ex vivo exposure of the organ of Corti to As at 0.3 µg/mL. (A,B) After ex vivo exposure of the organ of Corti to As at 0.3 µg/mL and no exposure (cont) for 48 and 72 hours, (A) inner hair cells (arrowhead) and outer hair cells (arrows) were stained with phalloidin and (B) spiral ganglion neurons (SGNs) and auditory neuron fibers (ANFs) were stained with anti-neurofilament 200 antibody. Scale bars: 50 µm. (C–F) Densities (means ± SD) of (C) inner hair cells, (D) outer hair cells, (E) auditory nerve fibers and (F) SGNs from the organ of Corti exposed to As (+, black bars, n = 10) and no exposure (−, white bars, n = 10) are presented. Significant difference (*p < 0.05, **p < 0.01, ***p < 0.0001) from the control was analyzed by the unpaired t-test.
Discussion
Since a huge number of people worldwide are exposed to As via drinking water, it is important to evaluate the health risk of oral exposure to As. In this epidemiological study, it was shown by multivariate analysis with adjustments for age, sex, BMI and smoking that consumption of tube well water contaminated with As was significantly associated with hearing loss. As has been shown to accumulate in hair after chronic exposure to As in mice orally exposed to As via drinking water13. Correspondingly, our epidemiological data also showed significant correlations between As levels in hair and hearing loss at 4 kHz (r = 0.4816, p < 0.0001), 8 kHz (r = 0.5885, p < 0.0001) and 12 kHz (r = 0.6432, p < 0.0001) in humans (Figure S1). Thus, our epidemiological study showed for the first time that oral exposure to As via drinking water causes hearing loss in young people.
Our epidemiological study showed that frequencies of As-mediated hearing loss in people aged 12–29 years were 4, 8 and 12 kHz but not 1 kHz, while our experimental study showed that all frequencies were affected in young mice orally exposed to As. These results partially correspond to the results of our previous study showing that all frequencies of hearing were affected in a genetically engineered mouse model of age-related hearing loss and in mice orally exposed to toxic elements, while frequencies of hearing loss in control mice were limited to higher frequencies14, 15. Thus, it is possible that oral exposure to As accelerates the onset of age-related hearing loss in young people. On the other hand, exposure to environmental stresses including As, smoking and noise has been shown to cause oxidative stress16,17,18. A previous study showed that exposure to smoking affected high frequency of hearing in young people11. Also, exposure to broadband noise has been shown to affect high frequency of hearing in young mice at 1 month of age19. Therefore, it is possible that hearing levels at higher frequencies are more susceptible to oxidative stress caused by environmental factors than are those at lower frequencies.
The present experimental study demonstrated that oral exposure of young mice to As caused hearing loss with accumulation of As in inner ears. Thus, our combined study showed that oral exposure to As via drinking water causes hearing loss in young people and young mice. In the experimental study, mice were exposed to As at 22.5 mg/L for 2 months via drinking water. We used the dose for mice at the similar order as 10 mg/L and 100 mg/L of As that were based on the exposure doses for humans in a previous study20, although the dose used in our study (22.5 mg/L) is about 100-times higher than the maximum level (221 µg/L) of As detected in tube well water in this study. On the other hand, our epidemiological study showed that the duration of drinking tube well water was also associated with hearing loss at 4 kHz (r = 0.2685; p = 0.0182), 8 kHz (r = 0.4545; p < 0.0001) and 12 kHz (r = 0.4689; p < 0.0001) (Figure S2), suggesting that duration of oral exposure to As via drinking well water is associated with hearing loss in people aged 12–29 years. We therefore calculated a total exposure dose of As with consideration of the duration of drinking water. In our calculation with the exposure period of 7.94 years for humans (mean duration of drinking tube wells) and the exposure period of 2 months for mice, the difference between total exposure doses of As for mice (281 mg/kg) and humans (34 mg/kg) may be decreased to a difference of about 8 times. Further investigation of not only the accumulation levels of As but also hearing levels in other lines of haired mice in addition to the hairless mice used in this study after oral exposure to As at less than 22.5 mg/L is needed.
In our ex vivo study, exposure of the organ of Corti to As at 0.3 µg/mL, which was detected in inner ears of mice orally exposed to As in vivo, significantly decreased the number of auditory fibers and SGNs but not hair cells. Thus, our results indicate the possibility that 0.3 µg/g of As that accumulated in inner ears caused a decrease in the numbers of the auditory nerve fibers and SGNs in vivo. In previous studies, aquaporins (AQPs) including AQP9 were shown to mediate cellular uptake of As in mammalian cells21. AQP9 was shown to be expressed in SGNs but not hair cells in the organ of Corti22. Therefore, it is possible that As accumulated in SGNs via AQPs caused a decrease in the numbers of auditory nerve fibers and SGNs. Previous studies also showed that As-mediated neurotoxicity was involved in apoptosis and demyelination of peripheral neurons including dorsal root ganglion (DRG) neurons23, 24. As-mediated neurotoxicity was also shown to be involved in increased levels of oxidative stress markers including heme oxiygenase 1 (HO-1) and decreased levels of procaspases 3 and 12 in DRG explants25. Further study is needed to determine whether in vivo exposure to As induces oxidative stress, apoptosis and demyelination in auditory neurons and to elucidate the molecular mechanism by which hair cells were not affected by exposure to As in this study.
In conclusion, our combined study suggests that exposure to As via drinking water causes hearing loss in young people and young mice. Hearing loss in young people has a negative impact on the quality of life. Therefore, further study is needed to determine the correlation between hearing loss and oral exposure to As via drinking water in order to prevent As-mediated hearing loss in humans.
Materials and Methods
Epidemiological study
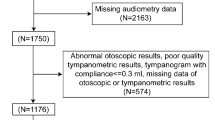
We performed a survey using self-administered questionnaire regarding age, smoking, sex, weight and height for 77 healthy subjects aged 12–29 years in Bangladesh. Informed consent in written form was obtained from all of the participants in the study. Subjects in the control group and subjects in the exposure group were drinking tap water and tube well water contaminated with As, respectively. Subjects with a drinking habit or a habit of using earphones to listen a music did not participate in this study. In addition, another ethnic group or race was not included as subjects in this study. In brief, BMI was calculated as weight (kg) divided by the square of height (m2). In this study, subjects were defined as being underweight if their BMI was less than 18.5 kg/m2, being of normal weight if BMI was 18.5–24.9 kg/m2 and being overweight if BMI was higher than 25 kg/m2 as set by the WHO26. We measured hearing thresholds at 1, 4, 8 and 12 kHz in the subjects by pure tone audiometry (PTA)10, 11, 27. For each frequency, the sound pressure level was increased from 0 dB to 90 dB in 5-dB steps until the subject showed a response. The epidemiological study was approved by Nagoya University International Bioethics Committee following the regulations of the Japanese government (approval number 2013-0070) and the Faculty of Biological Science, University of Dhaka (Ref. no. 5509/Bio.Sc).
Experimental study
Hairless mice having a C57BL/6 J mice background were purchased from Hoshino Laboratory Animal, Inc. The mice were maintained under specific pathogen-free (SPF) conditions at a fixed temperature (23 ± 2°C) and a 12-h light/dark cycle. Sodium arsenite (NaAsO2) purchased from Sigma-Aldrich was dissolved in distilled water. We newly prepared drinking water containing As and changed the drinking water every week. We gave distilled water to the non-exposure group. We started the exposure experiment at 1 month of age. ABR measurements (AD Instruments Pty. Ltd.) were performed as described previously14, 15, 28. Tone burst stimuli were measured 5 dB-stepwise from 0 dB SPL to 90 dB SPL. The threshold was judged by the appearance of the lowest level of I-IV waves of ABR. Data are presented as means ± SD. The experimental study was approved by the Institutional Animal Care and Use Committee in Nagoya University (approval number: 28251) and followed the Japanese Government Regulations for Animal Experiments.
Determination of As levels in biological samples
We collected biological samples (toenails, hair and urine) and performed measurements by ICP-MS (Agilent 7500cx) as described previously14, 15, 27. In brief, samples were placed in 15 ml tubes and ashed overnight at room temperature with 3 ml HNO3. Before ashing, nails were washed with detergent water and kept at room temperature overnight. After heating the samples at 80°C for 3 hours, 1–1.5 ml (0.5 ml for mice) H2O2 was added and the samples were immediately incubated at 80°C for 3 hours. After cooling, the samples were diluted by Milli-Q water. For urinary samples, a 15 ml tube was filled with 4 ml of a urine sample followed by addition of 1 ml HNO3, and the tube was left overnight. The samples were incubated at 80°C for 24–72 hours until the solution became clear. After centrifuging for 1 min, the samples were adjusted with Milli-Q water up to 5 ml, and the As levels were measured by ICP-MS. Urinary As levels were normalized for dilution by specific gravity (SG) adjustment using the following formula27:
where SGtarget is a population mean SG. In this study, the target SG used was 1.012 for subjects. The levels of As in inner ears were determined by the same protocol as that described above.
Ex vivo culture of the organ of Corti
We partially followed the previous method of ex vivo culture of the organ of Corti29. In brief, after dissection of the organs of Corti from C57BL6/J mice at postnatal days 4–6, the cochleas were placed in HBSS buffer. The cochlears were dissected and the organs of Corti containing IHC, OHC, auditory nerve fibers (ANFs) and SGNs were placed in a 6-well dish. D-MEM (low glucose) medium containing 10% FBS and 10 µg/mL of ampicillin was added to the dish. A 0.3 mg/mL stock solution of As was diluted in the culture medium to a final concentration of 0.3 µg/mL. The organ of Corti was placed in an incubator (37°C, 5% CO2). On the next day (day 1), we removed the medium and added a fresh medium for the control group and exposure group (0.3 µg/mL of As). On day 2 and day 3, the medium was removed and the organ of Corti was washed three times with 0.1 M PBS. Specimens were fixed with 10% formalin for 1 hour and rinsed with PBS. To detect SGNs and auditory fibers, specimens were immunolabeled with a solution containing 1 µL of rabbit anti-neurofilament 200 kD (Sigma, N4142) primary antibody, 40 µL of 10% Triton X-100, 6 µL of normal goat serum and 153 µL of PBS overnight at 4°C. On the second day, specimens were washed with PBS and immersed in a solution containing 1 µL of a secondary antibody, 20 µL of 10% Triton X-100, 6 µL of normal goat serum and 173 µL of PBS for 2 hours at room temperature. To evaluate hair cells, we labeled specimens with fluorescein-phalloidin (Wako, 068-06261). The specimens were immersed in a solution containing 1 µL of fluorescein-pholloidin (6.6 µM stock solution) and 199 µL of PBS. After washing with PBS, the specimens were mounted on glass sides, covered with antifade reagent, and observed with Super-resolution/confocal Microscopy (Carl Zeiss Microscopy LSM880-ELYRA PS.1). Hair cells were counted in the width (100 µm) of the field of view of the of microscope at 40X. The ANFs (length > 50 µm) and SGNs were counted in the field (100 µm × 100 µm) of view of the microscope at 40X. Ten specimens were examined for each group.
Statistical analysis
All statistical analyses were performed by JMP Pro (version 11.0.0; SAS Institute Inc., Cary, NC, USA) as described previously27. Since the Shapiro-Wilks test showed that As levels in biological samples and hearing thresholds for PTA and ABR of all frequencies were non-normal distributions, the Mann-Whitney U test to evaluate statistical differences of As levels and hearing levels between two groups. For multivariate analysis, we performed binary logistic regression analysis with adjustments for age8, sex9, BMI12 and smoking10, 11 as confounding factors. In this study, values of p < 0.05 were considered statistically significant.
References
Kumasaka, M. Y. et al. Enhanced carcinogenicity by coexposure to arsenic and iron and a novel remediation system for the elements in well drinking water. Arch Toxicol. 87, 439–447 (2013).
Yajima, I. et al. Arsenic-mediated promotion of anchorage-independent growth through increased level of placental growth factor. J Invest Dermatol 135, 1147–1156 (2015).
Paul, S. & Giri, A. K. Epimutagenesis: A prospective mechanism to remediate arsenic-induced toxicity. Environ Int. 81, 8–17 (2015).
Flanagan, S. V., Johnston, R. B. & Zheng, Y. Arsenic in tube well water in Banglasesh: health and economic impacts and implications for arsenic mitigation. Bull World Health Organ 90, 839–846 (2012).
Bencko, V. & Symon, K. Test of environmental exposure to arsenic and hearing changes in exposed children. Environ Health Perspect 19, 95–101 (1977).
Lalwani, A. K. & Gürtler, N. Sensorineural hearing loss, the aging inner ear, and hereditary hearing impairment. In CURRENT diagnosis & treatment in otolaryngology—head & neck surgery. 2nd edn (eds Lalwani, A.K.) 683–704 (McGraw-Hill, 2008).
Aly, S., Mousa, S., el-Kahky, M., Saleh, A. & el-Mofty, A. Toxic deafness. I. Histological study of the effect of arsenic, salicylates and quinine, on the organ of Corti of guinea pigs. J Egypt Med Assoc 58, 144–157 (1975).
Davis, A. et al. Aging and Hearing Health: The Life-course Approach. Gerontologist. 56(Suppl 2), S256–267 (2016).
Reschke, M. F. et al. Effects of sex and gender on adaptation to space: neurosensory systems. J Womens Health (Larchmt) 23, 959–962 (2014).
Sumit, A. F. et al. Cigarette smoking causes hearing impairment among Bangladeshi population. PLoS One. 10(3), e0118960 (2015).
Ohgami, N., Kondo, T. & Kato, M. Effects of light smoking on extra-high-frequency auditory thresholds in young adults. Toxicol Ind Health. 27, 143–147 (2011).
Kim, S. H. et al. Relationship between obesity and hearing loss. Acta Otolaryngol. 136, 1046–1050 (2016).
Kleiman, N. J., Quinn, A. M., Fields, K. G., Slavkovich, V. & Graziano, J. H. Arsenite accumulation in the mouse eye. J Toxicol Environ Health A 79, 339–341 (2016).
Ohgami, N. et al. Exposure to low-dose barium by drinking water causes hearing loss in mice. Neurotoxicology. 33, 1276–1283 (2012).
Ohgami, N. et al. Manganese-mediated acceleration of age-related hearing loss in mice. Sci Rep 6, 36306 (2016).
Samuel, S., Kathirvel, R., Jayavelu, T. & Chinnakkannu, P. Protein oxidative damage in arsenic induced rat brain: influence of DL-alpha-lipoic acid. Toxicol Lett. 155, 27–34 (2005).
Ahn, J. H. et al. Effects of cigarette smoking on hearing recovery from noise-induced temporary hearing threshold shifts in mice. Otol Neurotol. 32, 926–932 (2011).
Henderson, D., Bielefeld, E. C., Harris, K. C. & Hu, B. H. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 27, 1–19 (2006).
Lang, H. et al. Nuclear factor kappaB deficiency is associated with auditory nerve degeneration and increased noise-induced hearing loss. J Neurosci. 26, 3541–3550 (2006).
Perry, M. R., Wyllie, S., Raab, A., Feldmann, J. & Fairlamb, A. H. Chronic exposure to arsenic in drinking water can lead to resistance to antimonial drugs in a mouse model of visceral leishmaniasis. Proc Natl Acad Sci USA 110, 19932–19937 (2013).
Liu, Z. et al. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci USA 99, 6053–6058 (2002).
Miyoshi, T. et al. Quantitative analysis of aquaporin expression levels during the development and maturation of the inner ear. J Assoc Res Otolaryngol. 18, 247–261 (2017).
Windebank, A. J. Specific inhibition of myelination by lead in vitro; comparison with arsenic, thallium, and mercury. Exp Neurol. 94, 203–12 (1986).
Rai, N. K. et al. Exposure to As, Cd and Pb-mixture impairs myelin and axon development in rat brain, optic nerve and retina. Toxicol Appl Pharmacol. 273, 242–258 (2013).
Chou, Y. H. et al. Arsenite-induced cytotoxicity in dorsal root ganglion explants. Free Radic Biol Med 44, 1553–1561 (2008).
World Health Organization (WHO). Physical status: the use and interpretation of anthropometry. Available: http://www.who.int/iris/bitstream/10665/37003/1/WHO_TRS_854.pdf [accessed 21 January 2017] (1995).
Ohgami, N. et al. Epidemiological analysis of the association between hearing and barium in humans. J Expo Sci Environ Epidemiol 26, 488–493 (2016).
Ohgami, N. et al. c-Ret-mediated hearing loss in mice with Hirschsprung disease. Proc Natl Acad Sci USA 107, 13051–13056 (2010).
Ding, D. et al. Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear Res. 282, 196–203 (2011).
Acknowledgements
This study was supported in part by Grants-in-Aid for Scientific Research on Innovative Areas (24108001, 16H01639), Scientific Research (A) (15H01743 and 15H02588), (B) (16H02962) and (C) (25460178, 16K08343 and 16K11177) and Grant-in-Aid for Challenging Exploratory Research (26670525) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Japan Health Foundation, the Mitsui & Co., Ltd. Environment Fund, Foundation from Center for Advanced Medical and Clinical Research Nagoya University Hospital, the Mitsubishi Foundation, the Research Foundation for Health Sciences (The KENKO-KAGAKU Zaidan), Aichi Health Promotion Foundation, Nagono Medical Foundation, The Salt Science Research Foundation and AEON Environmental Foundation and Sumitomo Foundation (1631119). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
The project was conceived by X.L. and N.O. Epidemiological study and experimental studies were designed by M.K. and N.O. and performed by X.L., Y.O., I.Y., M.I., R.O., S.O., N.A., and A.A.A., X.L., N.O. and M.K. wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Ohgami, N., Omata, Y. et al. Oral exposure to arsenic causes hearing loss in young people aged 12–29 years and in young mice. Sci Rep 7, 6844 (2017). https://doi.org/10.1038/s41598-017-06096-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06096-0
This article is cited by
-
Multidisciplinary approach to assess the toxicities of arsenic and barium in drinking water
Environmental Health and Preventive Medicine (2020)
-
Hearing loss in humans drinking tube well water with high levels of iron in arsenic–polluted area
Scientific Reports (2019)
-
Improvement of balance in young adults by a sound component at 100 Hz in music
Scientific Reports (2018)
-
Clinical Symptoms, Neurological Signs, and Electrophysiological Findings in Surviving Residents with Probable Arsenic Exposure in Toroku, Japan
Archives of Environmental Contamination and Toxicology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.