Abstract
The MEN1 gene, which encodes the protein Menin, was investigated for its regulatory role in milk protein synthesis in mammary glands. Menin responds to nutrient and hormone levels via the PI3K/Akt/mTOR pathway. Bovine mammary epithelial cells and tissues were used as experimental models in this study. The results revealed that the milk protein synthesis capacity of mammary epithelial cells could be regulated by MEN1/Menin. The overexpression of Menin caused significant suppression of factors involved in the mTOR pathway, as well as milk protein κ-casein (CSNK). In contrast, a significant increase in these factors and CSNK was observed upon MEN1/Menin knockdown. The repression of MEN1/Menin on the mTOR pathway was also observed in mammary gland tissues. Additionally, MEN1/Menin was found to elicit a negative response on prolactin (PRL) and/or insulin (INS), which caused a similar downstream impact on mTOR pathway factors and milk proteins. Collectively, our data indicate that MEN1/Menin could play a regulatory role in milk protein synthesis through mTOR signaling in the mammary gland by mediating the effects of hormones and nutrient status. The discovery of Menin’s role in mammary glands suggests Menin could be potential new target for the improvement of milk performance and adjustment of lactation period of dairy cows.
Similar content being viewed by others
Introduction
The MEN1 gene, encoding the protein Menin which has 610 amino acid residues, was identified for its germline inactivating mutations, which cause the multiple endocrine neoplasia type 1 (MEN1) syndrome1. It is an autosomal dominant disorder characterized by tumors predominantly in the endocrine organs, including the parathyroid glands, pancreatic islets, and pituitary gland1,2,3. Thus, MEN1/Menin may play an indirect regulatory role in organism metabolism. For example, in the mammary gland, MEN1/Menin may function in the regulation of the endocrine hormone secretion of insulin (INS) or prolactin (PRL) on its target organs1,2,3.
In terms of its function in the mammary gland, Menin was found to directly interact with the estrogen receptor-α (ERα) in a hormone-dependent manner in breast cancer cells4, 5, thereby regulating the transcription of estrogen-responsive genes in breast cancer cells4, 5. Additionally, MEN1 loss of heterozygosity caused inherited breast-ovarian cancer in humans6 and hyperplasia of the breast (female) and prostate (male) cells in mice7, 8. The above discoveries indicated that Menin plays an important regulatory role in mammary gland tissue. The objective of the study was to uncover the molecular mechanism of MEN1/Menin in regulating milk synthesis in the mammary glands.
Milk synthesis is the primary and important function of the mammary gland in mammals. Milk protein is an important component of milk and a source of nutrition for human consumption. Its yield is tightly regulated by nutritional and endocrine factors (hormones). The phosphatidylinositol-3-kinase (PI3K)/protein kinase B (PKB, also known as Akt)/mammalian target of rapamycin (mTOR) signaling pathway plays a central role in regulating cell proliferation, cell growth, apoptosis and metabolism by responding to the nutrient level and/or the circulating concentration of specific hormones9, 10. Recent studies have indicated that the regulation of protein translation through the PI3K/Akt/mTOR signaling pathway by nutrients, energy and hormones may be important in determining the milk protein production in mammary epithelial cells of dairy cows11, 12. The mTOR signaling cascade integrates nutritional and endocrine signals to alter the expression levels or phosphorylation status of eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1), a translational repressor, and p70 ribosomal protein S6 kinase-1 (S6K1). The phosphorylation of S6K1 leads to the activation of several components of the protein translation apparatus in cells13.
Motivated by the search for the role of MEN1 as a regulator in the milk protein synthesis that mediated by the mTOR signaling pathway in mammary glands, this study investigated the direct effects of MEN1 in both the bovine mammary epithelial cell line MAC-T14,15,16 and bovine mammary gland tissues.
Results
MEN1 knockdown promoted the expression of genes that regulate milk protein synthesis
The factors involved in the PI3K/Akt/mTOR pathway have been known to participate in milk protein synthesis in the mammary gland12, 13. To assess the effect of MEN1/Menin overexpression, conversely, a knockdown system was applied in MAC-T cells (Supplementary Fig. S1). A 71.02% ± 0.38% knockdown efficiency for MEN1 was observed at 24 h post-transfection. The expression levels of several components of the PI3K/Akt/mTOR pathway, Akt, mTOR, S6K1 and 4E-BP1 were significantly increased at the mRNA level (Fig. 1A, P < 0.05). When their total and phosphorylated protein levels were detected, the protein Akt (phosphorylated at Ser473), mTOR (phosphorylated at Ser2448) and S6K1 (phosphorylated at Thr389) all showed increasing expression trend (Fig. 1B,C, P > 0.05), and it was markedly increased in some case (Fig. 1C). These results suggested that MEN1 silencing could promote milk protein synthesis by upregulating the expression of factors involved in the mTOR signaling pathway.
MEN1 knockdown promoted the expression of genes that regulate milk protein synthesis in mammary epithelial cells. The expression levels of factors involved in the PI3K/Akt/mTOR pathway (Akt, mTOR, S6K1 and 4E-BP1) that are associated with milk protein synthesis were assessed at the mRNA ((A) n = 3) and total protein ((B) n = 3) levels by quantitative real-time PCR (qRT-PCR) and WB, respectively, in MAC-T cells at 24 h after transfection with a non-specific control (control) or a MEN1 specific siRNA pool (siRNA). The phosphorylation levels of Akt at Ser473, mTOR at Ser2448, S6K1 at Thr389 and 4E-BP1 at Thr37/46 were detected simultaneously. The data are shown as the relative expression levels normalized to the internal control, β-actin. *P < 0.05, **P < 0.01. The horizontal dashed line represents the normalized level of their corresponding controls. Representative WB images of the expression of Menin, Akt, Akt phosphorylated at Ser473, mTOR, mTOR phosphorylated at Ser2448, S6K1 and S6K1 phosphorylated at Thr389 are shown in (C). PI3K, phosphatidylinositol-3-kinase; Akt, also known as protein kinase (B) Akt (Ser473), Akt phosplorylated at Ser473; mTOR, mammalian target of rapamycin; mTOR (Ser2448), mTOR phosplorylated at Ser2448; S6K1, p70 ribosomal protein S6 kinase-1; S6K1 (Thr389), S6K1 phosplorylated at Thr389; 4E-BP1, eukaryotic initiation factor 4E (eIF4E)-binding protein 1.
MEN1 overexpression suppressed the expression of factors that regulate milk protein synthesis
To confirm the promotive effect of MEN1/Menin in mammary epithelial cells, MAC-T cells were transfected with either a plasmid encoding bovine Menin or an empty vector. By 24 h after transfection, the expression of Menin at both the mRNA and protein level was significantly increased (Supplementary Fig. S2, P < 0.01). The mRNA expression levels of Akt, mTOR, S6K1 and 4E-PBP1, were found to significantly decrease upon Menin overexpression compared to the cells transfected with the empty vector (Fig. 2A, P < 0.01). The total and phosphorylated protein measurements of Akt, mTOR and S6K1 also showed averaged lower expression level upon Menin overexpression compared to the corresponding negative controls (Fig. 2B,C, P > 0.05), except for the phosphorylation level of Akt. For example, as shown in Fig. 2C, the total protein of Akt, mTOR and S6K1 were all inhibited upon Menin overexpression. This indicated that Menin may downregulate milk protein synthesis by suppressing the expression of factors involved in the mTOR signaling pathway.
MEN1 overexpression suppressed the expression of genes that regulate milk protein synthesis in mammary epithelial cells. The expression levels of factors involved in the PI3K/Akt/mTOR pathway (Akt, mTOR, S6K1 and 4E-BP1) that are associated with milk protein synthesis were assessed at the mRNA ((A) n = 3) and total protein ((B) n = 3) levels in MAC-T cells 24 h after transfection with the empty vector (Vector) or the MEN1 expression plasmid (MEN1). The phosphorylation levels of Akt at Ser473, mTOR at Ser2448 and S6K1 at Thr389 were detected simultaneously. The immunoblotting detection of the total and phosphorylated 4E-BP1 at Thr37/46 using primary antibodies purchased from Abcam (Cat No. ab2606) and Cell Signaling Technology (Cat No. 2855) did not yield clear and specific bands (data not shown). The data are shown as the relative expression levels normalized to the internal control, β-actin. *P < 0.05, **P < 0.01. The horizontal dashed line represents the normalized level of their corresponding controls. Representative WB images of the expression of Menin, Akt, Akt phosphorylated at Ser473, mTOR, mTOR phosphorylated at Ser2448, S6K1 and S6K1 phosphorylated at Thr389 are shown in (C). PI3K, phosphatidylinositol-3-kinase; Akt, also known as protein kinase B; Akt (Ser473), Akt phosplorylated at Ser473; mTOR, mammalian target of rapamycin; mTOR (Ser2448), mTOR phosplorylated at Ser2448; S6K1, p70 ribosomal protein S6 kinase-1; S6K1 (Thr389), S6K1 phosplorylated at Thr389; 4E-BP1, eukaryotic initiation factor 4E (eIF4E)-binding protein 1.
MEN1 may impact the expression of milk protein κ-casein
As the main components of the population of bovine milk proteins, acounting for approximately 80% of the total protein in bovine milk, the gene expression of the caseins, αs1-casein (CSNAS1), αs2-casein (CSNAS2), β-casein (CSNB) and κ-casein (CSNK), as well as β-lactoglobulin (LGB) and alpha-lactalbumin (LALBA), were evaluated with MEN1/Menin over- and under-expression in MAC-T cells. CSNK was the only detectable casein, along with LGB, in MAC-T cells (Supplementary Fig. S4 and Table S1). The expression of CSNK was found to be significantly decreased upon transient Menin overexpression (Fig. 3A, P < 0.05), but it was significantly increased upon transient Menin knockdown (Fig. 3B, P < 0.01), compared to their corresponding negative controls. Therefore, these results suggested that MEN1 could regulate milk protein synthesis by modulating the expression levels of the factors involved in mTOR signaling in MAC-T cells.
The RNA expression of CSNK (κ-casein) in mammary epithelial cells was downregulated upon MEN1/Menin overexpression and was upregulated upon MEN1/Menin knockdown. qRT-PCR was used to analyze the effect of MEN1/Menin on the expression of CSNK in MAC-T cells at 24 h after transfection with a MEN1-expressing plasmid (MEN1) or a MEN1-specific siRNA pool (siRNA) compared to their negative control cells transfected with the empty vector (Vector) or a non-specific control siRNA (control), respectively. The results, which are representative of three independent experiments, were used to determine statistical significance in both of the overexpression and knockdown system. CSNK was significantly suppressed upon transient MEN1/Menin overexpression (A), but it was significantly promoted (B) upon transient Menin knockdown. The data are shown as the relative expression levels normalized to the internal control, β-actin. *P < 0.05, **P < 0.01.
In mammary gland tissues, modulated MEN1 expression affected the expression of the genes regulating milk protein synthesis at different lactation periods
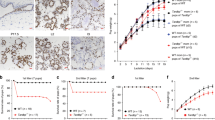
To assess the physiological relevance of the observations for MEN1 in the mammary epithelial cell line model, mammary gland tissues during two different lactation periods (Supplementary Table S2), the peak milk period and the dry period, were collected to detect the expression levels of MEN1 and the genes involved in the PI3K/Akt/mTOR pathway. The expression of Akt, mTOR, S6K1, and 4E-BP1, along with milk protein transcripts for CSNAS1, CSNAS2, CSNB, and CSNK, during the peak milk period (+55 days in milk, DIM) were increased compared with the dry period (-36DIM) (Fig. 4). Interestingly, the expression of MEN1 in the peak milk period (+55DIM) showed lower mRNA level than that in the dry period (-36DIM) (Fig. 4, P = 0.06), so as its protein Menin expression level (Supplementary Fig. S4). These results in tissues were consistent with the observations in mammary epithelial cells, suggesting that MEN1/Menin at least partially modulates casein synthesis by negatively regulating the expression of the genes that are involved in the mTOR signaling pathway.
The effect of MEN1/Menin expression on the process of protein synthesis was confirmed at different lactation periods in mammary gland tissue. Bovine mammary gland tissues at two different lactation periods, peak milk period (+55DIM, days in milking) and dry period before parturition (-36DIM), were collected and used to detect the expression levels of MEN1, genes involved in the PI3K/Akt/mTOR pathway and the milk protein transcripts CSNAS1 (αs1-casein), CSNAS2 (αs2-casein), CSNB (β-casein), CSNK (κ-casein), LGB (β-lactoglobulin) and LALBA (alpha-lactalbumin). The expression of MEN1 in the peak milk period (+55DIM) was lower (P = 0.06) than its expression in the dry period (-36DIM). The expression levels of Akt, mTOR, S6K1 and 4E-BP1, along with the 6 milk protein components, were increased in the peak milk period compared to those in the dry period. The results in mammary gland tissues were consistent with the observations from experiments conducted in the mammary epithelial cells.
MEN1 could respond to the hormones, such as prolactin or insulin, therefore makes effect on milk protein synthesis in mammary epithelial cells
Mammary epithelial cells quickly respond to the environment of the body and sense nutrient and hormonal levels, enabling them to tightly control milk protein synthesis and secretion. To determine the upstream expression regulators of MEN1 in mammary epithelial cells, the hormone prolactin (PRL) or insulin (INS) was used to treat MAC-T cells. Preliminary experiments were performed to obtain the optimal treatment concentration (Supplementary Fig. S5). The addition of prolactin or insulin to the culture of MAC-T cells markedly downregulated the expression of MEN1 mRNA and its protein Menin (Fig. 5A,B, P < 0.01). Simultaneously, the genes involved in the mTOR pathway, Akt, mTOR, S6K1 and 4E-BP1, and as well as CSNK, were markedly upregulated upon treatment with prolactin or insulin (Fig. 5C). These results suggested that MEN1/Menin participates in the regulatory process of CSNK synthesis by prolactin or insulin through the mTOR signaling pathway.
The endocrine hormones prolactin (PRL) and insulin (INS) modulated the expression of MEN1/Menin and its downstream factors involved in mTOR signaling. The MEN1 mRNA (A) and Menin protein (B) level were significantly inhibited in MAC-T cells after 8 h of treatment with prolactin and insulin, compared to their saline-treated controls. The factors in the mTOR pathway, Akt, mTOR, S6K1 and 4E-BP1, along with CSNK, were found to be upregulated upon prolactin (C) and insulin (D) treatment. As shown above, the data are relative expression levels normalized to the internal control β-actin. *P < 0.05, **P < 0.01. Akt, also known as protein kinase (B) mTOR, mammalian target of rapamycin; S6K1, p70 ribosomal protein S6 kinase-1; 4E-BP1, eukaryotic initiation factor 4E (eIF4E)-binding protein 1; CSNK, κ-casein.
Discussion
Recent studies have suggested a possible regulatory role for MEN1/Menin in different tissues through the PI3K/Akt/mTOR signaling pathway17,18,19. Menin was found to directly interact with Akt1 and suppresses its kinase activity by regulating its translocation in the cytoplasm and/or plasma membrane20, 21. MEN1 and the mTOR pathway genes were found to be frequently altered in pancreatic neuroendocrine tumors22. Menin might control insulin expression and/or secretion by modulating visinin-like protein 1 (VSNL1), which is a downstream target of the mTOR pathway23.
In mammary gland, it was suggested that MEN1/Menin played an important regulatory role in maintaining their normal function4, 6,7,8. In this study, we investigated how MEN1/Menin regulated the synthesis of milk protein in mammary epithelial cells. The bovine epithelial cell line MAC-T was used as a model for studying milk protein synthesis regulation14,15,16. The activation of the PI3K/Akt/mTOR pathway12, 13, 24 was revealed to be essential for the initiation of the transcription and translation of milk protein genes. Our results showed that the knockdown of MEN1 gene caused the significant increase in the mRNA expression of main factors of the PI3K/Akt/mTOR signaling pathway, including Akt, mTOR, S6K1 and 4E-BP1. Furthermore, as shown in Fig. 1C, the total and phosphorylated protein of Akt, mTOR and S6K1 also increased upon the lower Menin expression in mammary epithelial cells12, 13, 24, although the up-regulation range of these proteins by Menin was relatively various. Conversely, the overexpression of the MEN1 gene led to a significant reduction in the mRNA expression of these factors, so as the total and phosphorylated levels of mTOR and S6K1 upon increased Menin in some case (Fig. 2C). This led us to believe that MEN1/Menin could modulate the milk protein synthesis of mammary epithelial cells by inhibiting the PI3K/Akt/mTOR signaling pathway.
In vivo, we obtained similar results in the bovine mammary gland tissues. As expected, the RNA expressions of the factors involved in the PI3K/Akt/mTOR signaling pathway at peak milk stage were higher than that in the dry period stage, which was consistent with previous investigations in mammary tissues24,25,26. Interestingly, the expression of MEN1/Menin showed an opposite change at both the RNA and protein level. This further confirmed that MEN1/Menin negatively regulated the factors involved in the PI3K/Akt/mTOR signaling pathway in mammary epithelial cells, and this utimately had an effect on the synthesis of milk proteins, such as CSNAS1, CSNAS2, CSNB, CSNK, LGB and LALBA. Our data suggest that the lower expression level of MEN1/Menin at least partially causes of the higher milk protein yield at peak stage, yet we do not know whether other factors in addition to Menin play similar roles in milk protein synthesis.
Interestingly, MEN1/Menin was also found to negatively regulate STAT5 (signal transducer and activator of transcription 5) in both bovine mammary epithelial cells and bovine mammary tissues (Supplementary Fig. S6). STAT5 is one of the main factors in the JAK-STAT5 pathway, similar to the PI3K/Akt/mTOR pathway, which is another crucial signaling pathway that has been shown to regulate milk protein synthesis in mammary gland tissue14, 27, 28. With a lower level of MEN1/Menin at the peak milk stage in mammary gland tissues, the expression of STAT5 was shown to be higher than that during the dry period. It was reported that induced a higher expression of STAT5 could increase the milk yield of dairy cow mammary epithelial cells29. This further indicated the important mediating role of Menin in milk protein synthesis in the mammary gland.
Milk protein, as one of the main components of milk, is de novo synthesized in the mammary epithelial cells by taking free amino acids through the amino acid transporter (AT) and small peptide molecules ingested from the blood as raw materials (Fig. 6). The synthesis process has been shown to be regulated by the levels of nutrition, energy and hormones near mammary epithelial cells12, 30,31,32,33. We found that MEN1/Menin in the mammary epithelial cells could respond to the level of prolactin or insulin, by inhibiting the expression of milk protein upon hormone treatment. In the bovine mammary gland tissue, possibly as a response to the secreted hormone (such as PRL), MEN1/Menin expression was modulated accordingly throughout the lactation period (Shi et al., unpublished data). For example, the expression level of Menin in the dry period tissue was lower than in the peak milking tissue. Thus, we showed that MEN1/Menin could negatively respond to the hormones and possible nutrients from diets in mammary glands on the process of milk protein synthesis through the PI3K/Akt/mTOR pathway (Fig. 6). Interestingly, Menin was found to respond to feeding and diet in the proximal duodenum, and it plays a negatively regulatory role on the glucose-dependent insulinotropic polypeptide (GIP) by inhibiting of the PI3K/Akt signaling pathway19.
Mediation by Menin of milk protein synthesis through the PI3K/Akt/mTOR signaling pathway in mammary epithelial cells. Hormones such as insulin and prolactin that acts on epithelial cells modulate the MEN1/Menin expression level in cells, which further affects the abundance of the factors involved in mTOR signaling and eventually the synthesis of milk protein. Please see the text for further details. PI3K, phosphatidylinositol-3-kinase; Akt, also known as protein kinase (B) mTOR, mammalian target of rapamycin; IR, insulin receptor; IRS, IR substrate; PRLR, prolactin receptor; AT, amino acid transporter. The solid line represents fluxes; the dotted line represents possible effects or fluxes.
This inhibition response mode of MEN1/Menin for the hormones in mammary glands is consistent with previous studies conducted in pancreatic islets of pregnant mice with prolactin34 and in mouse hepatocytes with insulin35. MEN1/Menin was originally discovered for its tumor suppression function in the pituitary (anterior lobe) and pancreatic islets1,2,3, which are well-known endocrine organs that secrete prolactin and insulin, respectively. Additonally, prolactin and/or insulin could be downstream factors of Menin, regulating their gene transcription36, 37. We hypothesize that there could be a negative feedback inhibition effect between MEN1/Menin and hormones in regulating milk protein synthesis in the mammary gland, and possibly other metabolic systems19.
Overall, MEN1/Menin can negatively modulate the milk protein synthesis capacity of mammary epithelial cells through the regulation of the PI3K/Akt/mTOR pathway (Fig. 6). Moreover, MEN1/Menin in the mammary epithelial cells was shown to regulate MAC-T cell growth and induce cell apoptosis when the expression of Menin was low (Shi et al., unpublished data). The downregulation of Menin expression at the peak milking stage might be one possible reason for the decrease in cell number, but increase in the protein synthesis capacity of mammary epithelial cells in the mammary gland31. This further supports the hypothesis that MEN1/Menin plays a regulatory role in the milk protein synthesis and/or milk yield by mediating the effects of hormones, possible nutrients and energy in the mammary gland. The results of our study might aid in the understanding of the function of Menin in the metabolism of mammary glands, and supply a new potential target improving the milk production performance of dairy cows. However, the molecular mechanism of how MEN1/Menin modulates the PI3K/Akt/mTOR signaling pathway needs to be further studied.
Materials and Methods
Cell culture
Immortalized bovine mammary epithelial cells (MAC-T) were grown in Dulbecco’s modified Eagle’s medium-F12 (DMEM/F12; Gibco, Waltham, MA) containing 10% fetal bovine serum (FBS; Biological Industries, Beth-HaEmek, Israel), 100 IU/mL penicillin, and 100 μg/ml streptomycin (Sigma, St. Louis, MO) at 37 °C under 5% CO2. Prior to the experimental treatments, the cells were detached with 0.25%-trypsin (Gibco) and transferred to a 6-well plate at a density of 5 × 105 cells/well with antibiotic-free medium. For the hormone response experiment, MAC-T cells were treated using 5 μg/ml prolactin (PRL, Sigma) or insulin (INS,Sigma) for 8 h, which was followed by total RNA extraction and cell lysate production. Preliminary experiments were performed to optimize the treatment condition.
Construction of the MEN1 eukaryotic expression plasmid and transfection
The bovine MEN1 (accession No. NM_001076161) eukaryotic expression plasmid pEGFP-C2-bMEN1 was constructed using the pEGFP-C2-Vector (Clontech, BD Biosciences) and PCR, with the bovine mammary gland tissue cDNA as the template and the specific primers (Forward: 5′-cccaagcttgccaccatggggctgaaggctgcccagaaaacg-3′; Reverse: 5′-cggaattctcagagacccttgcgctgccgcttgag-3; the restriction enzyme recognition site for cloning is underlined). MAC-T cells were transfected with 1.2 μg/well of pEGFP-C2-bMEN1 (bMEN1), as well as the negative control pEGFP-C2-Vector (vector), using the LipofectamineTM 2000 transfection reagent (Invitrogen, Carlsbad, CA). The total RNA and protein were isolated from the transfected cells 24 h post-transfection for further analysis. Time-course preliminary experiments were performed to optimize the efficiency of MEN1/Menin overexpression (Supplementary Fig. S1).
Small interfering RNA transfection
Three bovine MEN1-specific siRNAs were designed and synthesized along with their negative controls (Ribobio, Guangzhou, China), targeting the sequences of 5′-ccgttgacctgtctctcta-3′, 5′-ccactgtcgtaaccgcaat-3′ and 5′-ccgagtgactacacgcttt-3′ in the MEN1 gene. MAC-T cells were transfected with 100 nM of either a pool of MEN1 siRNAs (siRNA) or negative control siRNA (control) using LF2000 (Invitrogen). Preliminary experiments were performed in order to obtain the highest knockdown efficiency (Supplementary Fig. S2).
Quantitative real-time PCR (qRT-PCR)
The total RNA was isolated from homogenized bovine mammary gland tissues (10–15 mg) or cultured MAC-T cells using the RNAsimple Total RNA Kit (Tiangen, Beijing, China), and 1 μg RNA was reverse transcribed using the PrimeScript TM RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Dalian, China). The specificity of the PCR product was confirmed by melting curve analysis. All qRT-PCR assays were performed in triplicates in three independent experiments. The primer sequences are listed in Supplementary Table S1. The expression levels of the target mRNAs were normalized to the internal control β-actin mRNA for each transfection, and the 2–ΔΔCT method was used to calculate the relative abundance of mRNA. The results are representative of at least three independent experiments to determine the statistical significance in both the overexpression and knockdown systems.
Western blot analysis
MAC-T cellular lysate extracted after 24 h of transfection or mammary gland tissue lysate (appoximately 20 μg of total protein) was separated on a 10% SDS-PAGE gel and transferred onto nitrocellulose membranes using 200 mA of constant current. This was followed by a normal standard western blot (WB) protocol. The primary antibody against Menin (A300-105A, Bethyl, Texas, USA) was used at a 1:2000 dilution. The antibodies against the total and site-specific phosphorylated Akt (Ser473) (4691, Cell Signaling Technology, Beverly, MA, USA; AF0908, Affinity Biosciences, Cincinnati, OH, USA, respectively), mTOR (Ser2448) (2983, Cell Signaling Technology, Beverly, MA, USA and ab109268, Abcam, Cambridge, MA, USA, respectively), S6K1 (Thr389) (ab32359, Abcam, Cambridge, MA, USA and 9234, Cell Signaling Technology, Beverly, MA, USA, respectively), and β-actin (AA128, Beyotime, Jiangsu, China) were used at a 1:1000 dilution. β-actin was used as the loading control. The HRP-conjugated secondary antibody (Beyotime, Jiangsu, China) was diluted by 1000 as the working solution. Chemiluminescence detection was performed using BeyoECL Plus (Beyotime, Beijing, China). The immunoblotting detection of the total and phosphorylated 4E-BP1 at Thr37/46 using primary antibodies purchased from Abcam (Cat No. ab2606) and Cell Signaling Technology (Cat No. 2855) did not yield clear and specific bands (data not shown). The data are shown as the relative expression level normalized to their corresponding negative controls. The results are representative of three independent experiments that were used to determine the statistical significance.
Ethics statement
All experiments were carried out according to the Regulations for the Administration of Affairs Concerning Experimental Animals published by the Ministry of Science and Technology (China, 2004) and approved by the Animal Care and Use Committee of Shandong Agricultural University (Shandong, China).
Mammary gland tissue collection
Mammary gland tissues from six healthy Holstein cows, with an average weight of 602 ± 19.2 kg, were collected at the Holstein Cattle Association Jiabao Farm in Shandong Province. The randomly selected rear mammary gland was sanitized, and a subcutaneous injection of 1% procaine was administered after the animal was bundled. Next, the skin was cut with an incision 0.5–1 cm in length, and the subcutaneous tissue was peeled off until the mammary gland tissue was exposed. Mammary tissues were biopsied using the Bard Magnum biopsy system (Bard Peripheral Vascular, Inc., Tempe, AZ, US) followed by surgical suturing of the skin. The animal was administered ketaprofen and penicillin G procaine by intravenous injection immediately after biopsy. The same injection protocol was performed for the next 3–5 days, and the animal was monitored for up to two weeks depending on its condition. Mammary tissue samples were immediately snap-frozen in liquid nitrogen and stored at −80 °C for later analysis. Tissue samples were collected on the day that corresponded with the peak milk period at +55DIM (55 ± 4.3 DIM, n = 3) and the dry period before parturition at −36DIM (−36 ± 2.8, n = 3). The milk production information for these cows is shown in Supplementary Table S2. Cows were housed in a free stall barn with constant access to water and feed. Diets were formulated to meet all NRC (2001) recommendations for mid-lactation and dry cows using the Cornell-Penn-Miner system (CPM-Dairy, version 3.0.7). Feed was provided ad libitum, and the cows were milked three times daily.
Statistical analysis
All the data were expressed as the mean ± S.E.M of at least three independent experiments or animals. The data statistics was analyzed using a t-test in the SAS 8.2 software (SAS Institute Inc., Cary, NC, USA). For each statistics, data sets obtained from at least three different biological repeats were used for analysis. The data for each biological repeat would make an individual difference. As for the qRT-PCR results, the individual difference would come from three pairs of CT values corresponding to three pairs of technical replicate reactions. Statistical differences were considered statistically significant if the P values were < 0.05 (*) or P < 0.01 (**).
References
Chandrasekharappa, S. C. et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276, 404–407 (1997).
Schussheim, D. H. et al. Multiple endocrine neoplasia type 1: new clinical and basic findings. Trends in Endocrinology and Metabolism 12(4), 173–178 (2001).
Agarwal, S. K. et al. Menin molecular interactions: insights into normal functions and tumorigenesis. Hormone and Metabolic Research 37, 369–374 (2005).
Dreijerink, K. M. et al. Menin links estrogen receptor activiation to histone H3K4 trimethylation. Cancer research 66(9), 4929–4935 (2006).
Imachi, H. et al. Menin, a product of the MENI gene, binds to estrogen receptor to enhance its activity in breast cancer cells: possibility of a novel predictive factor for tamoxifen resistance. Breast Cancer Research Treatment 122, 395–407 (2010).
Papi, L. et al. Germline mutations in MEN1 and BRCA1 genes in a woman with familial multiple endocrine neoplasia type 1 and inherited breast-ovarian cancer syndromes: a case report. Cancer Genetics and Cytogenetics 195(1), 75–9 (2009).
Seigne, C. et al. Characterisation of prostate cancer lesions in heterozygous Men1 mutant mice. BMC Cancer 10, 395–405 (2010).
Seigne, C. et al. High incidence of mammary intraepithelial neoplasia development in Men1-disrupted murine mammary glands. Journal of Pathology 229(4), 546–558 (2013).
Hara, K. et al. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4EBP1 through a common effector mechanism. Journal of Biological Chemistry 273(23), 14484–14494 (1998).
O’Neil, T. K., Duffy, L. R., Frey, J. W. & Hornberger, T. A. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mTOR following eccentric contractions. Journal of Physiology 587, 3691–3701 (2009).
Yang, X. et al. The mammalian target of rapamycin-signaling pathway in regulating metabolism and growth. Journal of Animal Science 86(Suppl. 14), E35–E50 (2008).
Burgos, S. A., Dai, M. & Cant, J. P. Nutrient availability and lactogenic hormones regulate mammary protein synthesis through the mammalian target of rapamycin signaling pathway. Journal of Dairy Science 93, 153–161 (2010).
Hayashi, A. A. et al. Initiation and elongation steps of mRNA translation are involved in the increase in milk protein yield caused by growth hormone administration during lactation. Journal of Dairy Science 92(5), 1889–1899 (2009).
Zhou, Y., Akers, R. M. & Jiang, H. Growth hormone can induce expression of four major milk protein genes in transfected MAC-T cells. Journal of Dairy Science 91(1), 100–108 (2008).
Hosseini, A., Sharma, R., Bionaz, M. & Loor, J. J. Transcriptomics comparisons of Mac-T cells versus mammary tissue during late pregnancy and peak lactation. Advances in dairy research 1(1), 103 (2013).
Suzuki, Y. et al. Chemerin is a novel regulator of lactogenesis in bovine mammary epithelial cells. Biochemical and Biophysical Research Communications 466, 283–288 (2015).
Wang, X. & Proud, C. G. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21, 362–369 (2006).
Ehehalt, F., Franke, E., Pilarsky, C. & Grutzmann, R. Molecular pathogenesis of pancreatic neuroendocrine tumors. Cancers (Basel) 2(4), 1901–10 (2010).
Angevine, K. R. et al. Menin and GIP are inversely regulated by food intake and diet via PI3K/AKT signaling in the proximal duodenum. Nutrition and Diabetes 2, e55 (2012).
Wang, Y., Ozawa, A. & Zaman, S. et al. The tumor suppressor protein Menin inhibits AKT activation by regulating its cellular localization. Cancer Research 71, 371–382 (2011).
Hughes, E. & Huang, C. Participation of Akt, menin, and p21 in pregnancy-induced beta-cell proliferation. Endocrinology 152(3), 847–855 (2011).
Jiao, Y., Shi, C. & Edil, B. H. et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331, 1199–1203 (2011).
Shi, K. R., Parekh, V. I., Swarnava, R., Desai, S. S. & Agarwal, S. K. The embryonic transcription 1 factor Hlxb9 is a Menin interacting partner that controls pancreatic β-cell proliferation and the expression of insulin regulators. Endocrine-Related Cancer 20(1), 111–122 (2013).
Toerien, C. A., Trout, D. R. & Cant, J. P. Nutritional stimulation of milk protein yield of cows is associated with changes in phosphorylation of mammary eukaryotic initiation factor 2 and ribosomal s6 kinase 1. Journal of Nutrition 140(2), 285–292 (2010).
Bionaz, M. & Loor, J. J. Gene network driving bovine mammary protein synthesis during the lactation cycle. Bioinformatics and Biology Insights 5, 83–89 (2011).
Shi, H. et al. Genes regulating lipid and protein metabolism are highly expressed in mammary gland of lactating dairy goats. Functional & Integrative Genomics 15, 309 (2015).
Akers, R. M. Lactation physiology: a ruminant animal perspective. Protoplasma 159, 96–111 (1990).
Yang, J., Kennelly, J. J. & Baracos, V. E. The activity of transcription factor Stat5 responds to prolactin, growth hormone, and IGF-1 in rat and bovine mammary explant culture. Journal of Animal Science. 78, 3114–3125 (2000).
Liu, X. F. et al. Stat5a increases lactation of dairy cow mammary gland epithelial cells cultured in vitro. In Vitro Cellular & Developmental Biology-Animal 48(9), 554–561 (2012).
Rius, A. G. et al. Regulation of protein synthesis in mammary glands of lactating dairy cows by starch and amino acids. Journal of Dairy Science 93, 3114–3127 (2010).
Capuco, A. V., Wood, D. L., Baldwin, R., Mcleod, K. & Paape, M. J. Mammary cell number, proliferation, and apoptosis during a bovine lactation: relation to milk production and effect of bST. Journal of Dairy Science 84, 2177–2187 (2001).
Appuhamy, J. A. D. R. N. et al. Effects of AMP-activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. Journal of Dairy Science 97, 419–429 (2014).
Arriola Apelo, S. I. et al. Isoleucine, leucine, methionine, and threonline effects on mammalian target of rapamycin signaling in mammary tissue. Journal of Dairy Science 97, 1047–1056 (2014).
Karnik, S. K. et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 318(5851), 806–809 (2007).
Wuescher, L. et al. Insulin regulates menin expression, cytoplasmic localization, and interaction with FOXO1. American. Journal of Physioloy. Endocrinology and Metabolism 301(3), E474–E483 (2011).
Namihira, H. et al. The multiple endocrine neoplasia type 1 gene product, Menin, inhibits the human prolactin promoter activity. Journal of Molecular Endocrinology 29, 297–304 (2002).
Sayo, Y. et al. The multiple endocrine neoplasia type1 gene product, menin, inhibits insulin production in rat insulinoma cells. Endocrinology 143, 2437–2440 (2002).
Acknowledgements
Thanks go to the two anonymous reviewers and the editor, whose comments and suggestions have greatly improved our paper. The authors would like to thank farm owner who generously allowed us to sample mammary gland tissue from their cattle. We are also thankful to Dr. Jifeng Zhong, Dr. Jianbin Li and Ms. Xiuxin Zhao at the Dairy Cattle Research Center, Shandong Academy of Agricultural Sciences, for their supports on the DHI data. We would like to thank Dr. Sunita K Agarwal (NIH/NIDDK), Mark Hanigan (Virginia Tech),Robin White (Virginia Tech), Dr. Ying Yu (China Agricultural University), Dr. Li Jiang (China Agricultural University) and Dr. Jinming Huang (Research Center of Dairy Cow, Shandong Academy of Agricultural Science) for their helpful discussions about the project and the manuscript; Ms. Zijuan Qin (Animal Husbandry Laboratory platform of Shandong Agricultural University) for her kind laboratory support on the routine experimental tests. This work was financially supported by the Natural Science Foundation of Shandong (ZR2013CM013), the National Natural Science Foundation of China (31402054), the Key Project of Agricultural Fine Breeding of Shandong Province (2016LZGC030), the Youth Innovation Fund of Shandong Agricultural University (24036), the Modern Agricultural Industry Technology System (CARS-37) and the Modern Agricultural Industry Technology System of Shandong Province (SDAIT-12-011-06).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: K.S., H.L. Performed the experiments: H.L. Analyzed the data: H.L., X.L., Q.C. Contributed reagents/materials/analysis tools: Z.W., X.L., Z.Y. Wrote the paper: K.S., H.L., M.Z.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Liu, X., Wang, Z. et al. MEN1/Menin regulates milk protein synthesis through mTOR signaling in mammary epithelial cells. Sci Rep 7, 5479 (2017). https://doi.org/10.1038/s41598-017-06054-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06054-w
This article is cited by
-
Menin orchestrates hepatic glucose and fatty acid uptake via deploying the cellular translocation of SIRT1 and PPARγ
Cell & Bioscience (2023)
-
Menin regulates lipid deposition in mouse hepatocytes via interacting with transcription factor FoxO1
Molecular and Cellular Biochemistry (2022)
-
Exploration of the lactation function of protein phosphorylation sites in goat mammary tissues by phosphoproteome analysis
BMC Genomics (2021)
-
AMPK-mTOR pathway is involved in glucose-modulated amino acid sensing and utilization in the mammary glands of lactating goats
Journal of Animal Science and Biotechnology (2020)
-
Menin Modulates Mammary Epithelial Cell Numbers in Bovine Mammary Glands Through Cyclin D1
Journal of Mammary Gland Biology and Neoplasia (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.