Abstract
Conduct disorder (CD) is a psychiatric disorder in children and adolescence. To investigate changes in the power distribution in brain networks between CD and typically developing (TD) groups, resting-state functional magnetic resonance imaging (rsfMRI) data of thirty-six subjects were first recorded, and then the data were preprocessed using DPARSF and SPM8. Meanwhile, the power of the blood oxygenation level-dependent (BOLD) signals of ninety brain regions was acquired using the integral of the Welch power spectral density (PSD). Additionally, the powers of the brain regions that reached significance (p < 0.05) were extracted using the bootstrap statistics, in which the standardized z-scores of the powers were used as a reference. The results of the analysis of the changes in power exhibited that there were significant power differences in some pairs of brain regions between the CD and TD groups, indicating a change in the power distribution. In addition, the results also suggest that the total power consumption of brain networks in CD patients is less than that observed in the TD group. Consequently, the study provided a paradigm for establishing quantifiable indicators via the power spectrum approach for the comparison and analysis of the BOLD signal power between CD patients and healthy controls.
Similar content being viewed by others
Introduction
Patients with conduct disorder (CD) may exhibit a repetitive and persistent pattern of aggressive and antisocial behaviors1,2,3. The symptoms of CD include but not limited to deception, theft, vandalism, violence, and serious violations of rules1,2,3,4. In addition, it is widely recognized that neural activity in the brain is accompanied by the changes in cerebral blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO2), which can be measured by using the functional magnetic resonance imaging (fMRI) technique5. In the past years resting-state fMRI (rsfMRI) has attracted extensive attention regarding the measurement of spontaneous neuronal activity without any specific task, making it a useful and powerful technique for non-invasive mapping the hemodynamic responses in the brain during rest6, 7. More importantly, the blood oxygen level-dependent (BOLD) signals in fMRI recordings are due to the changes in CBF and CMRO25. Consequently, the fluctuations of brain activity observed in the BOLD signals during the resting state play an essential role in exploring the neural mechanism of psychiatric and neurological disorders such as Alzheimer’s disease8 and epilepsy9. Interestingly, recent rsfMRI studies have been performed to explore the different brain activation patterns in CD1,2,3,4, 10, 11. However, most of work conducted focused on the investigation of the functional brain connectivity by examining the temporal correlation between the BOLD signals measured in different brain regions12.
In contrast to more commonly used functional connectivity analysis based on the correlation analysis between different brain regions, a power spectrum analysis method is proposed in this study, which allows us to investigate the oscillation power of brain regions. Power spectrum is a commonly used physical quantity that can quantitatively reflect the energy density changes of the object movement, which gives an intuitive sense of energy consumption changes in the dynamics. The adopted power spectrum method, such as the Welch spectrum method, has been validated to be able to improve the reliability of the analysis results of power spectral density (PSD)13, 14. In addition, it enables the neuroscientists to gain additional insight into the functional organization of the brain based on the generated power of BOLD signals within the whole brain regions. Based on the above reasons, we generated the hypothesis and concept for this study: 1) In resting state, whether or not the differences of BOLD signals in some brain regions are significant between CD and healthy subjects? 2) Can we use the improved power spectrum method to measure and quantitatively analyze the data from CD patients and healthy controls? 3) Can we identify the difference of brain region energy distributions between the two groups?
The purpose of the present study is to use the integrated power spectrum method to analyze the changes of the distribution of powers within different brain regions based on rsfMRI measurements. We will also examine whether the new paradigm can identify the difference of the powers of brain regions between young subjects with CD and young healthy controls. If succeeded, this model will provide us a tool towards an improved understanding of the neural mechanism of CD.
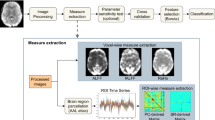
Materials and Methods
Subjects
Eighteen right-handed patients with pure CD (aged 15–17 years, males) were recruited from the Hunan province Youth Detention Center (YDC) in China. In addition, eighteen age-, gender-, and educational level-matched healthy subjects (typically developing (TD) group: aged 15–17 years, males) recruited from the community of Changsha, Hunan province and local schools participated in this study. By experimental design, none of the CD patients had current and lifetime comorbid psychiatric problems. More importantly, the K-SADS-PL (the Schedule for Affective Disorder and Schizophrenia for School-Age Children-Present and Lifetime)15,16,17, a semi-structured psychiatric interview based on DSM-IV criteria (the Diagnostic and Statistical Manual of Mental Disorders)1, was adopted in this study to exclude the additional psychiatric disorders by a professional interview psychiatrist. Consequently, all CD subjects met the K-SADS-PL criteria for CD and also the following criteria18,19,20,21: (1) satisfying the DSM-V criteria for CD; (2) no histories of neurological disorders; (3) no histories of other psychiatric disorders (e.g., attention deficit/hyperactivity disorder (ADHD), anxiety and depression disorders, affective disorders, obsessive-compulsive disorder, oppositional defiant disorder (ODD), mental retardation, alcohol- and drug-use disorder, and substance use disorder); (4) right handed; and (5) normal and corrected-to-normal vision. The subjects used for the present study were the same with the screened ones in the previous work18.
Informed consent and ethical approval
All subjects, as well as their parents or caregivers, completed the written informed consent before the experimental tests. The protocol for all clinical trial was approved by the Biomedical Ethics Board of The Second Xiangya Hospital of Central South University and was carried out in accordance with the relevant guidelines, including any relevant details.
Data acquisition
During the rsfMRI recordings, a foam padding with extendable padded head clamps was used to minimize the head motion, and the earplugs were used to minimize the effect of the scanning noise. All subjects were instructed to stay as still as possible, and to rest quietly with their eyes closed, and to relax without thinking of anything or falling asleep. The experimental tests were performed with a Siemens Allegra 3-T MR scanner at the Magnetic Resonance Center of Hunan Provincial People’s Hospital in Changsha, China. The echo planar imaging (EPI) settings were as follows: repetition time = 3.0 s; echo time = 30 ms; and flip angle = 90°. The contiguous axial slices aligned along the anterior commissure-posterior commissure line were acquired, and the imaging parameters were as follows: the number of slices = 36; field of view (FOV) = 256 mm × 256 mm; matrix size = 64 × 64; and slice thickness = 3 mm without a gap. For each subject, total 100 volume images were analyzed.
Image pre-processing
The first 10 images for each subject were discarded to allow for steady-state longitudinal magnetization22. The remaining images were then preprocessed using DPARSF (http://restfmri.net/forum/DPARSF) and SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) as follows: differences in image acquisition time between slices were corrected; the time-series of images were realigned to remove movement artifact; the images were normalized to a standard SPM8 EPI template which warps each individual subject into standard space with a resolution of 3 × 3 × 3 mm3 based on the Montreal Neurological Institute (MNI) template; and the images were smoothed with the full-width at half-maximum (FWHM) specified as 8 mm. Detrend and band-pass filtering (0.01~0.08 Hz) of the BOLD signals was performed to remove both low-frequency drift and high-frequency noise23,24,25. Additionally, the interferences were regressed out, which included the head motion parameters, white matter signal, cerebrospinal fluid signal and global mean signals.
Data analysis
The functional brain images for each subject were mapped to the automated anatomical labeling (AAL) brain template, which were further separated into ninety anatomical brain regions within the cortex and subcortex (excluding cerebellum)26. The time series from all the voxels within each brain region were extracted and averaged, and then the mean BOLD signal for each brain region was generated. To access the power of each brain region, the Welch PSD13, 14 of the BOLD signal was required to be first calculated by using the PWELCH function in Matlab. The parameters of the function were set as follows: a 75-point Hamming window was used; the parameter on samples of overlap was omitted according to the window; and the function returned the one-sided PSD of the BOLD signal. According to the acquired PSD, the power of each brain region was generated by using the following equation,
where P i,j (f) and P i,j are the Welch PSD and power of the ith brain region from the jth subject, respectively.
In addition, further processing was performed according to the power of the different brain regions:
-
1)
Every brain region had a single power value calculated using eq. 1 and those regions with significant power were identified by the bootstrap statistics for each subject27,28,29,30,31.
-
2)
Re-sampling was performed 5000 times to fit the normal distribution, and the mean and standard deviation of the distribution was calculated, in which the BOOTSTRP function in Matlab was utilized to implement this task. In particular, to obtain the power threshold corresponding to the statistical probability with p-value < 0.05, the inverse of the normal cumulative distribution with the corresponding mean and standard deviation needed to be calculated based on the following eqs 2 and 3. Here the inverse of the normal cumulative distribution was defined as:
where
Based on the symmetry of a normal distribution, we set the probability p value as 2 P. Meanwhile, μ and σ are the mean and standard deviation of the fitted normal distribution in bootstrap statistics, respectively. The NORMINV function in Matlab was used to calculate the inverse of the normal distribution, and the significance power threshold, corresponding to p-value < 0.05, was acquired using this function as well.
Consequently, for each subject we generated a very specific power threshold, and the power values of regions remained where they were greater than the corresponding threshold. In this way, the brain regions with the significant power were obtained for each subject (corresponding probabilities at p-value < 0.05). Then, the analysis returned the mean values of those significant powers for the eighteen subjects in the CD group and the brain regions with significant powers. The same operations were also performed for the TD group as well. The mean values of the significance powers from both groups were mapped to the AAL template, and the results were introduced into the BrainNet Viewer (http://www.nitrc.org/projects/bnv/)32 tool box to visualize the relationships between region structures and power network patterns with the significance level p < 0.05.
Further, the standardized indicators were used as a reference. P i,j was standardized as follows:
where P j is the data vector including all powers of ninety brain regions from the jth subject and \({P}_{j}=({P}_{1,j},{P}_{2,j},{P}_{3,j},\cdots ,{P}_{90,j})\), the mean(P j ) is the mean value of the data vector P j , and std(P j ) is the standard deviation of P j . Z i,j is the standardized z-scores of the ith brain region from the jth subject.
Results and Discussion
In this study, the bootstrap statistics was utilized to extract the significant power (p < 0.05) from ninety brain regions of each subject. Then, the mean values of the significant powers were generated for the eighteen subjects in the CD and TD group, respectively. The mean values and their distributions across different brain regions were compared between the CD and TD groups, in which Fig. 1 displayed the reconstructed three-dimensional (3D) network distribution by using the mean values of the significant powers. The relevant brain structural and functional information was provided in Table 1 for the power networks and associated brain regions in Fig. 1.
Network distribution of brain power with a nonparametric estimate p < 0.05: (a) CD; (b) TD. Panels (a,b) were generated by introducing the significant power values into the BrainNet Viewer (http://www.nitrc.org/projects/bnv/) tool box.
To the best of our knowledge, power analysis was used for the first time to identify the differences of the brain activation patterns between the CD and TD groups. Interestingly, we discovered from Fig. 1 and Table 1 that both the CD and TD groups have twenty-eight brain regions with significant power, and most of the regions exhibited the similar distribution. However, this is not the case for other brain regions, in which brain activity with significant power was only identified in Frontal_Mid_Orb_R (Label 26), Occipital_Sup_L, Occipital_Mid_R, Heschl_L and Heschl_R for CD group, whereas only in the Frontal_Inf_Orb_L, Rectus_L, Cingulum_Ant_R, Lingual_L and SupraMarginal_R only existed for TD group. Importantly, the identified orbital gyri is involved in the cognitive processing of decision-making, and is thought to represent emotion and reward in decision making in individuals with CD relative to healthy controls33,34,35,36,37. In addition, previous work has revealed that the superior frontal gyrus including Frontal_Mid_Orb_R plays an essential role in higher levels of cognitive processing, such as working memory11, 38. In contrast, the superior/middle occipital gyrus including Occipital_Sup_L and Occipital_Mid_R are recognized to be related to the low-level perceptual systems and low-order cognitive processing11, 39. Meanwhile, our findings based on energy exhibited that there existed significant correlation between the brain regions and higher-order/low-order cognitive function processes in CD patients. In particular, it is widely recognized that the cingulum forms the white matter core of the cingulate gyrus and the anterior cingulate cortex is linked to emotion, especially apathy and depression. The changes of power can cause the behavioral change since the function of anterior cingulate cortex is correlated with emotions40,41,42. Further, the lingual gyrus plays an important role in processing vision, which is also associated with logical reasoning43 and encoding visual memories44. Interestingly, a recent study showed that brain activation in lingual gyrus and cuneus was negatively correlated with risk-taking in CD individuals18, 45. The decreased activity of right supramarginal gyrus can causes individuals to be more egocentric whereas overcoming emotional egocentricity bias is associated with increased activation in the regions46. In addition, the most significant activation regions based on the power (Fig. 1) were identified to be correlated with the activated components identified by the independent component analysis (ICA) from previous studies11. Interestingly, we also found that the largest power value in the brain regions from CD group was smaller than that of the TD group.
More importantly, we also examined the power of the ninety brain regions for both CD and TD groups irrespective of whether the significant power was used or not. In Fig. 2(a), the blue color denoted the mean and standard error (SE) of the power of the brain regions from the eighteen subjects in the CD group, whereas the orange one represented those from the TD group. It was observed from Fig. 2 that the distribution of mean value of power from the CD group is similar to the TD group and the correlation coefficient between them is close to 0.93. In contrast, the sum of the power of the ninety brain regions from the CD group (61802.67) was smaller than that of the TD group (94131.96). In this figure, the numbers associated with the brain regions were used to simplify the figure, and the information of the labels of the ninety brain regions matching the AAL template was shown in Table 2. In addition, the standardized z-scores of the powers within the ninety brain regions were plotted in Fig. 2(b), in which we found that the distribution of mean value of the standardized power also exhibited the similarity between the CD and TD groups, and the correlation coefficient between them was 0.94.
Power of brain regions and the standardized z-scores (mean ± SE): (a) Power curves (the unit for 10 log10 Power is decibels); (b) Standardized z-score curves. The blue color represents the CD group whereas the orange line represents the TD group. The horizontal axes denote the ninety anatomical regions of interest from the AAL template. The vertical axes in (a,b) denote the power of brain regions and standardized z-scores of power values, respectively.
Although there are great morphological similarities between the power curves of the CD and TD groups in Fig. 2, the correlation coefficients are less than 1. This suggests that power migration exists among the brain regions when comparing the CD with the TD group. Consequently, this study investigated the differences in the powers of brain regions between the CD and TD groups. Figure 3 showed the brain regions in which there was a statistically significant difference in power between the CD and TD groups. In particular, after powers were transformed into the standardized z-scores, there were six brain regions that exhibited significant differences between the CD and TD groups, which were displayed in Fig. 3(b). Importantly, the brain regions identified in Fig. 3(b) are different from those revealed in Fig. 3(a).
Brain regions with statistically significant differences between the CD and TD groups: (a) Power (mean ± SE); (b) Standardized z-scores (mean ± SE) of power. The blue color represents the CD group whereas the orange color represents the TD group; the horizontal axes denote the anatomical regions of interest in the AAL template. The vertical axes denote the power of brain regions in (a) and standardized z-scores of power values in (b), respectively. *p < 0.05 and **p < 0.01 (the p values are from two-sample t-tests between CD and TD).
To survey the change in the distribution of power within the ninety brain regions in the CD and TD groups, we also calculated the mean power intensity for each of the ninety brain regions for the subjects from the CD and TD group, respectively. Then, the brain regions were sorted by the power intensity in descending order, which were shown in Fig. 4. We discovered from Fig. 4 that the powers of brain regions in the CD group were smaller than that in the TD group, and the order of the brain regions changed from the TD group in (b) to the CD group in (a).
The power analysis of the BOLD signals was performed to examine the energy consumption difference between the CD and TD groups. Interestingly, the new power spectrum approach involves the analysis of the power of the BOLD signal across the whole frequency bands rather than several frequencies, which can acquire more unique features of patients in the CD group. The power of fMRI BOLD signals directly reflects the degree of energy consumption within a region, which is able to quantify the energy consumption during a certain period of time. In this study, we explored the mean power of each brain region using the integrals of power spectral densities (PSDs) of the BOLD signals, in which PSDs were calculated by using the Welch spectrum method13, 14. The Welch method allows for the selection of a variety of window functions and improving the calculation of the PSD (PWELCH is a Matlab function which calculates the PSD using the Welch’s method). To balance the variance and resolution by reducing the sensitivity of the PSD to noise in the BOLD signals, the function was set to use the default segment parameters in Matlab, and then the BOLD signal was automatically segmented into eight sections of equal length, each with 50% overlap based on a Hamming window.
We think it is reliable to adopt the power spectrum to detect the abnormal brain functions. Firstly, the power spectrum of fMRI time series has been used to analyze brain functional activation in previous studies47,48,49,50,51, which can quantitatively determine the power changes of BOLD signals. As such, the previous work exhibited that the power spectrum analysis approaches are effective and reproducible in the analysis of BOLD signals. In particular, the Welch power spectrum used in this study is able to cover a wide variety of window functions, which can improve the quality of spectrum to an even better degree, and is recognized to be an accepted classical power spectrum estimation approach. And the brain power analysis is quite different from the correlation analysis since the power and correlation coefficient are two distinct measurements. The power analysis results in this study directly reflects the degree of energy consumption within the brain regions while the correlation coefficient from two time series is indicative of the functional connectivity between two nodes. In addition, the power distribution analysis uses the integral of the Welch power spectral density (PSD) to measure the power consumption of brain networks, and to compare the BOLD signal power between patients and healthy subjects. The method is also different from reported regional homogeneity (ReHo) analysis52,53,54. ReHo is used to measure the temporal synchrony of regional blood oxygen level-dependent (BOLD) signals as well as similarities in spontaneous neural activity52,53,54. It assumes that within a functional cluster, the hemodynamic characteristics of each voxel would be similar, or synchronous with that of each other, and such similarity could be changed or modulated by different conditions52, 54. Technically, ReHo uses the Kendall’s coefficient concordance (KCC) to measure the similarity of the time series of a given voxel to those of its nearest neighbors in a voxel-wise way52.
As it is very hard to recruit the CD subjects, only 18 patients were identified to participate in this study. Meanwhile, 18 heathy controls were also invited to take part in this work to balance the size of two samples. The small sample size may affect the statistical power for determining neural marks of CD although our findings indeed exhibit the significant difference in power between the two groups based on the small size sample. Although most brain regions with significant power were identified to be the same between the CD and TD groups, as displayed in Fig. 1 and Table 1, a few of the ninety brain regions analyzed did exhibit the difference between them. This indicates that a significant change in the distribution of power occurred between the two groups in a few of the ninety brain regions analyzed. The results shown in Fig. 1 and Table 1 also indicated that the significant powers for most of the brain regions in the CD group were lower than those of the TD group. Although these brain regions showed significant power during the resting state, most of the regions with significant power in the CD group were calmer than those in the TD group. Since the brain regions can interrelate and influence each other55, 56, we analyzed the relation of the powers in the ninety brain regions. In Fig. 2(a), by comparing the means of powers of the eighteen subjects from the CD group with that of the TD group, we discovered that the powers of the ninety brain regions in the TD group were larger than that in the CD group during the resting state, which implies that the brains of the TD group displayed a higher amount of activity. Further, the standardized z-score chart in Fig. 2(b) showed that the distributions of the two mean curves are highly similar. Thus, we further tested the statistically significant differences between the powers of brain-region pairs between the CD and TD groups. This study found that the powers of some brain-region pairs were significantly different between the CD and TD groups during resting state as shown in Fig. 3. The mean values of powers from the TD group were larger than that of the CD group during the resting state (shown in Fig. 3(a)), and statistically significant differences between standardized z-scores of the powers in the brain regions is also indicative of a change in the distribution of power between the TD and CD groups (shown in Fig. 3(b)). Moreover, we also sorted the brain regions of the TD and CD groups according to the strength of the power (shown in Fig. 4). The comparison of Fig. 4(a–c) reflect the change in power observed between the CD and TD groups. Based on these observed changes in the power in the brain regions of the CD group compared to the TD group, we can infer that functional activities related to these brain regions are observed in CD patients and the adjustment of these changes in power may provide a therapeutic strategy for the improvement and rehabilitation of CD. Clinically, the measure of power represents a specific level of brain activity, which can be used to identify the brain regions associated with different disorders or brain cognition functions, and to reflect the energy consumption within brain regions. These power indicators or neural marks can provide us additional clinical supplementary reference information for disease diagnosis and treatments.
Conclusions
Power spectrum estimation is one of the classic methods to analyze neural signals. In this study, power spectrum method was used to extract and analyze the power of functional brain regions in the CD and TD groups based on rsfMRI recordings. We discovered that there were significant differences in the power observed in brain regions in the CD and TD groups, indicative of a change in the distribution of power between the two groups, and the total power of the regions analyzed in the CD group was less than that of the TD group. The study provided a new paradigm for establishing quantifiable indicators via the power spectrum approach for the comparison and analysis of the BOLD signal power between patients and healthy subjects.
References
American Psychiatric Association. The diagnostic and statistical manual of mental disorders (5th ed.).Washington, DC: Author (2013).
Frick, P. J. Current research on conduct disorder in children and adolescents. South African Journal of Psychology 1–15, doi:10.1177/0081246316628455 (2016).
Passamonti, L. et al. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS One 7(11), e48789 (2012).
Sarkar, S., Daly, E., Feng, Y., Ecker, C. & Craig, M. C. Reduced cortical surface area in adolescents with conduct disorder. European child & Adolescent Psychiatry 24(8), 909–917 (2015).
Buxton, R. B., Uludağ, K., Dubowitz, D. J. & Liu, T. T. Modeling the hemodynamic response to brain activation. NeuroImage 23, S220–S233 (2004).
Huang, W. et al. Characterizing acupuncture stimuli using brain imaging with fMRI-a systematic review and meta-analysis of the literature. PLoS ONE 7(4), e32960, doi:10.1371/journal.pone.0032960 (2012).
Lee, M. H., Smyser, C. D. & Shimony, J. S. Resting-state fMRI: a review of methods and clinical applications. American Journal of Neuroradiology 34, 1866–72 (2013).
Sheline, Y. I. & Raichle, M. E. Resting state functional connectivity in preclinical Alzheimer’s disease. Biological psychiatry 74(5), 340–347 (2013).
Maneshi, M., Vahdat, S., Fahoum, F., Grova, C. & Gotman, J. Specific resting-state brain networks in mesial temporal lobe epilepsy. Frontiers in Neurology 5, doi:10.3389/fneur.2014.00127 (2014).
Dodge, K. A. Social-cognitive mechanisms in the development of conduct disorder and depression. Annual review of psychology 44, 559–84 (1993).
Lu, F. M. et al. Functional connectivity estimated from resting-state fMRI reveals selective alterations in male adolescents with pure conduct disorder. PLoS ONE 10(12), e0145668, doi:10.1371/journal.pone.0145668 (2015).
Murphy, K., Birn, R. M. & Bandettini, P. A. Resting-state fMRI confounds and cleanup. Neuroimage 80(15), 349–359 (2013).
Proakis, J. G. et al. Algorithms for statistical signal processing. Printiace Hall (2002).
Welch, P. D. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodogram. IEEE Trans. Audio and Electroacoustics AU 15(2), 70–73 (1967).
Kaufman, J. et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry 36(7), 980–988 (1997).
Shanee, N., Apter, A. & Weizman, A. Psychometric properties of the K-SADS-PL in an Israeli adolescent clinical population. Israel Journal of Psychiatry and Related Sciences (1997).
Zhou, J. et al. Psychiatric disorders in adolescent boys in detention: a preliminary prevalence and case–control study in two Chinese provinces. Journal of Forensic Psychiatry & Psychology 23(5–6), 664–675 (2012).
Zhou, J., Yao, N., Fairchild, G., Zhang, Y. & Wang, X. Altered hemodynamic activity in conduct disorder: a resting-state FMRI investigation. PloS one 10(3), e0122750, doi:10.1371/journal.pone.0122750 (2015).
Su, L., Wang, K., Fan, F., Su, Y. & Gao, X. Reliability and validity of the screen for child anxiety related emotional disorders (SCARED) in Chinese children. Journal of anxiety disorders 22(4), 612–621 (2008).
Zhou, J. et al. High impulsivity as a risk factor for the development of internalizing disorders in detained juvenile offenders. Comprehensive psychiatry 55(5), 1157–1164 (2014).
Zhou, J. et al. Anxiety, depression, impulsivity and substance misuse in violent and non-violent adolescent boys in detention in China. Psychiatry research 216(3), 379–384 (2014).
Chen, H., Yang, Q., Liao, W., Gong, Q. & Shen, S. Evaluation of the effective connectivity of supplementary motor areas during motor imagery using Granger causality mapping. Neuroimage 47, 1844–1853 (2009).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102, 9673–8 (2005).
Hayasaka, S. & Laurienti, P. J. Comparison of characteristics between region-and voxel-based network analyses in resting-state fMRI data. Neuroimage 50, 499–508 (2010).
van den Heuvel, M. P., Stam, C. J., Boersma, M. & Hulshoff Pol, H. E. Small-world and scale-free organization ofvoxel-based resting-state functional connectivity in the human brain. Neuroimage 43, 528–39 (2008).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRIsingle-subject brain. Neuroimage 15, 273–289 (2002).
Efron, B. & Tibshirani, R. J. An introduction to the bootstrap. CRC Press 45–139, 220–234 (1993).
Manly, B. F. J. Randomization, bootstrap and monte carlo methods in biology. CRC Press 41–79 (2006).
Nichols, T. E. & Holmes, A. P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping 15, 1–25 (2001).
Zhang, J., Chen, H., Fang, F. & Liao, W. Convolution power spectrum analysis for fMRI data based on prior image signal. IEEE Transactions on Biomedical Engineering 57(2), 343–352 (2010).
Zhang, J., Tuo, X. G., Yuan, Z., Liao, W. & Chen, H. F. Analysis of fMRI data using an integrated principal component analysis and supervised affinity propagation clustering approach. IEEE Transactions on Biomedical Engineering 58, 3184–3196 (2011).
Xia, M., Wang, J. & He, Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS ONE 8, e68910, doi:10.1371/journal.pone.0068910 (2013).
Fuster, J. M. The Prefrontal Cortex (3rd ed.). Raven Press, New York (1997).
Coccaro, E. F., McCloskey, M. S., Fitzgerald, D. A. & Phan, K. L. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry 62, 168–178 (2007).
Finger, E. C. et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Journal of Psychiatry 168, 152–162 (2011).
Matthys, W., Vanderschuren, L. J. & Schutter, D. J. The neurobiology of oppositional defiant disorder and conduct disorder: altered functioning in three mental domains. Development and psychopathology 25, 193–207 (2013).
Yang, Y. & Raine, A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Research: Neuroimaging 174, 81–88 (2009).
du Boisgueheneuc, F. et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain 129(12), 3315–28 (2006).
Shang, J. et al. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: a resting-state fMRI study. PLoS ONE 9(5), e96834, doi:10.1371/journal.pone.0096834 PMID: PMC4019529 (2014).
Brodal, P. The central nervous system: structure and function (4th ed.). Oxford University Press (2010).
Bush, G., Luu, P. & Posner, M. I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4(6), 215–222 (2000).
Posner, M. I. & DiGirolamo, G. J. Executive attention: Conflict, target detection, and cognitive control. R. Parasuraman, (Ed.) The Attentive Brain Cambridge: MIT Press (1998).
Takeuchi, H. et al. Resting state functional connectivity associated with trait emotional intelligence. Neuroimage 83, 318–328 (2013).
Roland, P. E. & Gulyas, B. Visual memory, visual imagery, and visual recognition of large field patterns by the human brain: functional anatomy by positron emission tomography. Cereb Cortex 5, 79–93 (1995).
Dalwani, M. S. et al. Default mode network activity in male adolescents with conduct and substance use disorder. Drug Alcohol Depend 134, 242–250 (2014).
Silani, G., Lamm, C., Ruff, C. C. & Singer, T. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. Journal of Neuroscience 33(39), 15466–15476 (2013).
Marchini, J. L. & Ripley, B. D. A new statistical approach to detecting significant activation. NeuroImage 12, 366–380 (2000).
Moritz, C. H., Rogers, B. P. & Meyerand, M. E. Power spectrum ranked independent component analysis of a periodic fMRI complex motor paradigm. Human Brain Mapping 18(2), 111–122 (2003).
Duff, E. P. et al. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Human Brain Mapping 29(7), 778–790 (2008).
Engel, S. A., Glover, G. H. & Wandell, B. A. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cerebral Cortex 7, 181–192 (1997).
Zhang, J., Yuan, Z., Huang, J., Yang, Q. & Chen, H. Quantification of the power changes in BOLD signals using Welch spectrum method during different single-hand motor imageries. Magnetic Resonance Imaging 32, 1307–1313 (2014).
Zang, Y., Jiang, T., Lu, Y., He, Y. & Tian, L. Regional homogeneity approach to fMRI data analysis. Neuroimage 22(1), 394–400 (2004).
Wu, Q., Zhang, X., Dong, D., Wang, X. & Yao, S. Altered spontaneous brain activity in adolescent boys with pure conduct disorder revealed by regional homogeneity analysis. European Child & Adolescent Psychiatry, doi:10.1007/s00787-017-0953-7 (2017).
Wu, T. et al. Regional homogeneity changes in patients with Parkinson’s disease. Human Brain Mapping 30, 1502–1510 (2009).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3), 1059–1069 (2010).
Zhang, J., Li, Y. Y., Chen, H. F., Ding, J. R. & Yuan, Z. An investigation of the differences and similarities between generated small-world networks for right- and left-hand motor imageries. Sci. Rep. 6, 36562, doi:10.1038/srep36562 (2016).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 61273361). This work was also supported in part by MYRG2014-00093-FHS, MYRG2015-00036-FHS and MYRG2016-00110-FHS grants from the University of Macau, and FDCT 026/2014/A1 and FDCT 025/2015/A1 grants from Macao government in Macau.
Author information
Authors and Affiliations
Contributions
J.Z. (Jiansong Zhou) designed the experiments. J.Z. (Jiang Zhang) and F.L. analyzed the data. J.Z. (Jiang Zhang) and Z.Y. wrote the manuscript. L.C., Y.H., H.C., Y.X. and G.Y. provided support to this study during the experiments.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Zhou, J., Lu, F. et al. Investigation of the Changes in the Power Distribution in Resting-State Brain Networks Associated with Pure Conduct Disorder. Sci Rep 7, 5528 (2017). https://doi.org/10.1038/s41598-017-05863-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05863-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.