Abstract
6-Sulfatoxymelatonin (aMT6s) is the main metabolite of melatonin in urine, and is a reliable surrogate biomarker reflecting the blood melatonin concentration. This meta-analysis assessed the association between urinary aMT6s level and BC incidence. The electronic databases PubMed, EMBASE, Cochrane Library, and Web of Science were searched. Risk ratios (RRs) were adopted to estimate the relative BC incidence. A total of 7 prospective case-control publications were included, and 6 of them were distinct studies. Pooled analysis of data from the 6 studies involving 1824 women with incident BC and 3954 matched control participants with no overlapping of subjects among studies indicated no significant association between the highest levels of urinary aMT6s and the incidence of BC (RR = 0.97, 95% CI, 0.88–1.08, P = 0.56). Negative associations were observed in postmenopausal women (RR = 0.88, 95% CI, 0.75–1.02, P = 0.10), estrogen receptor positive BC (RR = 0.83, 95% CI, 0.64–1.07, P = 0.15), and studies using 12-hour overnight urine (RR = 0.81, 95% CI, 0.61–1.07, P = 0.13), all with borderline significances. Lag time or invasive degree did not interfere with the results. There was no evident publication bias detected by the Egger’s test and the funnel plot. Conclusively, the current evidence did not support a significant association between urinary aMT6s level and BC risk.
Similar content being viewed by others
Introduction
Breast cancer (BC) is one of the most common malignancies and a leading cause of cancer-related mortality among women worldwide1, 2. Night-shift work has been suggested to be a risk factor for BC and was classified as a group 2 A carcinogen by the International Agency for Research on Cancer (IARC)3. Women who ever had night-shift work had a significantly increased risk of BC, compared to those who had normal sleep duration. Melatonin (N-acetyl-5-methoxytrptamine) is secreted primarily by the pineal gland in humans. It has an intricate role in chronobiology, regulating the circadian rhythm4. The long-term disruption of decreased nocturnal melatonin production in night-shift workers has been associated with modestly increased risk of BC and other cancer types5.
There has been no definite explanation of the mechanism by which melatonin affects the development of BC. One hypothesis suggests that a lower level of melatonin secretion at night may lead to increased estrogen levels6. It is likely to impact estrogen metabolism through the selective estrogen receptor modulator (SERM) and the selective estrogen enzyme modulator (SEEM) activities, resulting in increased turnover of breast epithelial stem cells, and in thus subsequently raised risk of malignant transformation7. Physiological concentrations of melatonin have been demonstrated to down-regulate the aromatase expression in the MCF-7 human BC cell lines, showing a synergistic anti-proliferative effect with tamoxifen8. Melatonin also appears to directly promote apoptosis9 and inhibit angiogenesis10. In addition, it seems to have immunopotentiating and oncostatic effects by increasing the activity of T and B lymphocytes, monocytes, natural killer cells, and immunoactive cytokines (interferon [IFN]-γ, interleukin [IL]-2, IL-6, and IL-12), providing a promising treatment for cancer patients5.
6-Sulfatoxymelatonin (aMT6s) is the primary urinary metabolite of melatonin. It is suggested that urinary aMT6s levels remain stable when sample processing is delayed for 24–48 hours11, and that urinary aMT6s in the morning is not influenced by sleeping pattern or by the storage time of urine, so it is selected to be a biomarker of plasma melatonin concentrations at the collection time12. As melatonin is mainly secreted at night, and its peak concentration occurs in the early morning, the detection of the first morning urinary aMT6s 12 hours overnight to assess the peak melatonin production is reliable13, 14. However, according to the secretion pattern of melatonin, levels of aMT6s collected at random time might not be useful as surrogates of nocturnal melatonin secretion. Although melatonin amplitudes show great variability, its range within the same person remains relatively stable, justifying singular melatonin measurements13. For our analysis, studies measuring the concentration of aMT6s in the first morning urine and 12-hour overnight samples were included.
Recent studies15,16,17,18,19,20,21,22 concerning the relationship of urinary aMT6s and BC provided mixed results. Travis et al.13 conducted the first prospective study implying no association between aMT6s and BC incidence. While Shernhammer et al.16,17,18 demonstrated a significantly inverse association between aMT6s level and BC risk, the remaining papers20,21,22 supported no evidence of such a negative association. To the best of our knowledge, up till now there is only 1 meta-analysis23 concerning this issue, which is however considered biased due to methodological dissonances and incomplete literature retrieval. Basler et al.23 found a weak but statistically significant inverse association between the urinary aMT6s level and BC risk (RR = 0.82, 95% CI, 0.68–0.99, P = 0.04) based on only 5 studies13, 16,17,18,19. While several factors (e.g., menopausal status and lag time) may influence the final conclusion potentially causing bias, with 5 studies Basler et al.23 could not do any subgroup analysis to provide the complete information. In addition, Basler et al.23 only searched one electronic database, suggesting an incomprehensive retrieval. Thus, their conclusions that melatonin affects BC incidence in women should be interpreted with caution and needs to be tested with the 3 emerging studies20,21,22. Herein an up-dated meta-analysis and systematic review was conducted to further assess the possible relationship between melatonin and the risk of BC based on studies investigating the urinary aMT6s concentrations.
Results
Study Selection
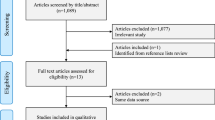
A total of 26 studies were retrieved from the electronic databases PubMed, Cochrane Library, EMBASE, and Web of Science for full review during primary search according to the inclusion criteria, and 7 prospective case-control publications16,17,18,19,20,21,22 including 6 distinct studies17,18,19,20,21,22 were finally selected (Fig. 1). Wu et al.24 carried out a prospective trial on Chinese women in 2013, but the urine samples for measuring aMT6s were randomly collected not following a pre-specified time schedule (e.g., first morning and 12-hour overnight). As melatonin is mostly secreted during nighttime, the level of aMT6s in spot urine void could not reflect the real melatonin secretion. So this study was excluded. Studies carried out by Travis et al.13 and Wang et al.21 were both based on the Guernsey III Study with the same enrollment time. In Travis et al.’s study13, the end follow-up date was October 31st 2001, while Wang et al.’s observation21 lasted until October 31st 2009. Due to the overlap of the same group of participants, we selected Wang et al.’s study21 only for our meta-analyses. Likewise, Shernhammer et al.’s16 and Brown et al.’s studies22 included participants both from the NHSII cohort, and they followed them up to May 31st 2001 and June 1st 2007, respectively. To avoid selecting the same participants twice, Brown et al.’s study22 with a larger number of participants was included for the overall analysis. But for subgroup analyses, data from the Schernhammer et al.’s study16 were included if they were useful and were not overlapped. Furthermore, although Schernhammer et al.17 and Schernhammer et al.19 reported results from the same study cohort (the ORDET cohort), they investigated participants with different menopausal statuses. Therefore there was no dispute when selecting both studies17, 19. The characteristics of each study were listed in Table 1.
Overall Analysis
The detailed highest and lowest levels in each study were defined in Table 2. Altogether, this meta-analysis included the overall data from 1824 women with incident BC and 3954 matched control participants in 6 studies17,18,19,20,21,22. The aMT6s levels in the BC and healthy control groups were listed in Table 3. When results from all the 6 distinct studies17,18,19,20,21,22 were pooled, the aggregate RR for BC was 0.97 (95% CI, 0.88–1.08; Z = 0.58; P = 0.56, Fig. 2), comparing women in the highest level of aMT6s concentration versus women in the lowest level, with no significant heterogeneity in estimates between the studies.
Forest plot for the association between urinary 6-sulfatoxymelatonin levels and breast cancer risk. Case subjects are defined as women who developed breast cancer after their enrollment in the study cohort, and matched healthy control subjects are randomly chosen, alive, and free of cancer at the time of diagnosis of the index case subject. Events indicate cases in the highest proportion or in the lowest proportion. Urinary 6-sulfatoxymelatonin levels are not significantly associated with overall breast cancer incidence.
Subgroup Analyses
Subgroup analyses were conducted for pre- and post-menopausal patients, for invasive and in situ BCs, for lag time shorter and longer than 4 years, for estrogen receptor-positive (ER+) and ER− BCs, and for studies using different urine samples (Table 4).
Menopausal status
aMT6s levels were not significantly associated with BC risk among premenopausal participants (highest level vs. lowest level, aggregate RR = 1.08, 95% CI, 0.84–1.38, Z = 0.59, P = 0.55), based on data from 2 studies19, 21. While in postmenopausal women, there was an inverse association between aMT6s level and BC risk with a borderline significance (RR = 0.88, 95% CI, 0.75–1.02, Z = 1.67, P = 0.10) based on data from 4 studies17, 18, 20, 21 (Fig. 3).
Forest plot for the association between urinary 6-sulfatoxymelatonin levels and breast cancer risk in pre- (upper sub-figure) and post-menopausal (lower sub-figure) women. 6-sulfatoxymelatonin levels are not significantly associated with breast cancer risk among premenopausal participants; while in postmenopausal women, there is an inverse association between 6-sulfatoxymelatonin level and tumor risk with a borderline significance.
BC pathological type
Three studies17, 18, 22 reported results for invasive and in situ BCs separately. The meta-analysis for aMT6s levels and invasive BC showed no association, and the RR for highest level vs. lowest level was 0.92 (95% CI, 0.80–1.07, Z = 1.07, P = 0.29), based on a fixed-effect model due to insignificant heterogeneity. The result for aMT6s and in situ BC was also insignificant, and the RR for highest level vs. lowest level was 0.91 (95% CI, 0.69–1.19, Z = 0.70, P = 0.48) (Fig. 4B).
Lag time
As the lag time differed within the selected studies15,16,17,18,19,20,21,22, we pooled data to find out whether different lag time influenced the final result. For lag time of less than 4 years, aMT6s levels were not associated with BC risk (highest level vs. lowest level, RR = 1.13, 95% CI, 0.64–1.99, Z = 0.41, P = 0.68, 3 studies16, 19, 21), using a random-effects model due to significant heterogeneity (χ 2 = 8.29, P = 0.02, I 2 = 76%). A similar result was seen in the subgroup where lag time was longer than 4 years (highest level vs. lowest level, RR = 0.93, 95% CI: 0.70–1.24, Z = 0.47, P = 0.64, 2 studies19, 20) (Fig. 5).
ER expression
When we restricted the analysis to women with ER+ BC, a negative association between aMT6s and BC risk with a borderline significance was shown (highest level vs. lowest level, RR = 0.83, 95% CI, 0.64–1.07, Z = 1.44, P = 0.15, 5 studies17,18,19,20, 22). However, the risk of ER− BC was not significantly lower in participants with the highest level of aMT6s (highest level vs. lowest level, RR = 0.96, 95% CI: 0.61–1.52, Z = 0.17, P = 0.87, 3 studies18, 19, 22) (Fig. 6).
Forest plot for the association between urinary 6-sulfatoxymelatonin levels and estrogen receptor positive (upper sub-figure) and negative (lower sub-figure) breast cancer risk. Urinary 6-sulfatoxymelatonin is inversely associated with estrogen receptor positive breast cancer with a borderline significance, but not with estrogen receptor negative tumors.
Urinary sample
The meta-analysis based on the 4 studies18, 20,21,22 using first morning urinary samples indicated no link between aMT6s and BC incidence (highest level vs. lowest level, RR = 0.97, 95% CI, 0.87–1.08, Z = 0.54, P = 0.59). On the contrary, for the 12-hour overnight urine samples, aMT6s was negatively associated with BC risk with a borderline significance (highest level vs. lowest level, RR = 0.81, 95% CI, 0.61–1.07, Z = 1.52, P = 0.13, 2 studies17, 19) (Fig. 7).
Bias Assessment
We performed funnel plot analysis and Egger’s test to evaluate the potential bias. Judging from the linear regression test of funnel plot asymmetry, it was suggested that the data were distributed evenly. The result from the Egger’s test showed that there was no indication of a bias for this meta-analysis (P = 0.412). Funnel plots further supported that there were not any significant biases. (Supplementary Figure 1)
Discussion
A total of 7 publications16,17,18,19,20,21,22 including 6 distinct studies17,18,19,20,21,22 that met the inclusion criteria were selected, and the evidence extracted from them was summarized quantitatively. To our surprise, there was no evidence of statistical association between urinary aMT6s and BC risk, which was inconsistent with the previous reports21, 23. Clinical investigations suggested an inverse association between nocturnal plasma melatonin level and the incidence of BC25,26,27. The meta-analysis performed by Basler et al.23 demonstrated a significant inverse association between aMT6s level and BC risk. And Yang et al.28 implied that an increase of 15 ng/mg creatinine in aMT6s reduced BC risk (RR = 0.86, 95% CI, 0.78–0.95), with a significant linear dose-response trend. In this analysis, we revealed no significant link between aMT6s and BC risk (RR = 0.97, P = 0.56). One of the possible explanations might be that melatonin may co-function with many other factors during breast carcinogenesis. Artificial light at night may induce melatonin suppression and vitamin D insufficiency, leading to BC29. And shiftwork was suggested to have a significant association with BC risk30 with reduction of 25-hydroxyvitamin D concentration31 and vitamin D deficiency32. Since low melatonin and low 25OHD concentration are both the results of night shiftwork, it is suggested that melatonin is not the only significant risk reduction factor for BC. Further studies investigating the association between urinary aMT6s and BC incidence should take these factors into consideration.
Of all the studies included, although Schernhammer was the first author in 4 included articles16,17,18,19, their data sets are distinct from each other due to discrepant cohorts or characteristics of participants. Schernhammer et al.17 and Schernhammer et al.19 selected premenopausal and postmenopausal participants respectively from the ORDET cohort. While Schernhammer et al.’ study16 was based on the NHSII cohort, and Schernhammer et al.’ study18 originated from the NHS cohort. For the studies based on the same cohorts15, 16, 21, 22, overlaps of study recruitment period was observed. The studies21, 22 with the most participants and longest follow-up period were included. Besides, useful data from Schernhammer et al.16 were included in subset analyses. Therefore there is no dispute about initially selecting all these 7 studies.
In 2014, Basler et al.23 performed a meta-analysis and found a negative association between the urinary melatonin level and BC risk. The electronic database searched by Basler et al.23 was only PubMed from 1989 to 2013. As meta-analysis requires a comprehensive, objective, and reproducible search of a range of sources to identify as many relevant studies as possible, we enlarged the retrieval using PubMed, Cochrane Library, EMBASE, and Web of Science and identified additional studies21, suggesting an inadequate retrieval by Basler et al.23. Furthermore, as melatonin secretion is affected by various factors, such as menopausal status33, 34 and some preclinical diseases, their meta-analysis was not sufficient without taking these factors into consideration. After that, 3 novel studies20,21,22 with a larger sample size were published, and the results of these papers were inconsistent with Basler et al.’s findings23. Compared with the former meta-analysis23, our meta-analyses had several strengths. First, more studies with a larger number of participants were included. Markedly more subgroup analyses were carried out. Second, all the included studies had high qualities according to the good research design and matched control selection criteria35. Third, no limitation was set during literature search, potentially decreasing selection bias. In addition, the 3 newly enrolled studies20,21,22 had significantly enlarged sample size, and 2 of them21, 22 could cover the previous reports with improved quality.
It was unclear whether methodological dissonances (some of the studies18, 20,21,22 use first morning urine samples and others17, 19 12-hour overnight samples) led to mixed results of all the included studies. So we carried out a subgroup analysis based upon diverse urine samples. Interestingly, pooled results from studies18, 20,21,22 using first morning urine samples showed no association between aMT6s and BC incidence, while an inverse association with a borderline significance was observed when we restricted samples to 12-hour overnight urine (RR = 0.81, 95% CI, 0.61–1.07). As many postmenopausal women may void during the night, so ‘first morning’ urine may not reflect a true overnight first void20. On the contrary, 12-hour overnight urine samples might well mirror the melatonin secretion. However this finding needs to be treated with caution as only 2 studies17, 19 were included.
Upon different cut-points of these studies, aMT6s concentrations were classified into quartiles or tertiles. The division of aMT6s was based on the distribution in the controls. In our study, the relative BC risks of the highest and lowest quartile (tertile respectively) were determined, compared, and used to calculate the corresponding odds ratios. All of the 6 studies17,18,19,20,21,22 used creatinine-adjust aMT6s to reflect the concentration of urinary aMT6s. As urinary creatinine concentration is influenced by many factors, including sex, ethnicity, and age, the mean aMT6s concentration varied.
Some of the identified studies17, 19, 20, 22 highlighted the lag time between urine collection and BC diagnosis, because of the possibility of preclinical tumors affecting urinary aMT6s levels. Schernhammer et al.17, 19 tested the trend of the association between aMT6s and BC occurrence with increasing lag time. They excluded cases that were diagnosed shortly after urine collection, using a stepwise approach. The association between urinary aMT6s level and breast cancer risk became increasingly inverse after excluding case patients who were diagnosed with invasive breast cancer within 2 years (OR for highest versus lowest quartile 0.68), 4 years (OR = 0.61), or 8 years after urine collection (OR = 0.17). However, the lag time analyses conducted by Sturgeon et al.20 suggested no significantly decreased risks of BC with higher urinary levels of melatonin when restricting analyses to those with lagged exposure by 4 or more years after urinary collection. Similar conclusion was also drawn by Brown et al.22. To address this question, we performed analysis based on 3 studies14, 17, 19 which provided data of lag intervals of less than 4 years, and the RR for BC risk on highest level vs. lowest level was insignificant. When we restricted the lag time to longer than 4 years17, 18, the RR was also insignificant. Therefore no trend of stronger association between aMT6s level and BC was observed with a longer lag interval. However, as a limited number of studies was included in this subgroup analysis, further studies with larger participants and various lag time are need for more in-depth investigation.
Although no overall link between aMT6s and BC incidence was elucidated, there existed an inverse association between aMT6s level and ER+ BC incidence with a borderline significance (RR = 0.83, 95% CI, 0.64–1.07). The mechanisms underlying melatonin’s protection against ER+ BC are becoming clearer36. Melatonin works through receptors and distinct second messenger pathways33, 37 to reduce cellular proliferation and to induce cellular differentiation. A physiological peak nighttime serum value of melatonin could delay and slow tumor progression via interfering with the malignant cell cycle, suppressing the proliferation of ER+ human BC cell lines significantly and directly38. Melatonin interferes with the estrogen-signaling pathways39, by suppressing the ERα mRNA expression and the estrogen-induced transcriptional activity. Besides, melatonin impacts the expression of growth inhibitory and apoptotic pathway modulators including TGF-α, Bax, and CaM39. Schernhammer et al.15,16,17 found that the significantly inverse association between aMT6s levels and tumor incidence remained when only ER+ BC was considered, which was moderately in accordance with the previous findings8, 29, 30. Likewise, Chottanapund et al.8 demonstrated melatonin as an aromatase inhibitor in the co-culture system. In 2001, Hansen et al.40 found that night work and melatonin were more strongly related to invasive than in situ BC risk. Similar results were presented by Thompson and Li41 investigating melatonin and invasive BC. The association was limited to postmenopausal women. In a study exploring levels of melatonin and sex hormones11, although melatonin appeared to be not directly associated with recent night work and estrogen levels, long-term night work did seem to increase the estrogen levels among postmenopausal women. These were in half agreement with our findings that there presented an inverse association between aMT6s levels and postmenopausal BC incidence, although with borderline significance (RR = 0.88, P = 0.10). However, we observed no association of aMT6s and invasive BC incidence. These call for further investigations due to the relatively limited number of studies in these subgroups.
This study is limited by the diverse classifications of the aMT6s levels, the different definition of lag time, the various urine collection ways, and the discrepant primary menopause statuses. And due to the limited number of included studies and some heterogeneities, our analyses should also be interpreted with caution. Besides, as many other potential confounding factors have been suggested to be associated with BC incidence, such as vitamin D, 25OHD, artificial light at night and shiftwork29, 30, 32, further studies need to take all these factors into consideration. Particularly, among the included studies, there is only one by Schernhammer et al.16 reporting the influence of night shiftwork. Although the corresponding subgroup analysis might not be possible in this case, the study found that results remained similar after excluding participants just having the night shift. Moreover, our analyses were majorly based on specific classifications of the aMT6s concentration only available in included studies, future investigations quantitatively defining BC-associated aMT6s concentrations might be warranted.
In conclusion, our meta-analysis showed that there was no significant association between the levels of urinary aMT6s and the risk of BC, while an inverse association with a borderline significance was observed in postmenopausal women and ER+ BC patients. The role of aMT6s in predicting BC risk might require further investigations. As the public interest has increasingly focused on the potential morbid risk of the light-at-night work schedules and the circadian disruption with emphasis on melatonin42, additional studies with further explorations are needed to validate the association between aMT6s/melatonin and BC risk.
Materials and Methods
Publication Search
This meta-analysis was guided by the Preferred Reported Items for Systematic Reviews and Meta-Analysis (PRISMA) statement issued in 200943. The electronic databases PubMed, Cochrane Library, EMBASE, and Web of Science were searched for relevant published studies up to November 6th 2016, using the following keywords: “melatonin/6-sulfatoxymelatonin/6-sulphatoxymelatonin” and “breast/mammary cancer/carcinoma”. The American Society of Clinical Oncology annual meeting abstracts have also been retrieved.
Inclusion Criteria
To be considered eligible for our meta-analysis, the relevant studies were carefully selected based on the following criteria: (1) available baseline status of enrolled women; (2) prospective studies; (3) BC incidence in relation to highest levels and lowest levels of first morning and 12-hour overnight urinary aMT6s; and (4) risk ratio (RR)/odds risk (OR) reported with a 95% confidence interval (95% CI).
Xu J and Huang L implemented the literature search, and identified eligible papers according to the inclusion criteria. In case of discrepancy, consensus was reached through discussion with Sun GP’s participation. Multiple articles covering the same research were identified. And for those overlapping publications, only the most recent publication or the one with the largest number of participants, most abundant information, and longest follow-up period was included. Data from the overlapping studies, if useful for subgroup analyses, were included as well where appropriate.
Data Extraction and Definition
Data extraction and quality assessment were conducted by Xu J and Huang L. The data extracted from each eligible study included authors’ names, years, study design, baseline characteristics, concentration of aMT6s (quartiles or tertiles), and urine samples. Case subjects were defined as women who developed BC after their enrollment in the study cohort, and matched healthy control subjects were randomly chosen, alive, and free of cancer at the time of diagnosis of the index case subject. Levels of urinary aMT6s remain stable when processing is delayed for 24–48 hours, so aMT6s resolution after sample collection is not a matter of concern13. Besides, the levels of aMT6s in first-spot morning and 12-hour overnight urine samples are both moderately associated with melatonin secretion12, 14, 44, suggesting them as reliable study samples. Lag time was defined as the interval from a participant’s enrollment to the diagnosis of BC. Concerning the urinary aMT6s levels, the first quantile corresponded to the lowest level, and the highest quantile to the highest level, as specified by each study.
Statistical Analysis
RRs were used to estimate the association between urinary aMT6s concentration and BC incidence. The 95% CIs were further calculated. The Mantel-Haenszel’s method was applied for meta-analyzing dichotomous results, and the Inverse Variance strategy was used for pooling RRs in the overall analysis as well as in the subgroup analyses based on ER status. The χ 2-based Q-test (P < 0.05 was considered significant) was applied to calculate the heterogeneity or the I 2 statistic was used to examine the extent of cross-study heterogeneity. Data were analyzed using the fixed-effect or the random-effects model based on data heterogeneity45. For analyses using the random-effects model, the Tau2 test46 was also performed to indicate heterogeneity. Funnel plot was drawn and Egger’s test47 was carried out to investigate potential publication bias. Sensitivity analyses were applied to estimate the influence of individual study on the overall effect. All statistical analyses were performed using RevMan 5.3 and Stata 11. All P values were two-sided.
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. Journal international du cancer 136, E359–386, doi:10.1002/ijc.29210 (2015).
Torre, L. A. et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians 65, 87–108, doi:10.3322/caac.21262 (2015).
Straif, K. et al. Carcinogenicity of shift-work, painting, and fire-fighting. The lancet oncology 8, 1065–1066 (2007).
Blask, D. E. et al. Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res 51, 259–269, doi:10.1111/j.1600-079X.2011.00888.x (2011).
Srinivasan, V., Spence, D. W., Pandi-Perumal, S. R., Trakht, I. & Cardinali, D. P. Therapeutic actions of melatonin in cancer: possible mechanisms. Integrative cancer therapies 7, 189–203, doi:10.1177/1534735408322846 (2008).
Grant, S. G., Melan, M. A., Latimer, J. J. & Witt-Enderby, P. A. Melatonin and breast cancer: cellular mechanisms, clinical studies and future perspectives. Expert Rev Mol Med 11, e5, doi:10.1017/S1462399409000982 (2009).
Cos, S. et al. Estrogen-signaling pathway: a link between breast cancer and melatonin oncostatic actions. Cancer detection and prevention 30, 118–128, doi:10.1016/j.cdp.2006.03.002 (2006).
Chottanapund, S. et al. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol In Vitro 28, 1215–1221, doi:10.1016/j.tiv.2014.05.015 (2014).
Korkmaz, A. et al. Combination of melatonin and a peroxisome proliferator-activated receptor-gamma agonist induces apoptosis in a breast cancer cell line. J Pineal Res 46, 115–116, doi:10.1111/j.1600-079X.2008.00635.x (2009).
Sainz, R. M. et al. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cellular and molecular life sciences: CMLS 60, 1407–1426, doi:10.1007/s00018-003-2319-1 (2003).
Schernhammer, E. S. et al. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 13, 936–943 (2004).
Crasson, M. et al. Serum melatonin and urinary 6-sulfatoxymelatonin in major depression. Psychoneuroendocrinology 29, 1–12 (2004).
Travis, R. C., Allen, N. E., Peeters, P. H., van Noord, P. A. & Key, T. J. Reproducibility over 5 years of measurements of 6-sulphatoxymelatonin in urine samples from postmenopausal women. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 12, 806–808 (2003).
Nowak, R., McMillen, I. C., Redman, J. & Short, R. V. The correlation between serum and salivary melatonin concentrations and urinary 6-hydroxymelatonin sulphate excretion rates: two non-invasive techniques for monitoring human circadian rhythmicity. Clin Endocrinol (Oxf) 27, 445–452 (1987).
Travis, R. C., Allen, D. S., Fentiman, I. S. & Key, T. J. Melatonin and breast cancer: a prospective study. Journal of the National Cancer Institute 96, 475–482 (2004).
Schernhammer, E. S. & Hankinson, S. E. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst 97, 1084–1087, doi:10.1093/jnci/dji190 (2005).
Schernhammer, E. S. et al. Urinary 6-sulfatoxymelatonin levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 100, 898–905, doi:10.1093/jnci/djn171 (2008).
Schernhammer, E. S. & Hankinson, S. E. Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses’ Health Study cohort. Cancer Epidemiol Biomarkers Prev 18, 74–79, doi:10.1158/1055-9965.EPI-08-0637 (2009).
Schernhammer, E. S. et al. Urinary 6-Sulphatoxymelatonin levels and risk of breast cancer in premenopausal women: the ORDET cohort. Cancer Epidemiol Biomarkers Prev 19, 729–737, doi:10.1158/1055-9965.EPI-09-1229 (2010).
Sturgeon, S. R. et al. Urinary levels of melatonin and risk of postmenopausal breast cancer: women’s health initiative observational cohort. Cancer Epidemiol Biomarkers Prev 23, 629–637, doi:10.1158/1055-9965.EPI-13-1028 (2014).
Wang, X. S. et al. First-morning urinary melatonin and breast cancer risk in the Guernsey Study. Am J Epidemiol 179, 584–593, doi:10.1093/aje/kwt302 (2014).
Brown, S. B. et al. Urinary melatonin concentration and the risk of breast cancer in Nurses’ Health Study II. Am J Epidemiol 181, 155–162, doi:10.1093/aje/kwu261 (2015).
Basler, M. et al. Urinary excretion of melatonin and association with breast cancer: meta-analysis and review of the literature. Breast care 9, 182–187, doi:10.1159/000363426 (2014).
Wu, A. H. et al. Sleep duration, spot urinary 6-sulfatoxymelatonin levels and risk of breast cancer among Chinese women in Singapore. International journal of cancer. Journal international du cancer 132, 891–896, doi:10.1002/ijc.27653 (2013).
Rondanelli, M., Faliva, M. A., Perna, S. & Antoniello, N. Update on the role of melatonin in the prevention of cancer tumorigenesis and in the management of cancer correlates, such as sleep-wake and mood disturbances: review and remarks. Aging Clin Exp Res 25, 499–510, doi:10.1007/s40520-013-0118-6 (2013).
Dauchy, R. T. et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer research 74, 4099–4110, doi:10.1158/0008-5472.CAN-13-3156 (2014).
Jung, B. & Ahmad, N. Melatonin in cancer management: progress and promise. Cancer Res 66, 9789–9793, doi:10.1158/0008-5472.CAN-06-1776 (2006).
Yang, W. S., Deng, Q., Fan, W. Y., Wang, W. Y. & Wang, X. Light exposure at night, sleep duration, melatonin, and breast cancer: a dose-response analysis of observational studies. Eur J Cancer Prev 23, 269–276, doi:10.1097/CEJ.0000000000000030 (2014).
Smolensky, M. H., Sackett-Lundeen, L. L. & Portaluppi, F. Nocturnal light pollution and underexposure to daytime sunlight: Complementary mechanisms of circadian disruption and related diseases. Chronobiol Int 32, 1029–1048, doi:10.3109/07420528.2015.1072002 (2015).
Fritschi, L. et al. The association between different night shiftwork factors and breast cancer: a case-control study. Br J Cancer 109, 2472–2480, doi:10.1038/bjc.2013.544 (2013).
Grant, W. B. Low 25-hydroxyvitamin D concentrations may explain the link between breast cancer risk and shift work. Int Arch Occup Environ Health 88, 819, doi:10.1007/s00420-014-1005-y (2015).
Benabu, J. C., Stoll, F., Gonzalez, M. & Mathelin, C. [Night work, shift work: Breast cancer risk factor?]. Gynecol Obstet Fertil 43, 791–799, doi:10.1016/j.gyobfe.2015.10.004 (2015).
Bellipanni, G., Bianchi, P., Pierpaoli, W., Bulian, D. & Ilyia, E. Effects of melatonin in perimenopausal and menopausal women: a randomized and placebo controlled study. Experimental gerontology 36, 297–310 (2001).
Bellipanni, G., F, D. I. M., Blasi, F. & Di Marzo, A. Effects of melatonin in perimenopausal and menopausal women: our personal experience. Ann N Y Acad Sci 1057, 393–402, doi:10.1196/annals.1356.030 (2005).
Heaney, R. P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 72, 48–54, doi:10.1111/nure.12090 (2014).
Nooshinfar, E., Safaroghli-Azar, A., Bashash, D. & Akbari, M. E. Melatonin, an inhibitory agent in breast cancer. Breast Cancer, doi:10.1007/s12282-016-0690-7 (2016).
Sanchez-Barcelo, E. J. et al. Melatonin-estrogen interactions in breast cancer. J Pineal Res 38, 217–222, doi:10.1111/j.1600-079X.2004.00207.x (2005).
Hill, S. M. et al. Molecular mechanisms of melatonin anticancer effects. Integrative cancer therapies 8, 337–346 (2009).
del Rio, B. et al. Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin. The Journal of biological chemistry 279, 38294–38302, doi:10.1074/jbc.M403140200 (2004).
Hansen, J. Increased breast cancer risk among women who work predominantly at night. Epidemiology 12, 74–77 (2001).
Thompson, C. L. & Li, L. Association of sleep duration and breast cancer OncotypeDX recurrence score. Breast cancer research and treatment 134, 1291–1295, doi:10.1007/s10549-012-2144-z (2012).
Navara, K. J. & Nelson, R. J. The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res 43, 215–224, doi:10.1111/j.1600-079X.2007.00473.x (2007).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 339, b2535, doi:10.1136/bmj.b2535 (2009).
Cook, M. R. et al. Morning urinary assessment of nocturnal melatonin secretion in older women. J Pineal Res 28, 41–47 (2000).
DerSimonian, R. & Kacker, R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials 28, 105–114, doi:10.1016/j.cct.2006.04.004 (2007).
Takkouche, B., Cadarso-Suarez, C. & Spiegelman, D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. American journal of epidemiology 150, 206–215 (1999).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634 (1997).
Acknowledgements
We would like to most sincerely thank the reviewers and editors for the thoughtful and constructive comments and suggestions. We are very grateful to Prof. Hong Su for the methodological and technical assistance, and to Miss Leah Liu for the language editing. This work was supported by the National Natural Science Foundation of China (Grants No. 81572430 and No. 81272739).
Author information
Authors and Affiliations
Contributions
J.X. and L.H.—designed the study, performed the literature search, conducted the statistical analysis, and drafted the manuscript; G.P.S.—designed the study, discussed the idea of the meta-analysis, and corrected the draft of the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, J., Huang, L. & Sun, GP. Urinary 6-sulfatoxymelatonin level and breast cancer risk: systematic review and meta-analysis. Sci Rep 7, 5353 (2017). https://doi.org/10.1038/s41598-017-05752-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05752-9
This article is cited by
-
Neratinib for HER2-positive breast cancer with an overlooked option
Molecular Medicine (2023)
-
Association between nocturnal light exposure and melatonin in humans: a meta-analysis
Environmental Science and Pollution Research (2023)
-
Association between urine 6-sulfatoxy-melatonin level and intravesical Bacillus Calmette-Guerin treatment–induced sleep quality deterioration in patients with non-muscle invasive bladder cancer
Supportive Care in Cancer (2022)
-
Protective role of melatonin in breast cancer: what we can learn from women with blindness
Cancer Causes & Control (2022)
-
Effects of melatonin on cardiovascular risk factors and metabolic syndrome: a comprehensive review
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.