Abstract
Traumatic brain injury-induced acute lung injury (TBI-ALI) is a serious complication after brain injury for which predictive factors are lacking. In this study, we found significantly elevated blood glutamate concentrations in patients with TBI or multiple peripheral trauma (MPT), and patients with more severe injuries showed higher blood glutamate concentrations and longer durations of elevated levels. Although the increase in amplitude was similar between the two groups, the duration was longer in the patients with TBI. There were no significant differences in blood glutamate concentrations in the patients with MPT with regard to ALI status, but the blood glutamate levels were significantly higher in the patients with TBI-ALI than in those without ALI. Moreover, compared to patients without ALI, patients with TBI showed a clearly enhanced inflammatory response that was closely correlated with the blood glutamate levels. The blood glutamate concentration was also found to be a risk factor (adjusted odds ratio, 2.229; 95% CI, 1.082–2.634) and was a better predictor of TBI-ALI than the Glasgow Coma Scale (GCS) score. These results indicated that dramatically increased blood glutamate concentrations were closely related to the occurrence of TBI-ALI and could be used as a predictive marker for “at-risk” patients.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is a common, major cause of disability and death that occurs secondarily to accidents, competitive sports and even war. A primary lesion forms at the point of impact, but there are also secondary injuries of TBI, including secondary brain injury and peripheral organ injuries1, 2, which are key factors influencing the clinical prognosis3. Previous studies have primarily focused on secondary brain injuries caused by rapidly elevated brain glutamate-induced excitotoxicity4, 5. However, acute lung injury (ALI) induced by brain injury, which is also known as traumatic brain injury-induced acute lung injury (TBI-ALI) or neurogenic pulmonary oedema (NPE), is another salient cause of patient death6, 7. Although it has been reported that the incidence of TBI-ALI might reach 50% or more8, this disease has not received sufficient attention in clinical practice because it is relatively unpredictable, has a lack of specific, aetiological diagnostic markers and has similar clinical manifestations to severe pulmonary infection. Thus the diagnosis of TBI-ALI is mainly dependent on exclusion, and it is often overlooked or misdiagnosed9, 10. In contrast to TBI-ALI, multiple peripheral trauma (MPT), which refers to multiple peripheral organ damage or tissue trauma that results from a variety of causes without central nervous system injury, and multiple peripheral trauma-induced acute lung injury (MPT-ALI) are easily induced by MPT-associated haemorrhagic shock, fat embolism, disseminated intravascular coagulation (DIC), or other causes11, 12.
Two classical theories, blast theory and permeability defect theory, have been proposed and are widely accepted as explanations of the pathogenesis of TBI-ALI13. However, our recent studies have shown that in addition to brain glutamate levels, blood glutamate levels increase dramatically after TBI, especially in patients with TBI-ALI14. Additionally, a previous report found that increased blood glutamate levels might cause peripheral organ damage15, but it remained unclear whether these increased levels were related to the occurrence of TBI-ALI.
Therefore, based on our preliminary findings, the aims of this study were to investigate the relationship between high concentrations of blood glutamate and TBI-ALI and to explore the possible role and the predictive power of blood glutamate in the development of TBI-ALI. We accomplished these goals by collecting more cases of TBI and MPT than in previous studies while taking into account that TBI patients often present with peripheral injury. Thus, MPT/MPT-ALI patients served as a trauma control group to eliminate the interference of peripheral injury in TBI/TBI-ALI patients, thus allowing for further clarification of the role of blood glutamate in this particular type of ALI.
Results
Patient characteristics
The patients ranged in age from 19 to 87 years, with an average age of 47 years. The cohort was predominantly male (79.3%) and was more likely to have been involved in traffic accidents (59.8%). In total, 78.3% (n = 72) of the patients underwent a surgical procedure. A total of 37 patients were also diagnosed with ALI, including 21 who were diagnosed with TBI-ALI and 16 who were diagnosed with MPT-ALI. The median hospital stay was 24 or 28 days for the patients with TBI or MPT, respectively, and 5 patients died more than 7 days after admission (Table 1). The detailed patient information is listed in Tables S1–S3; the details of the patients who presented with ALI are listed separately in Table 2. A single sample was obtained from 50 healthy volunteers (age range, 43–67 years; average age, 50 years; 68% men).
Blood glutamate concentration was obviously elevated in patients with TBI or MPT
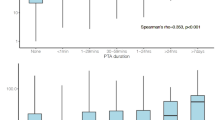
As shown in Fig. 1a and b, the mean blood glutamate concentration in patients with either TBI or MPT was significantly higher than in the controls (p = 0.008 and 0.004, respectively). However, there was no significant difference between the two patient groups (p > 0.05) (Fig. 1b). Moreover, we observed time-dependent changes in the blood glutamate concentrations in these two groups. The glutamate concentrations were significantly higher in the patients with TBI than in the controls at 1, 3, and 7 days after admission, and no significant difference was observed among these three time points (Figure S1). However, elevated blood glutamate concentrations were only observed on days 1 and 3 in the patients with MPT, and these levels quickly returned to baseline within 7 days (Figure S1).
Distribution of patients at each concentration interval (every 25 μM) (a) and the mean blood glutamate concentrations (b) in the control, MPT and TBI groups. The time-dependent changes in blood glutamate concentration in patients with different severities of TBI (c) or MPT (d) are shown. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control group; # p < 0.05, ## p < 0.01, and ### p < 0.001 compared between the two groups; NS, not significant. Data are expressed as the means ± SE. Significance was determined by ANOVA with Tukey-Kramer post hoc tests or nonparametric Mann-Whitney U tests. MPT, multiple peripheral trauma; TBI, traumatic brain injury.
Considering that different severities of TBI might result in various elevations of the blood glutamate concentration, we further analysed the relationship between disease severity and glutamate concentration. In the TBI patients, the blood glutamate concentrations significantly increased in those patients with severe injuries within 7 days and were consistently higher than the concentrations in patients with moderate or mild injuries. There was no significant difference between the latter two groups (Figs 1c and S1). However, elevated blood glutamate levels were only observed on the 1st and 3rd day in the patients with MPT (Fig. 1d), although higher blood glutamate concentrations were observed in patients with more severe injuries (Figure S1).
Elevated blood glutamate concentrations were closely related to the occurrence of TBI-ALI but not MPT-ALI
To analyse the relationship between blood glutamate concentration and ALI, we further divided the two patient groups into two subgroups according to whether the patient had ALI complications. In both situations, blood glutamate levels were always significantly higher in patients with TBI-ALI than in patients with TBI but without ALI (p < 0.001); however, in the MPT group, there was no significant difference in blood glutamate levels between patients with or without ALI (Fig. 2a). For all patients, blood glutamate concentrations were categorised according to the interquartile range (IQR), and the incidence of TBI-ALI in the high-level group was much higher than that in the medium- and low-level groups (p = 0.001); however, this trend was not observed in patients with MPT-ALI (Fig. 2b). Additionally, regression analysis showed that blood glutamate concentration upon admission was a risk factor for TBI-ALI (adjusted odds ratio (OR), 2.229; 95% CI, 1.082–2.634; p = 0.005) but not for MPT-ALI (adjusted OR, 0.996; 95% CI, 0.965–1.028; p = 0.802) (Table 3). Based on these results, we further hypothesised that blood glutamate concentrations might be closely related to the occurrence of TBI-ALI, but not MPT-ALI.
(a) Comparison of glutamate concentrations between subgroups (with or without ALI) of patients with TBI or MPT. Data are expressed as the means ± SE. (b) Incidence of ALI according to different blood glutamate levels in patients with TBI or MPT. *p < 0.05 and ***p < 0.001 for comparisons between the two groups using two-tailed Student’s t-tests or nonparametric Mann-Whitney U tests; NS, not significant. MPT, multiple peripheral trauma; TBI, traumatic brain injury.
Blood glutamate levels were closely associated with the inflammatory response in TBI-ALI
In the TBI group, all blood inflammatory markers (procalcitonin (PCT), C-reactive protein (CRP) and counts of white blood cells (WBCs) and neutrophils (NEUs)) were present at higher levels in the patients with ALI than in those without ALI (p < 0.05); however, no such difference was observed in the MPT group. Surprisingly, the patients with MPT-ALI had lower CRP levels than the patients without ALI (Table 4). Notably, these results were consistent with the blood glutamate concentrations (Fig. 2a). Concurrently, we found an obvious correlation between blood glutamate levels and inflammatory markers in patients with TBI-ALI (r = 0.593, 0.670, 0.659, 0.596 for PCT, CRP, WBCs and NEUs, respectively; p = 0.000) (Figure S2). We also noted that, although the intensity of inflammatory responses was similar between groups (Table 4), the patients with TBI-ALI had significantly higher blood glutamate concentrations than the patients with MPT-ALI (p = 0.018 for 1st day; p < 0.001 for the mean value) (Fig. 2a). These results further suggested that the blood glutamate concentration is closely related to TBI-ALI and might be associated with the inflammatory response in this condition.
The initial blood glutamate concentration was a better predictor of TBI-ALI than the Glasgow Coma Scale score
Because the occurrence of TBI-ALI is typically concealed but progresses rapidly (Table 2), we tested whether the Glasgow Coma Scale (GCS) score and the blood glutamate concentration could be used as predictors of patients with TBI within 24 hours of hospital admission. Although the differences in the incidence of TBI-ALI in patients with severe, moderate and mild brain injuries were significant (p = 0.002), regression analysis showed that the GCS score was not a risk factor for TBI-ALI (adjusted OR, 1.197; 95% CI, 0.981–1.459; p = 0.076) (Table 3). Furthermore, a correlation analysis showed no obvious correlation between the initial blood glutamate levels and the GCS score (r = 0.224, p = 0.154) (Fig. 3a), and the median GCS score was not higher in patients with ALI than in patients without ALI (p = 0.062) (Fig. 3b).
(a) There was no significant correlation between blood glutamate levels on admission and GCS score in patients with TBI (r = 0.224, p = 0.154). (b) No significant difference in GCS score was observed between patients with or without TBI-ALI (p = 0.062) using nonparametric Mann-Whitney U tests. (c). The AUC-ROCs showed that the predictive value of glutamate levels (AUC = 0.792; 95% CI, 0.710–0.873) was better than that of GCS score (AUC = 0.652; 95% CI, 0.565–0.739) (p = 0.022) (d). ROC curve showing the logistic regression model for the combination of glutamate levels and GCS score (AUC = 0.829; 95% CI, 0.706–0.953), which was better than GCS score (p = 0.039) but not glutamate concentration (p = 0.243). ALI, acute lung injury; GCS, Glasgow Coma Scale; Glu, glutamate; TBI, traumatic brain injury.
Based on the receiver operating characteristic (ROC) curve, the optimal cut-off value for the blood glutamate level at admission as an indicator for a diagnosis of TBI-ALI was 99.89 μM, which yielded an area under the receiver-operating characteristic curve (AUC-ROC) of 0.792 (95% CI, 0.710–0.873). For the GCS score, the AUC-ROC was 0.652 (95% CI, 0.565–0.739) (Fig. 3c). The likelihood ratio test showed a significant increase in the predictive value of the glutamate concentration compared with that of the GCS score (p = 0.022). A logistic regression resulted in a combined glutamate and GCS score model with an AUC-ROC of 0.829 (95% CI, 0.706–0.953). The predictive power of the combined model was better than the GCS score (p = 0.039), but not the blood glutamate level alone (p = 0.243) (Fig. 3d).
Discussion
Despite biological variations, blood glutamate concentrations are relatively stable under physiological conditions16, 17. However, in the present study, we found that the blood glutamate concentrations of patients with TBI or MPT were significantly higher than those in healthy volunteers and were related to injury severity in both patient groups, although the mechanisms involved may be different. In the patients with TBI, the elevated glutamate concentrations persisted for 7 days, whereas the patients with MPT showed a peak in blood glutamate levels on day 1 that quickly declined to baseline within 7 days. Although the mean glutamate levels were similar between the two patient groups, a subgroup analysis showed that the patients with TBI-ALI had much higher blood glutamate levels than the patients with MPT-ALI, and both subgroups had similar inflammatory response levels. Though the source of blood glutamate remains unclear, previous research found that blood cells, especially neutrophils18 and platelets19, and peripheral tissues20 might be the primary source of blood glutamate. However, considering that the blood-brain barrier (BBB) is effectively “opened twice” after brain injury21, the much higher concentrations of intracranial glutamate may contribute substantially to the prolonged increase in blood glutamate concentrations in patients with TBI22. By contrast, in patients with MPT, when the inflammatory response is attenuated and the tissue recovers from injury, the level of glutamate released into the blood might rapidly decrease.
TBI-ALI is the major complication following TBI, but its specific pathogenesis remains unclear10, 23. Our previous animal experiments revealed that the pathology of TBI-ALI may be related to blood glutamate levels14. In the present study, we observed that blood glutamate levels do not correlate with MPT-ALI but are closely related to the occurrence of TBI-ALI, which further confirmed our findings. These results also suggest that ALI alone is not a primary factor for elevated blood glutamate levels. In contrast, in our recent animal studies, blood glutamate did not play an important role in the development of MPT-ALI even if the glutamate levels have reached or exceeded those measured in TBI-ALI (data not shown).
The inflammatory response is the main pathological change during the development of ALI24, and previous studies have also found that glutamate signalling plays an important role in lung inflammation25,26,27. In this study, we found a close connection between the levels of blood glutamate and the inflammatory response in patients with TBI-ALI. However, we could not determine whether the elevations in blood glutamate levels were induced by or caused an inflammatory response or even whether there was a reciprocal causal relationship. Our previous research has shown that localised increases in glutamate levels could convert the anti-inflammatory response of adenosine 2A receptor (A2AR) to a pro-inflammatory response in the brain28. Thus, the polymerisation of metabotropic glutamate receptor 5 (mGluR5) and A2AR, which is activated by a high concentration of blood glutamate, is likely to further strengthen this pro-inflammatory effect14. This mechanism might offer a possible explanation for this finding. Recent studies have also highlighted the important role of neuroimmunologic modulation in ALI29, though the specific mechanism by which blood glutamate contributes to the occurrence and development of TBI-ALI remains unknown and requires further investigation.
It is common in clinical practice to use scores (such as the GCS score) as indicators or references for assessing patient prognoses. However, in this study, the GCS score was less accurate than the blood glutamate level in predicting TBI-ALI. Because the clinical manifestation of TBI-ALI lacks specificity and is often obscured by the primary disease, there is an urgent need for an objective and easily quantifiable parameter to predict the disease; therefore, blood glutamate levels make sense for use in clinical practice. Additionally, several studies have confirmed that lowering blood glutamate levels by haemofiltration30 or peritoneal dialysis31 exerts a protective effect on the brain. Based on the evidence revealed in this study, we believe that in the future, blood glutamate levels might be a good therapeutic target not only for brain injury but also for complicated lung injury in clinical practice.
Strengths and limitations
To the best of our knowledge, our study is the first to provide clinical evidence that elevated blood glutamate concentrations are correlated with the incidence of TBI-ALI. We also preliminarily discussed the possible mechanism underlying this relationship and suggested that the blood glutamate concentration could be used to identify and treat at-risk patients. However, because this study was observational in nature rather than a randomised control study, we could not identify the causal relationships between blood glutamate levels, inflammation and TBI-ALI. It is notable, however, that our previous research14 and recent animal studies (data not shown) have strongly suggested the triggering role of blood glutamate in TBI-ALI. Therefore, in future studies, it will be necessary to assess these relationships by examining how changes in blood glutamate levels in patients affect the development of ALI and by observing subsequent changes. Another limitation is shown in Table S3: specifically, aspartate transaminase (AST) and alanine aminotransferase (ALT) levels were significantly higher in the patients with MPT. As both of these proteins function as primary blood glutamate-scavenging enzymes, it is unknown whether this elevation was specific; a larger cohort of MPT patients may be needed for this determination. Finally, the relatively small sample size in this study precluded a more refined subgroup classification; therefore, even though the multiple logistic regression analysis was carried out on some major confounding factors (Table 3), there were many other hidden factors involved in this analysis. Thus, our current results must be carefully interpreted, and further studies with a larger sample size study and longer-term outcomes are needed to confirm our findings.
Conclusions
Blood glutamate concentrations dramatically increased following TBI and were closely related to the inflammatory response in TBI-ALI. Moreover, blood glutamate was found to be a good predictor of TBI-ALI and is a potential target for intervention during this condition.
Methods
Patients and controls
From August 2015 to June 2016, 92 hospitalised patients, including 50 diagnosed with TBI and 42 diagnosed with MPT, were recruited from the Neurosurgery and ICU Departments of Daping Hospital after admission. Clinical diagnosis of TBI and indicators for acute head injury were assessed according to National Institute of Health and Care Excellence (NICE) criteria, while MPT was defined as an Abbreviated Injury Scale (AIS) score greater than or equal to 3 in one or more body regions other than the head32; all diagnoses were confirmed by computed tomography (CT) or X-ray, and Marshall scores were also recorded33. The present study assessed only patients who were older than 16 years. Patients with any significant extracranial injury (AIS > 1), a blast-induced or penetrating head injury, or pre-existing chronic brain diseases (e.g., epilepsy or chronic subdural haematoma) were excluded from the TBI group. Other exclusion criteria for both groups included direct chest trauma or pulmonary contusion, pre-existing major diseases of the lung (e.g., pneumonia or chronic obstructive pulmonary disease (COPD)), liver (e.g., liver dysfunction), heart (e.g., coronary heart disease) or blood system (e.g., haemolytic anaemia)34, 35. Additionally, fifty healthy volunteers were selected as normal controls. After admission, the protocols used for treatment of the patients with TBI were based on international guidelines established by the Brain Trauma Foundation (BTF)36. The patients with MPT were managed using routine surgical or medical treatments. This study was approved by the ethics committee of the Research Institute of Surgery and Daping Hospital. All participants (or legal guardians) in this study provided written informed consent. All analyses and data handling were performed under the guidelines of the NIH and in accordance with the Declaration of Helsinki and its later amendments. Trial registration: http://www.chictr.org.cn (registered on 19 July, 2015). Unique identifier: ChiCTR-RPC-15006770.
Injury severity classification
Either the initial GCS score or the Abbreviated Injury Scale-Injury Severity Score (AIS-ISS) was obtained and recorded as appropriate by experienced staff at the time of patient arrival with or without resuscitation after injury. For the patients with TBI, the GCS scores for severe, moderate, and mild cases were 3–7, 8–12, and 13–15 points, respectively37, 38. Under the AIS-ISS, the patients with MPT were classified as mild (<16), moderate (16–25) or severe (>25)39, 40.
Diagnosis of complicated ALI
In this study, two types of ALI, TBI-ALI and MPT-ALI, were discussed. Patients meeting The Berlin Definition for ALI/ARDS were characterised by the following: rapidly progressing dyspnoea, cyanosis and other symptoms of respiratory failure, with varying decreases in PaO2 and increases in PaCO2 after admission; an oxygenation index (PaO2/FiO2) < 300 with pulmonary arterial wedge pressure (PAWP) < 18 mmHg; chest radiography (CT or X-ray) showing localised or diffused oedema or inflammatory changes; and with or without significantly increased blood inflammatory markers24, 41. To exclude interference from concurrent lung infection, we excluded those patients with confirmed pathogenic microbial infections42.
Clinical and laboratory assays
Venous blood samples (5 mL) were collected in heparinised tubes from the hospitalised patients and controls at the time of admission (examination) and at 6:00 am on the 3rd and 7th days after admission. Inflammatory markers (including PCT, CRP, and routine blood markers), indicators of hepatic function (e.g., AST, ALT and albumin) and indicators of renal function (e.g., creatinine and blood urea nitrogen (BUN)) were tested at the clinical laboratory, and the blood glutamate levels were analysed with our previously reported method43 at the Molecular Biology Center of our hospital by technicians blinded to the patient groups.
Statistical analysis
Data are expressed as the means ± standard errors (SE) in histograms and as the means (SE), medians (IQR) and n (%) in tables. Pearson’s chi-squared test was used to analyse categorical variables, and one-way analysis of variance (ANOVA) with Tukey-Kramer’s post hoc tests, two-tailed t-tests or nonparametric Mann-Whitney U tests were used to analyse continuous variables. Spearman’s correlation test and uni-/multivariate regression models were used to analyse the relationships between two groups; the results were reported as either the r value or the unadjusted/adjusted ORs with 95% confidence interval (CI), respectively. The AUC-ROCs were calculated to predict the occurrence of TBI-ALI44. A two-tailed value of p < 0.05 was considered statistically significant. Statistical analyses were performed using Sigma plot software (version 12.5, Systat Software Inc, San Jose, CA) by a statistician blinded to the study.
Data availability statement
All data generated or analysed during this study are included in this published article (and its Supplementary files).
References
Holland, M. C. et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J. Trauma 55, 106–111 (2003).
Oddo, M. et al. Acute lung injury is an independent risk factor for brain hypoxia after severe traumatic brain injury. Neurosurgery 67, 338–344 (2010).
Arbour, R. B. Traumatic brain injury: pathophysiology, monitoring, and mechanism-based care. Crit. Care Nurs. Clin. North Am. 25, 297–319 (2013).
Palmer, A. M. et al. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J. Neurochem. 61, 2015–2024 (1993).
Chamoun, R., Suki, D., Gopinath, S. P., Goodman, J. C. & Robertson, C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 113, 564–570 (2010).
Fontes, R. B. et al. Acute neurogenic pulmonary edema: case reports and literature review. J. Neurosurg. Anesthesiol. 15, 144–150 (2003).
Muroi, C. et al. Neurogenic pulmonary edema in patients with subarachnoid hemorrhage. J. Neurosurg. Anesthesiol. 20, 188–192 (2008).
Otero, H. J. & Pollock, A. N. Neurogenic pulmonary edema. Pediatr. Emerg. Care 30, 845–846 (2014).
Davison, D. L., Terek, M. & Chawla, L. S. Neurogenic pulmonary edema. Crit. Care 16, 212 (2012).
Sedy, J., Kunes, J. & Zicha, J. Pathogenetic mechanisms of neurogenic pulmonary edema. J. Neurotrauma 32, 1135–1145 (2015).
Wu, J. et al. Analysis of clinical risk factors associated with the prognosis of severe multiple-trauma patients with acute lung injury. J. Emerg. Med. 43, 407–412 (2012).
Harr, J. N. et al. Antiplatelet therapy is associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Crit. Care Med. 41, 399–404 (2013).
Pyeron, A. M. Respiratory failure in the neurological patient: the diagnosis of neurogenic pulmonary edema. J. Neurosci. Nurs. 33, 203–207 (2001).
Dai, S. S. et al. Plasma glutamate-modulated interaction of A2AR and mGluR5 on BMDCs aggravates traumatic brain injury-induced acute lung injury. J. Exp. Med. 210, 839–851 (2013).
Ortiz, G. G. et al. Monosodium glutamate-induced damage in liver and kidney: a morphological and biochemical approach. Biomed. Pharmacother. 60, 86–91 (2006).
Smith, Q. R. Transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr. 130, 1016S–1022S (2000).
Stegink, L. D., Filer, L. J. Jr. & Baker, G. L. Plasma, erythrocyte and human milk levels of free amino acids in lactating women administered aspartame or lactose. J. Nutr. 109, 2173–2181 (1979).
Collard, C. D. et al. Neutrophil-derived glutamate regulates vascular endothelial barrier function. J. Biol. Chem. 277, 14801–14811 (2002).
Aliprandi, A. et al. Increased plasma glutamate in stroke patients might be linked to altered platelet release and uptake. J. Cereb. Blood Flow Metab. 25, 513–519 (2005).
Graham, T. E., Sgro, V., Friars, D. & Gibala, M. J. Glutamate ingestion: the plasma and muscle free amino acid pools of resting humans. Am. J. Physiol. Endocrinol. Metab. 278, E83–E89 (2000).
Leibowitz, A., Boyko, M., Shapira, Y. & Zlotnik, A. Blood glutamate scavenging: insight into neuroprotection. Int. J. Mol. Sci. 13, 10041–10066 (2012).
Cohen-Kashi-Malina, K., Cooper, I. & Teichberg, V. I. Mechanisms of glutamate efflux at the blood-brain barrier: involvement of glial cells. J. Cereb. Blood Flow Metab. 32, 177–189 (2012).
Busl, K. M. & Bleck, T. P. Neurogenic pulmonary edema. Crit. Care Med. 43, 1710–1715 (2015).
Ards Definition, T. F. et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307, 2526–2533 (2012).
da Cunha, A. A. et al. N-methyl-D-aspartate glutamate receptor blockade attenuates lung injury associated with experimental sepsis. Chest 137, 297–302 (2010).
Said, S. I., Dey, R. D. & Dickman, K. Glutamate signalling in the lung. Trends Pharmacol. Sci. 22, 344–345 (2001).
Maillet, I. et al. Glufosinate aerogenic exposure induces glutamate and IL-1 receptor dependent lung inflammation. Clin. Sci. (Lond.) 130, 1939–1954 (2016).
Dai, S. S. et al. Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J. Neurosci. 30, 5802–5810 (2010).
Su, X., Matthay, M. A. & Malik, A. B. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J. Immunol. 184, 401–410 (2010).
Rogachev, B. et al. The effects of hemodialysis on blood glutamate levels in chronic renal failure: implementation for neuroprotection. J. Crit. Care 27, 743–e1 (2012).
Rogachev, B. et al. The effects of peritoneal dialysis on blood glutamate levels: implementation for neuroprotection. J. Neurosurg. Anesthesiol. 25, 262–266 (2013).
Yuan, Q. et al. Effects and clinical characteristics of intracranial pressure monitoring-targeted management for subsets of traumatic brain injury: an observational multicenter study. Crit. Care Med. 43, 1405–1414 (2015).
Marshall, L. F. et al. The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 9, S287–S292 (1992).
McQuistion, K. et al. Insurance status and race affect treatment and outcome of traumatic brain injury. J. Surg. Res. 205, 261–271 (2016).
Oresic, M. et al. Human serum metabolites associate with severity and patient outcomes in traumatic brain injury. EBioMedicine 12, 118–126 (2016).
Brain, T. Foundation, American Association of Neurological Surgeons & Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J. Neurotrauma 24, S1–S106 (2007).
Tian, H. L. et al. Risk factors for posttraumatic cerebral infarction in patients with moderate or severe head trauma. Neurosurg. Rev. 31, 431–437 (2008).
McNett, M. et al. The FOUR score and GCS as predictors of outcome after traumatic brain injury. Neurocrit. Care 21, 52–57 (2014).
Bolorunduro, O. B. et al. Validating the Injury Severity Score (ISS) in different populations: ISS predicts mortality better among Hispanics and females. J. Surg. Res. 166, 40–44 (2011).
Rowell, S. E. et al. Specific abbreviated injury scale values are responsible for the underestimation of mortality in penetrating trauma patients by the injury severity score. J. Trauma 71, S384–S388 (2011).
Yamagishi, T., Ochi, N., Yamane, H. & Takigawa, N. Neurogenic pulmonary edema after subarachnoid hemorrhage. J. Emerg. Med. 46, 683–684 (2014).
Kalil, A. C. et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 63, e61–e111 (2016).
Li, W. et al. Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience 151, 1198–1207 (2008).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1988).
Acknowledgements
We gratefully acknowledge the assistance and cooperation of the physicians, nurses and patients included in this study. We also thank the technicians and statisticians for their help and guidance. This study was partially supported by the National Natural Science Foundation of China (31171022).
Author information
Authors and Affiliations
Contributions
W.B., Y.-L.N. and Y.-G.Z. designed the research and drafted the manuscript. P.L. and Y.Z. analysed the data. N.Y., X.C. and Y.-L.J. performed the experiments. W.-L.Z., W.-Q.Y., D.-P.J. and L.-Y.C. were responsible for patients enrolment and management. Y.-G.Z. revised the manuscript and contributed reagents, materials and analysis tools. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
41598_2017_5574_MOESM1_ESM.pdf
Dramatic increases in blood glutamate concentrations are closely related to traumatic brain injury-induced acute lung injury
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bai, W., Zhu, WL., Ning, YL. et al. Dramatic increases in blood glutamate concentrations are closely related to traumatic brain injury-induced acute lung injury. Sci Rep 7, 5380 (2017). https://doi.org/10.1038/s41598-017-05574-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05574-9
This article is cited by
-
Glutamate triggers the expression of functional ionotropic and metabotropic glutamate receptors in mast cells
Cellular & Molecular Immunology (2021)
-
A systematic review of biomarkers multivariately associated with acute respiratory distress syndrome development and mortality
Critical Care (2020)
-
Tonic NMDA receptor signalling shapes endosomal organisation in mammalian cells
Scientific Reports (2020)
-
Brain-Derived Glia Maturation Factor β Participates in Lung Injury Induced by Acute Cerebral Ischemia by Increasing ROS in Endothelial Cells
Neuroscience Bulletin (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.