Abstract
Energy expenditure (EE) during walking includes energy costs to move and support the body and for respiration and circulation. We measured EE during walking under three different oxygen concentrations. Eleven healthy, young, male lowlanders walked on a treadmill at seven gait speeds (0.67–1.83 m s−1) on a level gradient under normobaric normoxia (room air, 21% O2), moderate hypoxia (15% O2), and severe hypoxia (11% O2). By comparing the hypoxia-induced elevation in heart rate (HR [bpm]), ventilation (VE [L min−1]) with the change in energy expenditure (EE [W]) at each speed, we were able to determine circulatory and respiratory costs. In a multivariate model combining HR and VE, respiratory costs were 0.44 ± 0.15 W per each L min−1 increase in VE, and circulatory costs were 0.24 ± 0.05 W per each bpm increase in HR (model adjusted r2 = 0.97, p < 0.001). These VE costs were substantially lower than previous studies that ignored the contribution of HR to cardiopulmonary work. Estimated HR costs were consistent with, although somewhat higher than, measures derived from catheterization studies. Cardiopulmonary costs accounted for 23% of resting EE, but less than 5% of net walking costs (i.e., with resting EE subtracted).
Similar content being viewed by others
Introduction
Humans’ bipedal walking gait is a defining feature of our lineage, and the metabolic costs of walking have figured prominently in discussions of human evolution and ecology1,2,3. The energy cost of walking must include energy expenditure (EE) to move and support the body and to fuel physiological changes, such as increased ventilation and circulation, needed to meet increased metabolic demand. To date, studies of walking cost have largely ignored the costs of ventilation and circulation, focusing instead on the factors known to affect the costs of movement such as walking speed, internal and external work4, 5, gradients6, 7, body mass8,9,10,11, and load carriage12,13,14,15. Locomotor cost is of central importance in human ecology16, 17, behavior18, 19, evolution1,2,3, and biomechanics research4, 5, 7, 12, and a full accounting of human walking cost and its determinants requires an understanding of the contribution of cardiopulmonary work to total metabolic cost.
Respiratory costs have been measured in humans and other vertebrates during rest and exercise20,21,22,23,24,25,26,27,28,29,30. Early studies of respiratory cost at rest, measured by having the subject breathe through a long tube to increase the dead space and thus the work needed to breathe, found that ventilation costs in humans at rest were quite low (<1 mlO2 per L VE)21, 27. Subsequent studies reported substantially greater respiratory costs during exercise, though still less than 10% of total exercise energy expenditure20, 22, 23, 25, 29, 30. However, these studies have generally used an experimental design in which respiratory costs are not measured during exercise, but rather during voluntary hyperpnea in which subjects match their respiratory frequency and tidal volume to levels observed during exercise20. The contribution of increased heart rate (HR) is generally ignored, although HR can increase substantially during hyperpnea31 and could contribute substantially to the measured increase in energy expenditure. Further, these studies have typically focused on running and other moderate- or high-intensity exercise. The contribution of cardiopulmonary work to walking cost may be higher, as the total cost of walking is considerably lower32.
Measuring walking costs under hypoxic conditions provides a potential experimental design for measuring cardiopulmonary costs during exercise. At high altitude, and under normobaric hypoxia, a reduction of alveolar PO2 limits pulmonary O2 diffusion capability, which induces a decrease in arterial O2 saturation (SpO2)33, 34. Under these conditions, pulmonary ventilation (VE) increases within minutes35, followed by increases in HR 36. These responses are generally considered part of the compensatory mechanisms against hypoxia with reduced oxygen delivery to the peripheral tissues. These results suggest that if hypoxic-induced increases in energy cost of ventilation and/or circulation are observed even at resting conditions, differential energy costs during walking under hypoxia would also be observed.
We developed and tested an experimental model to evaluate the energy cost of ventilation and circulation during walking using acute hypoxic exposure. We used pulmonary ventilation (\({{\rm{V}}}_{{\rm{E}}}\)) and heart rate (HR) as indices of respiratory and circulatory work, respectively. Healthy male adults, not acclimated to hypoxic or high-altitude conditions, walked over a range of speeds under three different oxygen concentrations (normoxia, moderate, and severe hypoxia) at level gradient. This design enabled us to vary the cardiopulmonary work done while keeping the energy costs to support and move the body (i.e., the mechanical cost of walking) constant across conditions. Our objective was to determine the costs of ventilation and circulation during walking, and to compare values measured during walking to those measured during standing at rest.
Results
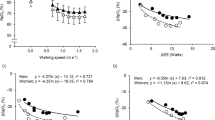
Mean (±SD) values for EE, VE, and HR at rest and each walking speed, and changes in these variables with hypoxia are given in Table 1. All three change variables, ΔEE, ΔVE, and ΔHR, increased with speed and degree of hypoxia, with severe hypoxia having a much stronger effect than moderate hypoxia (Fig. 1). Both walking speed and the degree of hypoxia were significant predictors for ΔEE (model adjusted r2 = 0.82, p < 0.001), ΔVE (adj. r2 = 0.66, p < 0.001), and ΔHR (adj. r2 = 0.92, p < 0.001) in multiple regression.
Mean values of differences in energy expenditure (EE: top panel), pulmonary ventilation (VE: middle panel), and heart rate (HR: bottom panel) between normoxic trial and moderate hypoxia (open circles), and between normoxic trial and severe hypoxia (filled circles) at rest and each walking speed. The number of 0.0 on the X axis indicate at rest.
Bivariate analyses of ΔEE revealed significant correlations with both ΔVE and ΔHR (Fig. 2). With the intercept set at 0, EE increased with ventilatory effort as ΔEE = 1.10 ± 0.12 ΔVE (r2 = 0.84, p < 0.001), or roughly 1 Watt for every 1 L min−1 increase in VE. This cost is equivalent to 3.3 mlO2 per L VE, assuming 20.1 J per mlO2. Energy expenditure increased with circulation as ΔEE = 0.36 ± 0.04ΔHR (r2 = 0.81, p < 0.001), or roughly 1 Watt for every 3 beats per minute increase in HR. Slopes changed less than 3% when the three hypoxia-speed conditions with negative ΔEE values were excluded from analysis (Fig. 2).
ΔVE and ΔHR were strongly correlated (r2 = 0.96, second order polynomial regression, Fig. 2), making it difficult to determine their independent effects on EE. When ΔVE and ΔHR were entered as independent variables in a multiple regression, ΔVE achieved significance (t(14) = 2.33, p = 0.036) but ΔHR did not (t(14) = 1.42, p = 0.178). However, when the three hypoxia-speed conditions with negative ΔEE values were excluded from the analysis, both ΔVE (t(11) = 2.87, p = 0.015) and ΔHR (t(11) = 4.65, p < 0.001) were significant, and the fit of the model was very strong (ΔEE = 0.44 ± 0.15ΔVE + 0.24 ± 0.05ΔHR, adj. r2 = 0.97, p < 0.001). Notably, this multivariate model reduces the estimated individual costs of ventilation and circulation compared to bivariate analyses. Ventilatory costs are approximately 1 Watt for every 2.3 L min−1 increase in VE; circulatory costs are approximately 1 Watt for every 4 beats per minute increase in HR. The fit of this predictive model for all data is shown in Fig. 3.
Observed ΔEE plotted against that predicted ΔEE from the least squares regression with VE and HR: ΔEE = 0.44ΔVE + 0.24ΔHR; see text. Line indicates y = x. This predictive fit model for data was calculated using the hypoxia-speed conditions with only positive ΔEE values (filled circles), but excluding the hypoxia-speed conditions with negative ΔEE values (open circles).
We used the combined model with ΔVE and ΔHR (i.e., EE = 0.44VE + 0.24HR) along with VE and HR values at each condition to estimate the energy cost of cardiopulmonary work at rest and during walking under normoxia, moderate hypoxia, and severe hypoxia. At rest, the estimated energy cost of breathing and circulation accounted for approximately one-fourth of total EE across conditions, rising slightly under severe hypoxia compared to normoxia and moderate hypoxia. As walking speed and EE increased, the percentage of EE attributed to cardiopulmonary work decreased under all conditions. This change was similar in moderate hypoxia and severe hypoxia (Table 2).
Next, we subtracted EE and estimated cardiopulmonary expenditure during resting from values measured during walking in order to calculate net walking EE and net walking cardiopulmonary work. Net cardiopulmonary energy expenditure accounted for 3% of net walking EE at 0.67 m s−1, rising to 5% at 1.83 m s−1 under both normoxia and moderate hypoxia. Under severe hypoxia, these values were slightly higher compared to normoixa and moderate hypoxia. (Table 2).
Finally, we applied the multivariate model with ΔVE and ΔHR to the VE and HR values at each condition to calculate the relative contributions of ventilatory and circulatory work to cardiopulmonary energy expenditure. Across normoxic and both hypoxic conditions, the estimated energy cost of circulation exceeded that of ventilation. Heart beat accounted for 80% of cardiopulmonary work at rest in all three conditions. The proportion of cardiopulmonary work attributable to HR decreased regularly with walking speed under all conditions (Fig. 4).
Discussion
Both breathing and circulation have measureable effects on EE during both resting and walking. While our findings are consistent with studies using hyperpnea at rest to mimic the costs of ventilation during exercise20, 22, 23, 25, 29, 30, results here also indicate that previous work has greatly underestimated the contribution of the heart to cardiopulmonary costs. Our bivariate analysis of VE and EE (Fig. 2) gives a ventilation cost of 3.3 mlO2 per L VE (assuming 20.1 J per mlO2), consistent with hyperpnea studies reporting 2–4 mlO2 per L VE during exercise20, 26, 30. However, HR and VE increase together during exercise (Fig. 2) and during enforced hyperventilation at rest31. Consequently, analyses that ignore HR and attribute cardiopulmonary costs entirely to breathing will artificially inflate the estimated cost of ventilation. This inflation may be substantial. Ventilation costs decreased 60%, to 1.3 mlO2 per L VE, and the heart accounted for more than half (60–80%) of cardiopulmonary energy cost, when ventilatory and circulatory costs were considered together in our multivariate model. Future studies using forced hyperpnea to estimate ventilation costs should include simultaneous measures of HR in order to assess the potential contribution of circulatory costs.
Measuring cardiopulmonary costs by induced hypoxia, as done here, enabled us to measure the energy expenditure of the heart non-invasively. To our knowledge, the only previous in vivo measures of the heart’s energy expenditure were conducted by monitoring oxygen concentrations in arterial blood supply and venous return of a catheterized heart37. Catheterization methods produce estimates of 7 W for heart energy expenditure in adult males lying supine, at rest37. Assuming a resting HR of ~60 bpm, this estimate translates to ~7 J per beat. HR cost in our multivariate model was 0.24 ± 0.05 W per bpm, which translates to 14 ± 3 J per beat, twice that of catheterization methods. Given the very different experimental designs, these estimates are reasonably similar. One reason that our approach may produce higher estimates is that our measurements were taken primarily during exercise, which increases blood pressure and stroke volume compared to resting, and presumably increases the energy cost per heartbeat. More work is needed to refine non-invasive estimates of HR cost and to reconcile them with catheterization studies.
The present study focused on walking, rather than the running or cycling tests typical of previous work on cardiopulmonary cost, e.g., refs 20, 22,23,24, 26, 30. As a result, we examined a relatively low range of VE. Maximum VE in the present study was 59 L min−1, which falls at the very low end of the range examined in most previous works. The lower range of VE in this study may explain why we did not detect the exponential increase in VE cost at higher ventilation rates reported by those studies26, 30.
Cardiopulmonary effort accounts for a substantial portion of resting EE, approximately 23% in normoxic conditions. In contrast, cardiopulmonary energy expenditure accounts for only 3 to 5% of net walking EE (i.e., with resting EE subtracted). The small contribution of cardiopulmonary effort to net walking cost is in line with previous work in humans and a range of other vertebrates25. These results lend support to analyses of locomotor cost that focus on musculoskeletal energy expenditure, ignoring cardiopulmonary work, e.g., refs 3, 6, 7, 38,39,40. Even under severe hypoxia, cardiopulmonary energy cost never exceeded 8% of net walking EE, suggesting that the primary cost of walking at altitude remains the musculoskeletal effort needed to support and propel the body.
Of note, we found small (~3%) reductions in EE with moderate hypoxia compared to normoxia at rest and during walking at slow speeds (0.67 and 0.83 m s−1). In our original study41, we found that VO2 and VCO2 in these conditions trended lower (without achieving statistical significance; see Table 1 and Fig. 1 in Horiuchi et al.41). Similarly, the small reductions reported here for EE, which is calculated from VO2 and VCO2, do not reach statistical significance. These results are nonetheless unexpected and we can only speculate regarding the cause of this phenomenon, but there are three general possibilities. One is that these small differences are spurious, reflecting measurement error and the limits of precision in our experimental set up. A second possibility is that the body fueled some small percentage of physiological activity using anaerobic metabolism in these conditions. In this case, the aerobic measures of EE employed here would fail to capture some small portion of total energy expenditure in these (and possibly other) conditions, leading to an underestimation of EE and ΔEE. Third, the body may be adjusting to hypoxic conditions by reducing EE to some unspecified set of physiological activities. Given the small size of the effect (~3% or less of EE), we do not expect that this phenomenon greatly affects the results of the currently analyses. Nonetheless, additional research into this phenomenon is warranted.
Another analytical concern here is the effect of CO2 stores at the onset of exercise on our measures of VCO2, and hence EE. As the generation of CO2 increases at the beginning of exercise, initial production will be absorbed in the bloodstream and accumulate there, prior to being exchanged in the lungs and expired. Consequently, measures of expired air, such as those employed here, may underestimate VCO2 at the beginning of an exercise bout. However, previous work has demonstrated that the CO2 stores were in equilibrium by ~2 min of exercise42. As we discarded the first 3 minutes of each trial and used only the last 1 min data of VO2 and VCO2 at each gait speed in our analyses, the effect of CO2 accumulation in the blood should be negligible for this study.
Limitations of this study include the absence of women in our sample and the relatively small range of VE, HR, and EE examined. Our multivariate analyses also underscore the inherent difficulties and limitations in parsing the effects of HR and VE on EE, given the physiological linkage between HR and VE. Additional work, examining a broader range of subjects and activities, is needed to improve our estimates of HR and VE cost and to determine whether these costs vary with body size, sex, age, or other factors.
Imposing greater cardiopulmonary effort through induced hypoxia provides a non-invasive approach for measuring cardiac and ventilatory costs during locomotion. The advantage of this approach is the ability to measure VE and HR during a given activity, non-invasively, rather than mimicking these costs separately during forced hyperpnea. Results here suggest that previous studies may have overestimated the cost of respiration during locomotion by ignoring the cost of increased HR. Induced hypoxia experiments may prove useful in determining respiratory and circulatory costs across a broader range of activities and conditions.
Methods
Participants
The present report represents additional data from investigations that were published previously41, examining the interaction of speed and hypoxia on walking cost. Of the 12 subjects who completed the entire protocol in the previous study, 11 completed the experiment in this study. We used the data from these 11 subjects for this study. Subjects (n = 11) were male athletes (sprinters, middle-distance runners, and soccer and baseball players) who engaged in strenuous daily training (2 hours per day, 5–6 days per week). Their mean age, height, and body weight were 24 ± 8 years, 174 ± 6 cm, and 70 ± 9 kg, respectively (values are mean ± standard deviation [SD]). Before this study, we explained all procedures, possible risks, and benefits of participation to the subjects, and all subjects signed an informed consent form. They were asked to refrain from intense physical activity 2 days before testing and to refrain from drinking any alcohol or caffeinated beverages the day before testing. This study was approved by an ethical committee at the Mount Fuji Research Institute according to the Declaration of Helsinki (No: ECMFRI-03-2014).
Exercise Protocols
All experiments were carried out on a motor-driven treadmill, 2.21 m long and 0.88 m wide (T7000, Johnson Health Tech. Co., Ltd., Taichung Hsein, Taiwan). During an experiment, the subject was allowed to choose his stride and step frequency freely during walking. Subjects wore underwear, shirts, socks, shorts, and lightweight training shoes43. They practiced at least three times at several gait speeds to familiarize themselves with treadmill walking while wearing a gas-collection mask. Inspired oxygen concentrations were set at normobaric normoxia (21% room air), moderate hypoxia (FiO2; 15%), and severe hypoxia (FiO2; 11%). Each trial was performed on a different day in random order with a single-blind study design. First, each subject sat on a chair for 10 minutes, then stood for 5 minutes on the treadmill for baseline measurement. Thereafter, he began to walk on the treadmill. As described in our previous study, seven speeds were set incrementally at 0.67, 0.86, 1.06, 1.25, 1.44, 1.64, and 1.83 m s−1, and each speed lasted for 4 minutes41, 44.
Measurements
Pulmonary ventilation (\({{\rm{V}}}_{{\rm{E}}}\)) and gas-exchange variables were measured with a computerized breath-by-breath system (AE-310S, Minato Ltd., Osaka, Japan). The standard, known gases (O2 15.23%, CO2 4.999%, and N2 balance) and room air were used to calibrate the gas analyzer. Inspired and expired gas volumes were measured with a hot wire respiratory flow system. Flow signals were electrically integrated for the duration of each breath to calculate minute ventilation. The expired fractions of O2 and CO2 were analyzed with a zirconium solid electrolyte oxygen analyzer and an infrared carbon dioxide analyzer, respectively. Each oxygen concentration gas was supplied via a 200-L Douglas bag with a hypoxic gas generator system (Everest summit II, Will Co. Ltd., Tokyo, Japan). HR was measured throughout the study with a commercial HR monitor (POLAR RC800X, POLAR Electro, Tokyo, Japan).
SpO2 was monitored with a pulse oximeter on the left middle finger every 1 minute throughout the study (TM-2564G, A&D, Tokyo, Japan).
Data Analysis
To calculate energy expenditure, EE, at rest and at each gait speed, \({{\rm{V}}{\rm{O}}}_{2}\) and \({{\rm{V}}\text{CO}}_{2}\) were measured with the following equation.
Energy Expenditure (Watts) = [(3.869 × \({{\rm{V}}{\rm{O}}}_{2}\)/1000) + (1.195 × \({{\rm{V}}\text{CO}}_{2}\)/1000)] × 4.186/60 × 100045, 46.
At baseline, all physiological values (i.e., gas exchange variables, HR, and SpO2) were averaged for the last 2 minutes of standing prior to the start of walking. During walking, a single sample with an average final 1 minute of physiological data was also obtained.
Calculating Ventilatory and Heart Rate Costs
To evaluate respiratory and circulatory cost, total walking energy expenditure at each speed (including rest) under normoxia (EEnorm) was subtracted from values under moderate or severe hypoxia (EEhyp) to calculate a ΔEE value. We then compared ΔEE to the change in ventilation rate, ΔVE = (VE hyp − VE norm) or change in heart rate, ΔHR = (HRhyp − HRnorm) between conditions. First, we examined the change in EE with respect to VE and HR separately, in a bivariate regression model (Ventilation Cost: ΔEE ~ ΔVE. Circulation Cost: ΔEE ~ ΔHR). Next we used multiple regression to test for the independent, additive effects of VE and HR on EE (Cardiopulmonary Cost: ΔEE ~ ΔVE + ΔHR). These regressions were evaluated with the intercept set at 0. This approach assumes that any change in EE between conditions at a given speed is attributable to the change in cardiopulmonary work. This design yielded n = 16 comparisons (1 rest + 7 walking speeds for 2 hypoxic conditions).
Statistics
All data are presented as mean ± SD. A two-way repeated ANOVA (oxygen level × walking speed) was conducted for comparison in EE, VE, and HR. As stated above, a bivariate regression model was used for ventilation or circulation cost. Further, multiple regression analysis was also used to test for the independent, additive effects of VE and HR on EE. All statistical analysis were performed using R ver. 2.13.1 (R Core Team, 2014). A P value less than 0.05 was considered statistically significant.
References
Pontzer, H., Raichlen, D. A. & Rodman, P. S. Bipedal and quadrupedal locomotion in chimpanzees. J Hum Evol 66, 64–82 (2014).
Rodman, P. S. & McHenry, H. M. Bioenergetics and the origin of hominid bipedalism. Am J Phys Anthropol 52, 103–106 (1980).
Sockol, M. D., Raichlen, D. A. & Pontzer, H. Chimpanzee locomotor energetics and the origin of human bipedalism. Proc Natl Acad Sci USA 104, 12265–12269 (2007).
Cavagna, G. A. & Kaneko, M. Mechanical work and efficiency in level walking and running. J Physiol 268, 467–481 (1977).
Willems, P. A., Cavagna, G. A. & Heglund, N. C. External, internal and total work in human locomotion. J Exp Biol 198, 379–393 (1995).
Abe, D., Muraki, S. & Yasukouchi, A. Ergonomic effects of load carriage on energy cost of gradient walking. Appl Ergon 39, 144–149 (2008).
Horiuchi, M., Endo, J., Horiuchi, Y. & Abe, D. Comparisons of energy cost and economical walking speed at various gradients in healthy active younger and older adults. J Exerc Sci Fit 13, 79–85 (2015).
Browning, R. C., Baker, E. A., Herron, J. A. & Kram, R. Effects of obesity and sex on the energetic cost and preferred speed of walking. J Appl Physiol 100, 390–398 (2006).
Browning, R. C. & Kram, R. Energetic cost and preferred speed of walking in obese vs. normal weight women. Obes Res 13, 891–899 (2005).
Peyrot, N. et al. Why does walking economy improve after weight loss in obese adolescents? Med Sci Sports Exerc 44, 659–665 (2012).
Wall-Scheffler, C. M. & Myers, M. J. Reproductive costs for everyone: how female loads impact human mobility strategies. J Hum Evol 64, 448–456 (2013).
Abe, D., Muraki, S. & Yasukouchi, A. Ergonomic effects of load carriage on the upper and lower back on metabolic energy cost of walking. Appl Ergon 39, 392–398 (2008).
Bastien, G. J., Schepens, B., Willems, P. A. & Heglund, N. C. Energetics of load carrying in Nepalese porters. Science 308, 1755 (2005).
Bastien, G. J., Willems, P. A., Schepens, B. & Heglund, N. C. Effect of load and speed on the energetic cost of human walking. Eur J Appl Physiol 94, 76–83 (2005).
Maloiy, G. M., Heglund, N. C., Prager, L. M., Cavagna, G. A. & Taylor, C. R. Energetic cost of carrying loads: have African women discovered an economic way? Nature 319, 668–669 (1986).
Bornstein, M. H. & Bornstein, H. G. The pace of life. Nature 259, 557–559 (1976).
Raichlen, D. A. et al. Evidence of Levy walk foraging patterns in human hunter-gatherers. Proc Natl Aca Sci USA 111, 728–733 (2014).
Shadmehr, R., Huang, H. J. & Ahmed, A. A. A Representation of Effort in Decision-Making and Motor Control. Curr Biol: CB 26, 1929–1934 (2016).
Selinger, J. C., O’Connor, S. M., Wong, J. D. & Donelan, J. M. Humans Can Continuously Optimize Energetic Cost during Walking. Curr Biol: CB 25, 2452–2456 (2015).
Aaron, E. A., Johnson, B. D., Seow, C. K. & Dempsey, J. A. Oxygen cost of exercise hyperpnea: measurement. J Appl Physiol 72, 1810–1817 (1992).
Campbell, E. J., Westlake, E. K. & Cherniack, R. M. Simple methods of estimating oxygen consumption and efficiency of the muscles of breathing. J Appl Physiol 11, 303–308 (1957).
Coast, J. R. et al. Ventilatory work and oxygen consumption during exercise and hyperventilation. J Appl Physiol 74, 793–798 (1993).
Levison, H. & Cherniack, R. M. Ventilatory cost of exercise in chronic obstructive pulmonary disease. J Appl Physiol 25, 21–27 (1968).
Margaria, R., Milic-Emili, G., Petit, J. M. & Cavagna, G. Mechanical work of breathing during muscular exercise. J Appl Physiol 15, 354–358 (1960).
Markley, J. S. & Carrier, D. R. The cost of ventilation in birds measured via unidirectional artificial ventilation. Comp Biochem Physiol A, Mol Integr Physiol 155, 146–153 (2010).
Marks, D. et al. Oxygen cost of ventilation and its effect of the VO2 plateau. J. Exerc. Physiol. Online 8, 1–12 (2005).
Milici-Emili, G. & Petit, J. M. Mechanical efficiency of breathing. J Appl Physiol 15, 359–362 (1960).
Murray, J. F. Oxygen cost of voluntary hyperventilation. J Appl Physiol 14, 187–190 (1959).
Scano, G., Grazzini, M., Stendardi, L. & Gigliotti, F. Respiratory muscle energetics during exercise in healthy subjects and patients with COPD. Respir Med 100, 1896–1906 (2006).
Vella, C. A., Marks, D. & Robergs, R. A. Oxygen cost of ventilation during incremental exercise to VO2 max. Respirology 11, 175–181 (2006).
Alexopoulos, D. et al. Hemodynamic response to hyperventilation test in healthy volunteers. Clin Cardiol 18, 636–641 (1995).
Rubenson, J. et al. Reappraisal of the comparative cost of human locomotion using gait-specific allometric analyses. J Exp Biol 210, 3513–3524 (2007).
Calbet, J. A. & Lundby, C. Air to muscle O2 delivery during exercise at altitude. High Alt Med Biol 10, 123–134 (2009).
Schoene, R. B. Limits of human lung function at high altitude. J Exp Biol 204, 3121–3127 (2001).
Dempsey, J. A. & Forster, H. V. Mediation of Ventilatory Adaptations. Physiol Rev 62, 262–346 (1982).
Naeije, R. Physiological adaptation of the cardiovascular system to high altitude. Prog Cardiovasc Dis 52, 456–466 (2010).
Elia, M. Organ and tissue contribution to metabolic rate. In Energy Metabolism: Tissue Determinants and Cellular Corollaries. J. M. Kinney and H. N. Tucker eds pp. 19–60, Raven Press, New York, NY, USA, (1992).
Hubel, T. Y. & Usherwood, J. R. Children and adults minimise activated muscle volume by selecting gait parameters that balance gross mechanical power and work demands. J Exp Biol 218, 2830–2839 (2015).
Pontzer, H. A unified theory for the energy cost of legged locomotion. Biol Lett 12, 20150935 (2016).
Usherwood, J. R. Constraints on muscle performance provide a novel explanation for the scaling of posture in terrestrial animals. Biol Lett 9, 20130414 (2013).
Horiuchi, M., Handa, Y., Abe, D. & Fukuoka, Y. Walking economy at simulated high altitude in human healthy young male lowlanders. Biol Open 5, 1408–1414 (2016).
Chuang, M. L. et al. Aerobically generated CO2 stored during early exercise. J Appl Physiol 87, 1048–1058 (1999).
Abe, D., Yanagawa, K. & Niihata, S. Effects of load carriage, load position, and walking speed on energy cost of walking. Appl Ergon 35, 329–335 (2004).
Abe, D., Fukuoka, Y. & Horiuchi, M. Economical Speed and Energetically Optimal Transition Speed Evaluated by Gross and Net Oxygen Cost of Transport at Different Gradients. PLoS One 10, e0138154 (2015).
Brouwer, E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (Oxygen intake and carbonic acid output) and urine-N. Acta Physiol Pharmacol Neerl 6, 795–802 (1957).
Masschelein, E. et al. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J Appl Physiol 113, 736–745 (2012).
Author information
Authors and Affiliations
Contributions
M.H. and H.P. conceived and designed the research. M.H. and Y.H. performed the experiments. M.H. Y.H., D.A., Y.F. and H.P. analyzed and intepreted data. Y.H. prepared tables and figures. M.H. and H.P. wrote the first manuscript. All authors edited, revised and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horiuchi, M., Fukuoka, Y., Handa, Y. et al. Measuring the Energy of Ventilation and Circulation during Human Walking using Induced Hypoxia. Sci Rep 7, 4938 (2017). https://doi.org/10.1038/s41598-017-05068-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05068-8
This article is cited by
-
Hypoxic-induced resting ventilatory and circulatory responses under multistep hypoxia is related to decline in peak aerobic capacity in hypoxia
Journal of Physiological Anthropology (2022)
-
Avoiding the effects of translocation on the estimates of the metabolic rates across an elevational gradient
Journal of Comparative Physiology B (2022)
-
Sex differences in respiratory and circulatory cost during hypoxic walking: potential impact on oxygen saturation
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.