Abstract
Etanercept is a TNFα receptor Fc fusion protein used for the treatment of rheumatic disease and psoriasis. Physicochemical and functional investigation of process fractions during development of the etanercept biosimilar GP2015 (Erelzi®) revealed a correlation between reduced potency and incorrect disulfide bridging between specific cysteines in the receptor domain. This novel structure-function relationship was found to be the molecular basis for reduced potency in recent Enbrel® batches, which exhibit higher levels of incorrect disulfide bridging. Interestingly, incorrect disulfide bridging was found to be reversible under serum-like redox conditions, restoring potency to normal levels. This redox dependent reversibility suggests that these variants are likely not relevant for clinical efficacy once the drug enters the bloodstream. Nonetheless, incorrect disulfide bridging in etanercept represents a new quality attribute that is critical for biopharmaceutical functionality and should thus be carefully monitored and controlled to guarantee patient safety.
Similar content being viewed by others
Introduction
Tumor necrosis factor alpha (TNFα) is a potent cytokine involved in immune response regulation and elicitation of inflammatory response1, 2. The protein is produced by cells as a 26 kDa membrane bound precursor (mTNFα) that can be subsequently processed by cell surface metalloproteinase known as TNFα converting enzyme (TACE) or ADAM 17 to generate a soluble 17 kDa form (sTNFα). Both membrane bound and soluble TNFα can bind and activate TNF receptors 1 or 2 to elicit pro-inflammatory cytokine cascades, tissue destruction and cell death, which, if exacerbated, can result in a variety of inflammatory, rheumatic or dermatological diseases3,4,5,6. The development of biopharmaceutical drugs, such as etanercept, that can specifically and potently bind and neutralize TNFα have dramatically improved the ability to treat a number of pathological conditions including rheumatoid arthritis, ankylosing spondylitis and psoriasis7,8,9.
Etanercept is a ~125 kDa fusion protein, consisting of a TNF receptor 2 domain coupled to the Fc portion of human IgG1 10 (data not shown). Each amino acid chain of the molecular dimer contains three N-glycans as well as up to fourteen sites that can be differentially modified by O-glycosylation11 (data not shown). Further, the etanercept molecule contains thirteen intra-chain and three inter-chain disulfide bonds, the majority of which are located in the TNF receptor region of the molecule12, 13. The size and complexity of biopharmaceuticals such as etanercept present an exceptional challenge for companies aiming to produce follow on biosimilar products, which must comprise highly similar structural and functional properties and exhibit no clinically meaningful differences to the original reference prodcut14,15,16. To achieve this regulatory standard, extensive physicochemical and functional characterization of both the biosimilar and the reference product is required to understand the product characteristics and to allow targeted control of variants that impact the structure and function of the molecule.
In the course of process development for the etanercept biosimilar GP2015 (Erelzi®) at Sandoz a loss of potency, as measured by TNFα inhibition reporter gene assay, was observed in late eluting fractions during hydrophobic interaction chromatography (HIC) purification (see Fig. 1). This loss of potency could not be correlated with product related variants known to influence etanercept potency, such as low molecular weight degradation products (LMW variants) or high molecular weight aggregates (HMW variants), which were only moderately enriched in the late eluting fraction (see Fig. 1).
Potency loss in late eluting large scale HIC fractions. Large scale HIC separation of GP2015 (Erelzi®) resulted in the separation of a main peak from a late eluting shoulder peak. Assessment of potency in these two fractions revealed near 100% in the main peak while the late eluting fraction was found to be inactive. Although high and low molecular weight variants were slightly enriched in the late eluting fraction, this moderate decrease in purity could not explain the loss of potency.
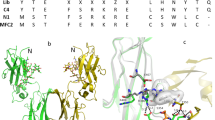
The TNF receptor domain of etanercept is stabilized by eleven disulfide bonds. While no direct evidence demonstrating an impact of incorrect disulfide bridging on potency has yet been reported, there has been speculation based on similar observations that late eluting HIC fractions exhibit low potency without observable aggregation, degradation or modification13, 17,18,19,20. To characterize disulfide bridging in GP2015 (Erelzi®) and Enbrel® samples, an LC-MS based native peptide map approach using three distinct proteolytic enzymes was developed (‘triple digest’, see methods and Supplementary Table S1 for details). Using this “triple digest” assay, we identified each of the eleven intra-chain disulfide bonds in the receptor region of etanercept, which correspond with those identified by X-ray crystallography12 (see Fig. 2). In addition, four incorrect disulfide bridged variants, C18-C74, C71-C88, C78-C88 and C71-C74, were identified which have not yet been reported in the literature. These incorrect disulfide bonds all localize to the TNFα binding region of etanercept (see Supplementary Fig. S1 for details) and were ubiquitously present in all GP2015 (Erelzi®) and Enbrel® batches.
Disulfide bridge structures in etanercept. X-ray crystallography of the GP2015 TNF-receptor domain (blue) in conjugation with TNFα (green) was achieved and provides important structural information for etanercept. The eleven disulfide bridge structures in the receptor domain are in line with those previously reported in the literature for crystal structures of etanercept12. The LC-MS based triple digest peptide map (see methods for details) was able to identify each of the eleven disulfide bridge structures as well as four additional incorrect disulfide bonds between cysteines C18-C74, C71-C88, C78-C88 and C71-C74 in both GP2015 (Erelzi®) and the reference product (Enbrel®).
To assess whether incorrect disulfide bridging directly impacts etanercept potency, we developed a non-reducing peptide map approach for quantitation of the incorrectly bridged C78-C88 peptide relative to an internal peptide standard21, 22 (see methods section for details). Compared to the other three incorrect disulfide bridges, quantitation of the peptide containing C78-C88 was found to provide the most accurate and repeatable results, likely due to the robust chromatographic resolution of the relevant peptide and its refraction to misscleavage or modification during sample preparation (see Supplementary Tables S2 and S3 for details). To allow accurate C78-C88 quantitation, the non reducing peptide map was developed under conditions which repress disulfide shuffling23, 24. This was achieved in part by using a mild detergent (RapiGest SF) in the presence of iodoacetamide during the denaturing step to quench nonspecific reactions with unpaired disulfide bonds. In addition, the enzymatic digestion was carried out under moderately acidic conditions which were found to stably suppress disulfide bridge shuffling to approximately 1% in GP2015 (Erelzi®) drug substance samples (see Table 1).
To quantitatively assess how the relative abundance of incorrectly bridged C78-C88 correlates with the overall amount of incorrect disulfide bridging in the etanercept molecule, an analytical HIC method was additionally developed which chromatographically separates incorrectly bridged structures based on changes in secondary and tertiary structure. Etanercept variants containing incorrect disulfide bridge structures were found to elute as a post peak in the analytical HIC together with high molecular weight aggregates. The correlation of non reducing peptide map and HIC results can be used to approximate how the relative percent C78-C88 peptide values translates into the overall amount of incorrect disulfide bridging present in a sample (see Fig. 3). Based on these results, samples with 1% C78-C88 are essentially free of incorrectly bridged structures, and can thus be considered artifact level incorrect bridging generated by residual shuffling of disulfide bridges during sample preparation. Samples containing 2% C78-C88 contain approximately 10% incorrectly bridged structures as measured by HIC. As described below, the percent incorrectly bridged structures estimated using the analytical HIC values correspond to the relative loss of potency, suggesting a direct correlation between incorrect disulfide bridging and potency loss.
Approximation of overall incorrect disulfide bridging using analytical hydrophobic interaction chromatography. Samples with different potencies, such as GP2015 (Erelzi®) drug substance, process intermediates and late eluting HIC fractions were analyzed using the analytical HIC and the non reducing peptide map methods. The abundance of incorrect disulfide bridging assessed by these methods was found to correlate, allowing approximation of how the relative amount of C78-C88 assessed by the non reducing peptide map corresponds with overall incorrect disulfide bridging in etanercept, as reflected by the HIC post peak.
To assess whether a real causation between incorrect disulfide bridging and potency exists, the C78-C88 peptide was quantified in an array of GP2015 process intermediates, late eluting HIC fractions and reference product samples exhibiting a wide range of potencies. As shown in Fig. 4, a strong inverse correlation was observed between potency and the abundance of the peptide containing C78-C88, indicating a 10% decrease in potency for every 1% increase in the relative amount of incorrectly bridged variant. The functional relationship between C78-C88 abundance and etanercept potency is underlined by the high correlation value. The high corollary impact of this incorrectly bridged variant on etanercept function has important implications for companies seeking to market biosimilar versions of this molecule, as matching abundance of incorrectly bridged C78-C88 peptide in biosimilar and the reference products must be considered essential for guaranteeing comparable potency.
Correlation of incorrect disulfide bridge C78-C88 and potency. The relative amount of C78-C88 was quantified by non reducing peptide map analysis in different samples including reference product (Enbrel®), GP2015 (Erelzi®), process intermediates and late eluting HIC fractions. Evaluation of incorrect disulfide bridging relative to potency in these samples revealed a strong inverse correlation between potency and the relative abundance of C78-C88.
It has been noted in the literature that redox conditions, mimicking those found in human serum, can result in disulfide bridge reshuffling into energetically stable and correct structures23, 25,26,27. To assess whether serum-like redox conditions can result in the reshuffling of incorrectly bridged variants in etanercept, selected process intermediate GP2015 and reference product samples containing higher amounts of C78-C88, were incubated with physiologically relevant levels of cysteine for 48 hours at low temperature (see methods for details). As shown in Table 2, incubation under physiological redox conditions mediated an almost complete elimination of incorrectly bridged C78-C88 variant, resulting in subsequent restoration of potency. Following redox treatment, the adjusted levels of incorrectly bridged variant and potency continued to correlate along the linear regression, indicating that the observed improvements in potency are mediated exclusively through the correction of disulfide bridging (data not shown). These results suggest that while the abundance of incorrectly bridged C78-C88 predicts the degree of potency in vitro, in vivo efficacy would likely not be impacted by this incorrect disulfide bridging due to reshuffling once the drug is exposed to physiological redox conditions in the blood stream.
Assessment of reference product Enbrel® batches over time revealed reduced potency levels in more recent batches, likely resulting from a process change (see Fig. 5). This tightening of potency values at the lower range of the overall batch to batch variability was found to correlate with a consistently higher relative amount of incorrectly bridged C78-C88 variant in selected batches. Manufacturing process changes for biopharmaceuticals are a common occurrence and can impact the type and abundance of quality attributes critical for protein structure and function28,29,30. Reference product process changes are particularly relevant for biosimilar companies whose aim is to develop a follow on product with the same safety and efficacy profile. Indeed, biosimilar development normally begins with the analysis of multiple batches of the reference product to define its batch to batch variability and to establish the reference product target ranges. These target ranges are the basis for selection of an appropriate production cell clone and for the development of a manufacturing process that delivers a highly similar product with matching structural and functional properties. Therefore, even in cases where the biosimilar candidate is indistinguishable from the initially tested reference product batches, post approval process changes carried out by the reference product company can result in measurable analytical differences in some quality attributes. Importantly, health authorities approve process changes only if they do not result in clinically meaningful differences. To achieve this, they apply the same principles for evaluation of comparability that govern an evaluation of biosimilarity, assuring through extensive analytical and, if necessary, confirmatory clinical testing, that pre- and post-change batches exhibit the same safety and efficacy31. Thus, regardless of whether a reference product manufacturer implements process changes, demonstrating that functionally critical structural attributes of a biosimilar remain within the clinically verified range of the reference product is central to assuring the same safety and efficacy profile. As shown in Fig. 5, the Sandoz biosimilar etanercept GP2015 (Erelzi®) was developed with consistently low levels of incorrectly bridged variant, resulting in stable potency levels with low batch to batch variability that lie well within the overall clinically verified range of the reference product batches.
Assessment of potency variability over time. Assessment of potency in reference product Enbrel® batches sourced from both the EU and US since 2008 reveals a broad distribution with a tightening of values toward the lower end over time, likely due to one or more process changes. Assessment of potency for GP2015 (Erelzi®) batches over time reveals low batch to batch variability well within the clinically verified range of the reference product. Manufacturing dates are given as month/year.
Overall, this paper describes the characterization of a novel structure-function relationship between disulfide bonding and potency in etanercept. All batches of the recently FDA approved etanercept biosimilar GP2015 (Erelzi®) were found to contain identical incorrect disulfide bridge structures as Enbrel®, at levels within the clinically verified range of the reference product. Using a quantitative non reducing peptide map method, the relative amount of incorrectly bridged C78-C88 peptides could be inversely correlated with the degree of potency in GP2015 (Erelzi®) and Enbrel® samples. This approach was used to explain a potency shift in reference product Enbrel®, which could be associated with increased levels of incorrect disulfide bridging at C78-C88. Interestingly, incorrect disulfide bridging in etanercept was found to be reversible under redox conditions similar to those found in serum, suggesting that while critical for potency in vitro, such variants are likely not relevant for efficacy in vivo. We believe the insights and methodologies described in this manuscript will be important for pharmaceutical companies seeking to manufacture biosimilar versions of etanercept, as these companies cannot rely on long term manufacturing experience but instead must leverage detailed knowledge of quality attributes critical for molecular function to specifically control related variants and ensure that product safety and consistency are maintained. The functional relationship between incorrect disulfide bridging and potency demonstrated in this manuscript suggests that companies seeking to produce biosimilar versions of etanercept should establish comprehensive characterization and control of disulfide bridge variants to ensure robust manufacturing of a safe and efficacious product.
Methods
Triple digest LC-MS peptide map
This multi-enzyme non reducing peptide map allows characterization of correct and incorrect disulfide bridge structures present in etanercept using mass spectrometry. Samples containing 100 µg of etanercept were transferred into a sodium acetate buffer pH 5.0 and treated with 60 mU of sialidase (Roche) and 4 U pNGaseF (Roche) for 16 hours at 37 °C to remove sialic acid and N-glycans, respectively. Samples were subsequently transferred into 50 mM Tris buffer pH 8.0 containing 0.5% RapiGest SF (Waters) and 50 mM iodoacetamide and denatured for 30 min at 50 °C. Samples were then buffer exchanged into 50 mM Tris buffer pH 8.0 containing 0.1% Rapigest SF solution and digested with 0.1 µg/µL AspN (Roche), 1 µg/µL Trypsin (Promega Gold) and 1 µg/µL chymotrypsin (Roche) for 16 hours at 37 °C. Digestion was terminated by addition of formic acid and acetonitrile to a final concentration of 0.3% and 3%, respectively. To achieve optimal peak resolution, an Ascentis Express Peptide ES-C18 column was equilibrated for 15 min with 0.0375% ammonium formate in HPLC water. Following sample application, peptides were eluted from the column by applying a gradient of 0.0345% ammonium formate and 90% acetonitrile in water over a 110 min time frame. A Q Exactive mass spectrometer (Thermo scientific) was used for identification of the disulfide bridge peptides in the sample (see Supplementary Table S1 for details).
Non-reducing peptide map
This non reducing peptide map allows quantitation of a tryptic peptide T7 containing the incorrectly disulfide bridge C78-C88 relative to an internal peptide standard T27 in etanercept samples. Samples containing 150 µg of etanercept were denatured using 0.1% RapiGest SF (Waters) in Tris buffer pH 8.0 containing 50 mM iodoacetamide at 50 °C for 40 minutes. Samples were subsequently buffer exchanged into 50 mM MES buffer containing 0.05% RapiGest SF pH 6.0 and digested with 12 µg Trypsin (Promega Gold) for 17 hours at 37 °C. Digestion was terminated by addition of formic acid and acetonitrile to a final concentration of 0.3% and 3%, respectively. Separation was performed using an Ascentis Express Peptide ES-C18 column. To achieve optimal peak resolution, the column was flushed for 15 min with buffer B (0.1% TFA in 90% Acetonitrile and 10% HPLC water) and 10 min with buffer A (0.1% TFA in HPLC water) before use. Peptides were eluted using a gradient of 0–16% buffer B over 25 min, 18% buffer B at 28 min and 100% buffer B at 33 min. The UV absorption at 215 nm was recorded by a UV detector. Example chromatograms and mass spectra are shown in Supplementary Figs S2–S4. Examples of tests used demonstrate that the method is scientifically sound and fit for purpose are shown in Supplementary Tables S2 and S3.
Analytical hydrophobic interaction chromatography
GP2015 variants were separated according to their hydrophobicity using hydrophobic interaction chromatography (HIC). Samples were diluted by addition of water to a target concentration of 2.0 mg/mL and chromatographically resolved using a Tosoh, TSKgel Buty-NPR column with a gradient consisting of 1.2 M (NH4)2SO4 in 0.1 M phosphate buffer, pH 7.0 and 0.1 M phosphate buffer, pH 7.0. A fluorescence detector with an excitation wavelength of 278 nm and an emission wavelength of 350 nm was used for detection and quantification.
Potency
Binding and functional neutralization of TNF-α by GP2015 and Enbrel® samples was measured using HEK293 cells stably transfected with an NFκB-luciferase reporter gene. In a microtiter plate, 20,000 cells per well were stimulated with 4 ng/mL TNF-α in the presence of graded amounts of GP2015 or Enbrel®. After overnight incubation, cells were lysed and luciferase activity was quantified in the lysates using a luminogenic substrate. The potency of GP2015 or Enbrel® was determined by comparison to a reference standard and relative potency was calculated using a parallel line assay according to the European Pharmacopoeia.
Serum-like reducing conditions
Incorrect disulfide bridges of Enbrel® and GP2015 drug product, drug substance, process intermediates and late eluting HIC fractions were reshuffled by incubation under physiologically relevant cysteine levels. Samples were diluted to 4 mg/ml in redox-buffer (0.83 mM cysteine, 0.17 mM cystamine in 200 mM Tris buffer pH 8.0) and incubated for 48 hours at 2–8 °C. Samples were subsequently dialyzed in citrate buffer (25 mM citrate pH 6.3) using Slide-A-Lyzer 10000 MW dialysis cassettes (Thermo Scientific). Dialysis buffer was exchanged three times every 20 minutes. Dialyzed samples were diluted to 2 mg/ml with citrate buffer prior to analysis.
X-ray crystallography
The receptor domain of etanercept was released by papain digestion of the full-length dimeric fusion protein. The Fc fragments were removed by a Protein A column and the receptor domain was incubated with TNFα. The yielded complex was further purified on a S-200 gel filtration column and fractions containing the TNFα etanercept receptor complex was additionally concentrated using a 50 kDa Vivascience ultrafiltration device. Obtained crystals were flash-frozen and measured at a temperature of 100 K. The X-ray diffraction data sets were collected for GP2015 and Enbrel® at the Swiss Light Source (SLS, Villigen, Switzerland) using cryogenic conditions. Crystals belong to space group H 3 2. The data were processed using the programs XDS and XSCALE.
References
Aggarwal, B. B., Gupta, S. C. & Kim, J. H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 119, 651–665 (2012).
Sedger, L. M. & McDermott, M. F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev. 25, 453–742 (2014).
Black, R. A. et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 385, 729–733 (1997).
Moss, M. L. et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 385, 733–736 (1997).
Neurath, M. F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342 (2014).
Brenner, D., Blaser, H. & Mak, T. W. Regulation of tumour necrosis factor signalling: live or let die. Nat. Rev. Immunol. 15, 362–374 (2015).
Kuek, A., Hazleman, B. L. & Ostör, A. J. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad. Med. J. 83, 251–260 (2007).
Taylor, P. C. & Feldmann, M. Anti-TNF biologic agents: still the therapy of choice for rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 578–582 (2009).
Molinelli, E., Campanati, A., Ganzetti, G. & Offidani, A. Biologic Therapy in Immune Mediated Inflammatory Disease: Basic Science and Clinical Concepts. Curr. Drug Saf. 11, 35–43 (2016).
Tracey, D., Klareskog, L., Sasso, E. H., Salfeld, J. G. & Tak, P. P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 117, 244–279 (2008).
Houel, S. et al. N- and O-glycosylation analysis of etanercept using liquid chromatography and quadrupole time-of-flight mass spectrometry equipped with electron-transfer dissociation functionality. Anal. Chem. 86, 576–584 (2014).
Mukai, Y. et al. Solution of the structure of the TNF-TNFR2 complex. Sci. Signal. 16, ra83 (2010).
Cho, I. H. et al. Evaluation of the structural, physicochemical, and biological characteristics of SB4, a biosimilar of etanercept. MAbs. 8, 1136–1155 (2016).
Section 7002(b) (3) of the Affordable Care Act, adding section 351(i)(2) of the PHS Act (2009).
European Commission (EC). Consensus Information Document: What you need to know about Biosimilar Medicinal Products http://ec.europa.eu/DocsRoom/documents/8242/attachments/1/translations/en/renditions/native (Date of access: 11/04/2017) (2013).
Guideline on similar biological medicinal products. CHMP/437/04 Rev 1 (2014).
Fung, V. P., inventor. Immunex Corporation. Method for producing recombinant proteins. United States patent US 7,294,481 B1. Nov. 13 2007.
Sassenfeld, H. M., Remmele, R. L., McCoy, R. E. Inventors. Immunex Corporation. Increased recovery of active proteins. United States patent US 7,544.784 B2. Jun. 09 2009.
Haverick, M., Mengisen, S., Shameem, M. & Ambrogelly, A. Separation of mAbs molecular variants by analytical hydrophobic interaction chromatography HPLC: overview and applications. MAbs. 6, 852–858 (2014).
Min, K. H. & Lee, G. M. Purification of TNFR-Fc produced in recombinant CHO cells: Characterization of product related impurities. Process Biochemistry. 50, 1313–1317 (2015).
Rupprechter, A. et al. Inventors. Sandoz AG. Quantification of misfolded tnfr2:fc. United States patent US 9,598,718 B2. Mar. 21 2017.
Strand, V. et al. The totality-of-the-evidence approach to the development and assessment of GP2015, a proposed etanercept biosimilar. Curr. Med. Res. Opin. In press (2017).
Liu, H. & May, K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. MAbs. 4, 17–23 (2012).
Sung, W. C. et al. Evaluation of disulfide scrambling during the enzymatic digestion of bevacizumab at various pH values using mass spectrometry. Biochim. Biophys. Acta. 1864, 1188–1194 (2016).
Banks, D. D. et al. Removal of cysteinylation from an unpaired sulfhydryl in the variable region of a recombinant monoclonal IgG1 antibody improves homogeneity, stability, and biological activity. J Pharm. Sci. 97, 775–790 (2008).
Dillon, T. M. et al. Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. J. Biol. Chem. 283, 16206–16215 (2008).
Ouellette, D. et al. Studies in serum support rapid formation of disulfide bond between unpaired cysteine residues in the VH domain of an immunoglobulin G1 molecule. Anal. Biochem. 397, 37–47 (2010).
Schiestl, M. et al. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol. 29, 310–312 (2011).
Schneider, C. K. Biosimilars in rheumatology: the wind of change. Ann. Rheum. Dis. 72, 315–318 (2013).
Vezér, B., Buzás, Z., Sebeszta, M. & Zrubka, Z. Authorized manufacturing changes for therapeutic monoclonal antibodies (mAbs) in European Public Assessment Report (EPAR) documents. Curr Med. Res. Opin. 32, 829–834 (2016).
International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Harmonized Tripartite Guideline: Comparability of biotechnological/biological products subject to changes in their manufacturing process. Q5E. (2004).
Acknowledgements
We would like to thank Marco Dehling for his assistance in generating and interpreting X-ray crystallography figures.
Author information
Authors and Affiliations
Contributions
A.S., H.T. and M.S. designed the study. W.C.L., R.E.M., M.F., A.R., and J.H. developed the disulfide bridge methods. F.H. developed the redox methods. C.F. developed the bioassay. C.V. developed the HIC method. W.C.L., R.E.M. and J.H., wrote the manuscript with input and review from all authors.
Corresponding author
Ethics declarations
Competing Interests
Erelzi® is a registered trademark of Novartis AG and is, at the time of this submission, approved exclusively in the USA. The Erelzi trademark is registered worldwide. Enbrel® is a registered trademark of Immunex Corporation and Wyeth LLC. The authors are employees and shareholders of the Novartis group of companies, which are developing, manufacturing and marketing biopharmaceuticals, including biosimilar products.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lamanna, W.C., Mayer, R.E., Rupprechter, A. et al. The structure-function relationship of disulfide bonds in etanercept. Sci Rep 7, 3951 (2017). https://doi.org/10.1038/s41598-017-04320-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04320-5
This article is cited by
-
Future Evolution of Biosimilar Development by Application of Current Science and Available Evidence: The Developer’s Perspective
BioDrugs (2023)
-
Studying protein structure and function by native separation–mass spectrometry
Nature Reviews Chemistry (2022)
-
Comparability of Biologics: Global Principles, Evidentiary Consistency and Unrealized Reliance
BioDrugs (2021)
-
Assessment of biosimilarity under native and heat-stressed conditions: rituximab, bevacizumab, and trastuzumab originators and biosimilars
Analytical and Bioanalytical Chemistry (2020)
-
Similarity assessment of quality attributes of biological medicines: the calculation of operating characteristics to compare different statistical approaches
AAPS Open (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.