Abstract
Brown adipose tissue takes up large amounts of glucose during cold exposure in mice and humans. Here we report an induction of glucose transporter 1 expression and increased expression of several glycolytic enzymes in brown adipose tissue from cold-exposed mice. Accordingly, these genes were also induced after β-adrenergic activation of cultured brown adipocytes, concomitant with accumulation of hypoxia inducible factor-1α (HIF-1α) protein levels. HIF-1α accumulation was dependent on uncoupling protein 1 and generation of mitochondrial reactive oxygen species. Expression of key glycolytic enzymes was reduced after knockdown of HIF-1α in mature brown adipocytes. Glucose consumption, lactate export and glycolytic capacity were reduced in brown adipocytes depleted of Hif-1α. Finally, we observed a decreased β-adrenergically induced oxygen consumption in Hif-1α knockdown adipocytes cultured in medium with glucose as the only exogenously added fuel. These data suggest that HIF-1α-dependent regulation of glycolysis is necessary for maximum glucose metabolism in brown adipocytes.
Similar content being viewed by others
Introduction
There are two main types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT), which both store energy in the form of triglyceride. WAT mobilizes its triglyceride stores for the benefit of other tissues when energy supplies are scarce. BAT, on the other hand, utilizes stored triglyceride to fuel heat production. Upon cold exposure, sympathetic nerve fibers release noradrenaline at the surface of brown adipocytes, stimulating their β-adrenergic receptors. This stimulation leads to lipolysis and thermogenic gene expression. Long-chain fatty acids released by lipolysis activate the brown adipocyte-specific uncoupling protein 1 (UCP1). Activated UCP1 uncouples the proton gradient across the inner mitochondria membrane, thereby releasing its accumulated electrochemical energy as heat1, 2. The capacity of brown adipocytes to produce heat is termed thermogenic potential, and a β-adrenergically induced change in oxygen consumption is often used as a surrogate measure of this.
Following cold exposure of mice and humans, large amounts of glucose are taken up by BAT3, 4. Glucose uptake is mediated by glucose transport proteins (GLUTs), of which the two most prominent in adipose tissue are the insulin-independent GLUT1 and the insulin-dependent GLUT43, 5. Glucose is metabolized to two molecules of pyruvate in glycolysis through 10 enzymatic steps6. Glycolysis can be divided into two parts: the first part is energy-consuming and converts glucose to two triose sugars; in the second part, energy is harvested by converting the triose sugars to pyruvate6. The first reaction of glycolysis is catalyzed by hexokinase (HK), which is the major regulator of glycolytic flux in cancer cells7. Of the four HK isoforms, at least HK2 is known to be expressed at high levels in adipose tissue8. A second key flux-controlling step is the phosphofructokinase (PFK) reaction. The final reaction of glycolysis is catalyzed by pyruvate kinase (PK). There are several isoforms of PK, including two muscle isoforms: muscle pyruvate kinase 1 and 2 (Pkm1 and Pkm2). Studies with rodents have demonstrated a cold-induced increase in activity of HK, PFK and PK in BAT9,10,11. Consistent with this, we have reported increased expression of many glycolytic enzymes in BAT from cold-exposed mice8.
The transcription factor hypoxia inducible factor-1α (HIF-1α) induces the expression of most glycolytic enzymes during hypoxia. At normoxia, HIF-1α has a short half-life and its degradation is induced by prolyl-4-hydroxylases. During hypoxia, prolyl-4-hydroxylase activity is decreased and HIF-1α stability is increased12. At normoxia, HIF-1α activity has been suggested to depend not primarily on protein stability, but on transcriptional regulation of the Hif-1α gene13. HIF-1α forms a heterodimer with the more stable HIF-1β subunit, and the complex binds to hypoxic responsive elements to induce gene expression12. The role of HIF-1α in adipose tissue has been examined in several studies; however, whether HIF-1α has beneficial or detrimental functions is not clear. Some studies suggest that HIF-1α has effects in adipose tissue that negatively impact whole-body metabolism, e.g. by suppressing fatty acid oxidation and energy expenditure as well as by disturbing insulin sensitivity and glucose homeostasis14,15,16,17,18,19. Other mouse models suggest that adipose HIF-1α has beneficial effects on metabolic health, e.g. by protecting against high fat diet-induced obesity, insulin resistance and glucose intolerance as well as by augmenting mitochondrial biogenesis, energy expenditure and thermogenesis20,21,22. Thus, the functions of HIF-1α in adipose tissue are important and many.
Here we report the results of a comprehensive time course study of mice exposed to cold for up to eight days. Increased expression of Glut1, Glut4 and several glycolytic enzymes was observed in interscapular BAT (iBAT). These changes were largely recapitulated in cultured brown adipocytes after β-adrenergic stimulation. In addition, expression of Glut1 and several glycolytic enzymes was induced by hypoxia in brown adipocytes. Basal glycolytic gene expression as well as β-adrenergically- or hypoxia-induced increases in glycolytic gene expression was reduced by Hif-1α knockdown. In addition, knockdown of Hif-1α diminished glucose uptake, lactate secretion and glycolytic capacity as well as β-adrenergically stimulated oxygen consumption.
Results
Increased expression of genes encoding glucose transporters and glycolytic enzymes in BAT from cold-exposed mice
To study the expression of glucose transporters and glycolytic enzymes in iBAT during cold exposure, mice kept at room temperature were transferred to 4 °C for 3 h, 6 h, 12 h, 24 h, 2 days, 4 days or 8 days. In addition, a group of mice were housed at thermoneutrality (30 °C) for 8 days. Gene expression was determined by RT-qPCR (Fig. 1A–C). Ucp1 expression increased transiently, peaking after 2 days of cold exposure (Fig. 1A). Moreover, iBAT mass increased during cold exposure (Fig. 1D). Expression of the two glucose transporters Glut1 and Glut4 increased during the cold challenge (Fig. 1A). Glut1 expression increased ~2-fold after 3 h in the cold and remained high throughout the study. We observed a bell-shaped expression profile for Glut4 with a maximum induction of 1.4-fold after 2 days in the cold (Fig. 1A). We observed a ~2-fold decrease in Glut4 expression of mice kept at thermoneutrality, a condition that unexpectedly increased Glut1 expression ~2-fold (Fig. 1A).
Expression levels of genes associated with glycolysis in iBAT, iWAT and eWAT of mice exposed to cold or thermoneutrality. Total RNA was isolated from iBAT, iWAT and eWAT of control mice kept at room temperature (RT, white bars) (n = 12), mice kept at thermoneutrality for 8 days (TN, black bars) (n = 6) and mice exposed to cold for 3 h, 6 h, 12 h, 24 h, 2 d, 4 d or 8 d (grey bars) (n = 6). Relative gene expression was measured in iBAT by RT-qPCR for: (A) Ucp1, Glut1, Glut4 and Ldha; (B) Hk1, Hk2, Pfkl and Pfkp; (C) Tpi1, Pgk1, Pkm1 and Pkm2. (D) Mass (g) of iBAT and iBAT mass as percent of body weight. (E) Fold change in gene expression level in iWAT and (F) eWAT. The mRNA expression levels were normalized to TATA-binding protein (Tbp). Data represents mean + SEM. *p < 0.05 versus RT.
Lactate production is an important anaerobic pathway linked to glycolysis6. Lactate dehydrogenase A (Ldha) is the Ldh isoform with the highest expression in iBAT (data not shown). Ldha expression was induced ~2-fold in mice exposed to cold for 1, 2, 4 or 8 days (Fig. 1A). Contrary, expression of Ldha was down-regulated ~2-fold in iBAT from mice kept at thermoneutrality.
Expression of several enzymes catalyzing the first (Fig. 1B) and second (Fig. 1C) part of glycolysis was increased progressively in iBAT during cold exposure. Hk1 expression was increased ~2-fold from 12 h of cold exposure and onwards (Fig. 1B). Thermoneutrality did not affect Hk1 expression. Hk2 expression was significantly downregulated after 3 h of cold exposure and at thermoneutrality, but was otherwise not significantly affected by cold (Fig. 1B). Pfkl expression was induced ~2-fold in iBAT from mice exposed to cold for 2, 4 or 8 days (Fig. 1B). Pfkp expression increased more rapidly with a ~2-fold induction after 6 h and a maximum induction after 8 days cold exposure. Expression of Pfkl and Pfkp was decreased 2-3-fold in mice kept at thermoneutrality (Fig. 1B). Expression of triosephosphate isomerase 1 (Tpi1) and phosphoglycerate kinase 1 (Pgk1) increased modestly in a progressive manner during cold exposure with a significant maximum induction of 1.6- and 1.9-fold, respectively, at day 4 (Fig. 1C). Thermoneutrality caused a ~2-fold down-regulation of both. The expression profiles of Pkm1 and Pkm2 resembled that of Pfkl, except that Pkm1 expression was significantly downregulated after 6 h in the cold (Fig. 1C). Overall these data show a general increase in expression of glycolytic enzymes in iBAT during cold exposure and a decrease in expression of the same genes at thermoneutrality compared to room temperature.
In inguinal and epididymal WAT, cold exposure induced the expression of Glut4, Ldha, Pfkl and Pkm1 (Fig. 1E and F). Ucp1 and Hk1 mRNAs were selectively increased in inguinal compared with epididymal WAT.
β-Adrenergic stimulation causes induction of several glycolytic enzymes in cultured brown adipocytes
To test whether the expression of the glucose transporters and glycolytic enzymes was recapitulated in cultured brown adipocytes in response to β-adrenergic stimulation, we used the brown pre-adipocyte cell line WT-123 and primary brown adipocytes. We stimulated mature WT-1 adipocytes at day 8 with the pan-β-adrenergic agonist isoproterenol (ISO) or vehicle for 3, 6, 12 or 24 h before harvesting. Expression of Ucp1 was induced at all time points with a maximum induction of ~50-fold after 6 h (Fig. 2A). Glut1 expression was significantly induced after ISO stimulation for 6, 12 and 24 h with maximal induction of 4-fold after 6 h (Fig. 2A). Contrary, ISO stimulation caused an up to 2-fold down-regulation of Glut4 expression. Expression of Ldha was increased ~2-fold from 6 h of ISO stimulation (Fig. 2A).
Expression levels of genes associated with glycolysis in brown adipocytes after β-adrenergic stimulation in vitro. (A–C) Mature brown adipocytes (WT-1) were treated with vehicle (white bars) or 0.1 μM isoproterenol (ISO, black bars) for 3, 6, 12 or 24 h. Total RNA was harvested and analyzed by RT-qPCR (n = 4). Relative gene expression was measured for: (A) Ucp1, Glut1, Glut4 and Ldha; (B) Hk1, Hk2, Pfkl and Pfkp; (C) Tpi1, Pgk1, Pkm1 and Pkm2. (D) Mature primary brown adipocytes were treated with vehicle or 0.1 μM isoproterenol for 3 h (black bars), 6 h (grey bars) or 12 h (white bars). Relative gene expression was measured and presented as fold change relative to the corresponding vehicle-treated samples (n = 4). mRNA expression levels were normalized to Tbp. Data represent mean of means + SEM. *p < 0.05 versus vehicle controls.
The cold-induced changes in expression of glycolytic enzymes were to a large extent mimicked in vitro by β-adrenergic stimulation. The expression profile of Hk1 was bell-shaped with a maximum ~4-fold induction after 6 h of ISO stimulation (Fig. 2B). Hk2 expression was modestly increased after 3 and 6 h of stimulation. Expression of Pfkl and Pfkp was induced after 6, 12 and 24 h of stimulation with a maximum induction of ~3-fold, however, only the induction of Pfkl reached statistical significance (Fig. 2B). The enzymes catalyzing the second part of glycolysis followed a similar expression pattern with an induction after 6 h and a 2-3-fold increase in expression after 12 to 24 h of ISO stimulation (Fig. 2C).
To confirm these results in primary cells, we stimulated primary brown adipocytes with ISO for 3, 6 and 12 h (Fig. 2D). Expression of Ucp1, Glut1 and Ldha was induced by ISO stimulation, together with the enzymes catalyzing the first part of the glycolysis. The enzymes catalyzing the second part of glycolysis followed a similar expression pattern with significant induction observed after 6 h of ISO stimulation (Fig. 2D).
Overall, this demonstrates a similar expression profile of glycolytic enzymes after cold exposure in vivo and β-adrenergic stimulation in vitro, although the kinetics of induction is faster in vitro.
Mitochondrial uncoupling and hypoxia can induce the expression of glycolytic enzymes
β-Adrenergic stimulation might induce the expression of glycolytic enzymes directly through signal transduction-dependent activation of transcription factors or indirectly through increased energy demands in response to the mitochondrial uncoupling. To distinguish between these two possibilities, WT-1 brown adipocytes were treated either with ISO or the chemical uncoupler FCCP (Fig. 3A). FCCP significantly increased expression of Glut1 as well as Ldha, Pfkl, Pfkp, Tpi1, Pgk1 and Pkm2 compared to vehicle-treated cells (Fig. 3A). However, FCCP was a less potent inducer of glycolytic gene expression than ISO, even though experiments on a Seahorse XF96 Flux Analyzer established that 1 µM FCCP was sufficient to cause a more pronounced mitochondrial uncoupling than 0.1 µM ISO in these cells (Fig. 3B). Thus, these data indicate that the increased energy demand after mitochondrial uncoupling can explain some, but not all of the increased expression of glycolytic enzymes in response to β-adrenergic stimulation.
Expression levels of genes associated with glycolysis during chemical uncoupling or hypoxia. (A) Total RNA was isolated from mature brown adipocytes (WT-1) treated with vehicle, 0.1 μM isoproterenol (ISO) (black bars) or 1 μM of the chemical uncoupler FCCP (white bars) for 12 h. Relative gene expression was analyzed by RT-qPCR (n = 4) and presented as fold change relative to the corresponding vehicle-treated samples. (B) Representative fold induction in oxygen consumption rate (OCR) 30 min after stimulation of WT-1 brown adipocytes with 0.1 μM ISO (black bar) or 1 μM FCCP (white bar) on a Seahorse XF96 Flux Analyzer (n = 3) (C) Total RNA was isolated from mature brown adipocytes (WT-1) treated with vehicle or 0.1 μM ISO (black bars) or exposed to hypoxia (1% O2) (white bars) for 12 h. Relative gene expression was analyzed by RT-qPCR (n = 5) and presented as fold change relative to the corresponding control samples. (D) Total RNA was isolated from mature brown adipocytes (WT-1), which were reverse transfected with scramble siRNA or siRNA against Hif-1α at day 6 after initiation of differentiation. Mature cells at day 10 were exposed to normoxia or hypoxia (1% O2) for 12 h. Relative gene expression was analyzed by RT-qPCR (n = 3) and presented as fold change relative to the corresponding normoxia control samples. (E) Immunoblotting analyses of HIF-1α in brown WT-1 adipocytes transfected with scramble siRNA or siRNA against Hif-1α. The mRNA expression levels were normalized to Tbp. Gene expression data represent mean of means + SEM. *p < 0.05 versus vehicle control. #p < 0.05 versus ISO stimulated cells (panel A and C) or scramble siRNA transfected cells (panel D). Transcription factor II B (TFIIB) was used as loading control in panel E. Full-length blots for panel E are shown in Supplementary Figure S1.
One mechanism by which mitochondrial uncoupling might induce changes in expression of the glycolytic genes is through hypoxia. To test whether hypoxia mimicked the effect on glycolytic gene expression caused by ISO stimulation, we exposed cells to hypoxia (1% O2) or ISO for 12 h. Hypoxia caused a significant induction of Glut1, Ldha, Hk1, Hk2, Pfkl, Pfkp, Tpi1, Pgk1 and Pkm2 (Fig. 3C). The expression of Hk1, Pfkl, Pfkp, Tpi1, Pgk1, Pkm1 and Pkm2 was induced to a similar level by hypoxia and ISO stimulation (Fig. 3C). This indicates that hypoxia in response to uncoupling might induce glycolytic gene expression in response to ISO stimulation.
The most likely candidate regulating glycolytic gene expression in response to hypoxia is HIF-1α. To test whether HIF-1α is responsible for regulating glycolytic gene expression in brown adipocytes in response to hypoxia, we knocked down Hif-1α by siRNA in mature WT-1 adipocytes. Four days after siRNA transfection, the cells were exposed to normoxia or hypoxia for 12 h, and gene expression was analyzed. The siRNA reduced both Hif-1α mRNA (~80%) (Fig. 3D) and HIF-1α protein levels (Fig. 3E). The induction of glycolytic gene expression by hypoxia was completely blunted by knockdown of Hif-1α (Fig. 3D). This shows that HIF-1α is responsible for hypoxia-induced expression of the glycolytic enzymes in brown adipocytes.
Regulation of HIF-1α levels in cultured brown adipocytes
The HIF-1α-dependent induction of many glycolytic genes after hypoxia prompted us to measure levels of HIF-1α in response to β-adrenergic stimulation of cultured brown adipocytes. Increased Hif-1α mRNA levels were detected in WT-1 (Fig. 4A) and primary brown adipocytes (Fig. 4B) in response to ISO stimulation. Immunoblotting demonstrated a transient accumulation of HIF-1α protein in response to ISO stimulation in WT-1 (Fig. 4C) and primary brown adipocytes (Fig. 4D). The changes in HIF-1α protein levels were much more pronounced than Hif-1α mRNA levels, indicating increased stability of HIF-1α in response to ISO stimulation. HIF-1α protein levels also accumulated in a human brown adipocyte cell model after ISO stimulation (Fig. 4E).
Hif-1α mRNA and HIF-1α protein levels. (A) Total RNA was isolated from mature brown adipocytes (WT-1). Relative mRNA expression was measured by RT-qPCR for Hif-1α in cells treated with vehicle (white bars) or 0.1 μM ISO (black bars) for 3, 6, 12 or 24 h (n = 4). (B) Total RNA was isolated from primary mature brown adipocytes. Relative mRNA expression was measured by RT-qPCR for Hif-1α in cells treated with vehicle (white bars) or 0.1 μM ISO (black bars) for 3, 6 or 12 h (n = 4). Immunoblotting analysis of HIF-1α in (C) WT-1 brown adipocytes and in (D) primary brown adipocytes stimulated with vehicle or ISO for 1, 3, 6 and 12 h. (E) Immunoblotting analysis of HIF-1α in human brown adipocytes stimulated with vehicle or ISO for 3 or 6 h. (F) Relative gene expression for Hif-1α (n = 4) and (G) immunoblotting analyses of HIF-1α were performed on mature brown adipocytes (WT-1) treated with vehicle, 0.1 μM ISO or 1 μM FCCP for 12 h (gene expression) or 4 h (immunoblotting). (H) Relative gene expression for Hif-1α (n = 4) and (I) immunoblotting analysis of HIF-1α were performed on mature brown adipocytes (WT-1) treated with vehicle, 0.1 μM ISO or exposed to hypoxia (1% O2) for 12 h (gene expression) or 3 h (immunoblotting). (J) Immunoblotting analysis for HIF-1α and UCP1 was performed on mature brown adipocytes (WT-1) or (K) in mature primary brown adipocytes, which were reverse transfected with a scramble siRNA or siRNAs against Hif-1α at day 6 after initiation of differentiation or 8 days after isolation, respectively. Four days later, the adipocytes were stimulated with vehicle or 0.1 μM ISO for 3 h. mRNA levels were normalized to Tbp. (L) Immunoblotting analysis for HIF-1α was performed on mature brown adipocytes (WT-1), which were pretreated or not with 5 μM MitoQ for 1 h, followed by stimulation with vehicle or 0.1 μM ISO for an additional 3 h. Data represent mean of means + SEM. *p < 0.05 versus vehicle (panel A, B, F and H). TFIIB was used as loading control for immunoblotting (panel C–E, G and I–L). Full-length blots for panels C–E, G and I–L are shown in Supplementary Figure S1.
Treatment with FCCP did not increase Hif-1α mRNA levels in WT-1 adipocytes, but caused a modest increase in HIF-1α protein levels (Fig. 4F and G). Hypoxia increased both Hif-1α mRNA and HIF-1α protein levels in WT-1 adipocytes (Fig. 4H and I). To study the importance of uncoupling for HIF-1α stability further, we knocked down Ucp1 expression in mature WT-1 adipocytes and in mature primary adipocytes with siRNA (Fig. 4J and K). Four days after siRNA transfection, the cells were stimulated with vehicle or ISO for 3 h, and protein levels were analyzed. Knockdown of Ucp1 blunted the increase in HIF-1α levels after ISO stimulation in both WT-1 and primary adipocytes (Fig. 4J and K). Of notice, even without exposure to ISO, FCCP or hypoxia, low levels of HIF-1α protein were present in human and mouse brown adipocytes (Fig. 4C–E,G,I and J–L). Altogether, these results highlight the importance of UCP1, and thereby mitochondrial uncoupling, for ISO-induced HIF-1α stabilization. However, the modest effect of FCCP compared to ISO on HIF-1α stabilization (Fig. 4G) indicates that uncoupling-induced hypoxia is not the only mechanism through which β-adrenergic stimulation stabilizes HIF-1α. It was recently demonstrated that β-adrenergic stimulation induced production of mitochondrial reactive oxygen species (ROS) in brown adipocytes24. Since ROS is capable of stabilizing HIF-1α in a human hepatoblastoma cell line25, we hypothesized that β-adrenergic stimulation could also stabilize HIF-1α through increased ROS production. To test this, we pretreated WT-1 adipocytes with the mitochondria-targeted antioxidant MitoQ for 1 h, followed by stimulation with ISO or vehicle for an additional 3 h (Fig. 4L). MitoQ treatment abolished the β-adrenergically induced stabilization of HIF-1α. This indicates that mitochondrial ROS production plays an essential role in stabilizing HIF-1α in response to β-adrenergic stimulation.
Regulation of basal and β-adrenergically induced glycolytic gene expression by HIF-1α in cultured brown adipocytes
To investigate the involvement of HIF-1α in basal and β-adrenergically induced glycolytic gene expression, we knocked down Hif-1α in mature WT-1 adipocytes with two different siRNAs (siRNA 1 and 2). Four days post-transfection, the cells were stimulated with vehicle or ISO for 12 h, and gene expression was analyzed. The two siRNAs reduced both Hif-1α mRNA (~90%) (Fig. 5A) and HIF-1α protein levels (Fig. 5B). Knockdown of Hif-1α with siRNA 1 and/or 2 reduced basal expression of Glut1, Glut4, Ldha (Fig. 5C) and all the glycolytic enzymes measured, except Hk1 (Fig. 5D and E). Similarly, Hif-1α knockdown with siRNA 1 and/or 2 diminished expression of the same set of genes, excluding Glut4, after ISO stimulation (Fig. 5C–E). Finally, ISO stimulation did no longer significantly induce expression of Hk2 and Pfkp after Hif-1α knockdown with siRNA 1 and/or 2 (Fig. 5C). Of notice, basal expression of the glycolytic genes was consistently higher when cells were transfected with siRNA compared to the non-transfected cells in Fig. 2. This might be due to several factors: the cells being replated during differentiation; the cells being cultured in 96-well format compared to the larger well formats used in all non-siRNA experiments; or the transfection procedure itself. Due to this higher basal expression, the ISO-induced expression of glycolytic genes was less pronounced in the experiments where the cells were siRNA-transfected.
Involvement of HIF-1α in regulation of expression of genes associated with glycolysis in a brown adipocyte cell line. Mature brown adipocytes (WT-1) were reverse transfected with two different siRNAs against Hif-1α at day 6 after initiation of differentiation. Mature cells at day 10 were treated with vehicle (white bars) or 0.1 μM ISO (black bars) for 12 h. Total RNA was harvested and analyzed by RT-qPCR (n = 3). Relative gene expression was measured for: (A) Hif-1α; (C) Ucp1, Glut1, Glut4 and Ldha; (D) Hk1, Hk2, Pfkl and Pfkp; (E) Tpi1, Pgk1, Pkm1 and Pkm2. mRNA expression levels were normalized to Tbp. Data represent mean of means + SEM. *p < 0.05 versus vehicle-treated cells transfected with the same siRNA. #p < 0.05 versus scramble siRNA transfected cells treated in the same way (vehicle or ISO). (B) Immunoblotting analysis of HIF-1α in brown adipocytes (WT-1) reverse transfected with scramble siRNA or two different siRNAs against Hif-1α. Four days after the transfection, adipocytes were treated with vehicle or 0.1 μM ISO for 3 h. TFIIB was used as loading control. Full-length blots for panel B are shown in Supplementary Figure S1.
To validate the results from WT-1 cells, Hif-1α was also knocked down in primary brown adipocytes. Mature primary brown adipocytes were transfected 8 days after isolation with scramble siRNA or the two siRNAs against Hif-1α. Four days later, the cells were stimulated with vehicle or ISO for 12 h, and gene expression was analyzed. Hif-1α expression was reduced with ~90% and ~80% by siRNA 1 and 2, respectively (Fig. 6A), and the protein levels of HIF-1α was reduced as well (Fig. 6B). Knockdown of Hif-1α with siRNA 1 and/or 2 reduced the basal expression of Glut1, Glut4, Ldha, Pfkl, Tpi1 and Pgk1 (Fig. 6C–E). After ISO stimulation, the expression of Glut1, Glut4, Ldha, Hk2, Pfkl, Tpi1 and Pgk1was reduced by Hif-1α knockdown compared to control cells (Fig. 6C–E).
Involvement of HIF-1α in regulation of expression of genes associated with glycolysis in primary brown adipocytes. Primary mature brown adipocytes were reverse transfected with scramble siRNA or two different siRNAs against Hif-1α at day 8 after isolation. Four days later the primary adipocytes were treated with vehicle (white bars) or 0.1 μM ISO (black bars) for 12 h. Total RNA was harvested and analyzed by RT-qPCR (n = 4). Relative gene expression was measured for: (A) Hif-1α; (C) Ucp1, Glut1, Glut4, and Ldha; (D) Hk1, Hk2, Pfkl and Pfkp; (E) Tpi1, Pgk1, Pkm1 and Pkm2. The mRNA expression levels were normalized to Tbp. Data represent mean of means + SEM. *p < 0.05 versus vehicle-treated cells transfected with the same siRNA. #p < 0.05 versus scramble siRNA transfected cells treated in the same way (vehicle or ISO). (B) Immunoblotting analysis of HIF-1α in primary brown adipocytes transfected with scramble siRNA or two different siRNAs against Hif-1α. Four days after transfection, adipocytes were treated with vehicle or 0.1 μM ISO for 3 h. TFIIB was used as loading control. Full-length blots for panel B are shown in Supplementary Figure S1.
In summary, HIF-1α is important for basal expression of glycolytic genes as well as their expression after β-adrenergic stimulation in both primary and immortalized brown adipocytes.
HIF-1α promotes glycolysis and β-adrenergically stimulated thermogenesis in cultured brown adipocytes
Next, we examined whether HIF-1α influenced glucose consumption, lactate secretion and glycolysis in cultured brown adipocytes. Glucose consumption and lactate secretion were determined by measuring their concentrations in the cell culture medium. To determine the impact of HIF-1α, Hif-1α was knocked down in WT-1 brown adipocytes at day 6 (on average 83% knockdown at the mRNA level, data not shown). Four days after siRNA transfection, glucose uptake and lactate secretion was determined after 6 h of treatment with vehicle or ISO (Fig. 7). Hif-1α knockdown significantly decreased basal glucose uptake (Fig. 7A). Silencing of Hif-1α expression also diminished glucose uptake during 6 h of ISO stimulation (Fig. 7A). Furthermore, Hif-1α knockdown significantly decreased lactate secretion during exposure to both vehicle (basal secretion) and β-adrenergic stimulation, suggesting an attenuated glycolytic flux (Fig. 7B). To elaborate on the effect of HIF-1α on glycolysis under basal conditions we performed a glycolysis stress test on mature adipocytes transfected with either scramble siRNA or Hif-1α siRNA (Fig. 7C). Knockdown of Hif-1α decreased non-glycolytic ECAR, glycolysis and glycolytic capacity. These results highlight the importance of HIF-1α for glycolysis in brown adipocytes under basal conditions.
The importance of HIF-1α for β-adrenergically stimulated glycolysis and thermogenesis. Mature brown adipocytes (WT-1) were reverse transfected with scramble siRNA or two different siRNAs against Hif-1α. Four days later, the adipocytes were stimulated with vehicle or 1 μM ISO for 6 h, after which glucose (A) and lactate (B) measurements were performed on the medium. (C) Representative results from Seahorse XF Glycolysis Stress Tests performed on mature adipocytes (WT-1), which were reverse transfected with scramble siRNA or an siRNA against Hif-1α (siRNA 1) (n = 3). (D–G) Extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) were measured four days after transfection (n = 5). (D) Representative time-course of ECAR during basal conditions and after injection of 1 μM ISO. (E) Fold change in ECAR between the basal level and the measurement 30 min after ISO stimulation. (F) Representative time-course of OCR during basal conditions and after injection of 1 μM ISO. (G) Fold change in OCR between the basal level and the measurement 30 min after ISO stimulation. (H–K) ECAR and OCR were measured at day 8 on a Seahorse XF96 Flux Analyzer after treatment with 5 μM MitoQ or vehicle (n = 4). (H) Representative time-course of ECAR during basal conditions and after injection of 1 μM ISO. (I) Fold change in ECAR between the basal level and the measurement 30 min after ISO stimulation. (J) Representative time-course of OCR during basal conditions and after injection of 1 μM ISO. (K) Fold change in OCR between the basal level and the measurement 30 min after ISO stimulation. (L) Total RNA was isolated from mature adipocytes (WT-1) transfected with scramble siRNA or two different siRNAs against Hif-1α. Relative gene expression was analyzed by RT-qPCR and presented as fold change relative to the scramble siRNA-transfected cells. The genes measured were Cyc1, CoxII, Cs, Pgc-1α and Tfam (n = 3). mRNA expression levels were normalized to Tbp. Data represent mean + SEM (panel C-K) or mean of means + SEM (panel A, B and L). *p < 0.05 versus vehicle-treated cells. #p < 0.05 versus scramble siRNA transfected cells.
This prompted us to measure the effect of Hif-1α knockdown on ISO-stimulated glycolysis and thermogenesis using cell culture medium containing 5 mM glucose as the only exogenously added fuel. ECAR was measured under basal conditions and after addition of 1 μM ISO (Fig. 7D and E). Knockdown of Hif-1α (on average 93% knockdown at the mRNA level, data not shown) reduced ISO-stimulated ECAR by 22% (Fig. 7D and E). β-Adrenergically stimulated OCR was reduced by 18% in response to Hif-1α knockdown in the same experiment (Fig. 7F and G). To further investigate the importance of mitochondrial ROS production for the HIF-1α-dependent effects on glucose metabolism in response to β-adrenergic stimulation, we measured the effect of MitoQ treatment on ISO-stimulated glycolysis and thermogenesis using the same conditions as above (Fig. 7H–K). MitoQ treatment reduced β-adrenergically stimulated ECAR by 12% (Fig. 7H and I) and OCR by 40% (Fig. 7J and K). This indicates that mitochondrial ROS production is even more important for β-adrenergically stimulated thermogenesis than Hif-1α knockdown. Contrary, mitochondrial ROS production was less important for ISO-induced glucose metabolism than HIF-1α depletion.
The decrease in OCR after knockdown of Hif-1α might not necessarily be due to changes in glycolytic flux, but could be due to dysregulated expression of mitochondrial genes, since mice expressing an adipose-specific dominant negative form of HIF-1α display decreased mitochondrial gene expression21. However, we did not observe significantly decreased expression of the mitochondrial genes cytochrome c oxidase subunit 2 (CoxII), citrate synthase (Cs), cytochrome c-1 (Cyc1), peroxisome proliferator-activated receptor gamma coactivator-1α (Pgc-1α) and mitochondrial transcription factor A (Tfam) four days after Hif-1α knockdown in mature WT-1 adipocytes (Fig. 7L). Overall, these results emphasize an important role of HIF-1α in controlling the expression of glycolytic enzymes and glycolysis in brown adipocytes in vitro.
Discussion
An inverse correlation between BAT activity and blood glucose concentrations in humans has been reported26, suggesting a potentially important role for BAT in glucose homeostasis, and BAT transplants have been shown to diminish high fat diet-induced insulin resistance in mice27. We have previously shown an increased expression of enzymes linked to glucose metabolism in BAT in response to cold8. In the present study, we have investigated the transcriptional regulation of glycolytic gene expression and its importance for glucose consumption and glycolysis in brown adipocytes.
Cold exposure and β-adrenergic stimulation cause an increase in glucose uptake in BAT3, 4. Glucose uptake in brown adipocytes is believed to be mediated primarily by GLUT1 and GLUT4. GLUT1 translocate to the plasma membrane after β-adrenergic stimulation of cultured brown adipocytes5, 28, 29, and both cold-induced up- and down-regulation of Glut1 in BAT have been reported3, 30. GLUT4 levels and translocation to the plasma membrane increase after cold exposure in vivo 31. Here, we observed increased expression of both Glut1 and Glut4 in iBAT of mice exposed to cold (Fig. 1). The increase in Glut1 expression was recapitulated in β-adrenergically stimulated cultured brown adipocytes, however, this was not the case for Glut4 (Fig. 2). The inverse regulation of Glut1 and Glut4 expression after β-adrenergic stimulation of cultured brown adipocytes has been reported by others5.
We observed increased expression of the enzymes catalyzing both the first and second part of glycolysis in iBAT of mice exposed to cold and in cultured brown adipocytes stimulated with a β-adrenergic agonist (Figs 1 and 2). The increased expression of enzymes catalyzing the second part of glycolysis indicates an increased flow all the way through glycolysis and not only through the first 4 steps, which are required for conversion of glucose into glycerol. A high glycolytic flux in BAT is supported by the prominent expression of the Pkm2 isoform (Fig. 1) since its activity is induced allosterically by positive feedback loops when glycolytic flux is high32. A substantial flow through glycolysis after cold exposure is also supported by studies demonstrating increased glucose conversion to CO2 in BAT of cold-exposed mice and in brown adipocytes after β-adrenergic stimulation33,34,35.
The increased expression of Ldha after cold exposure or β-adrenergic stimulation (Figs 1 and 2) and the increased lactate release in response to β-adrenergic stimulation imply increased reduction of pyruvate to lactate. A high degree of lactate production in BAT is emphasized by a study in rats in which lactate and pyruvate release accounted for 33% to 88% of the glucose taken up by iBAT after stimulation with increasing doses of noradrenaline36. Moreover, overexpression of UCP1 or treatment with FCCP induces an increased glycolytic flux and an increased lactate production in white adipocytes33. Accordingly, we observed increased expression of Ldha in response to FCCP treatment. This indicates that mitochondrial uncoupling by itself induces lactate production.
Chronic treatment with FCCP has been reported to induce glucose uptake and glycolytic flux in white adipocytes33. Accordingly, β-adrenergically induced glucose uptake in BAT appears to depend on uncoupling since β-adrenergic stimulation does not induce glucose uptake in BAT of Ucp1 knockout mice29, although this observation is still a matter of discussion34. However, since treatment with FCCP only partly phenocopied the effect of ISO stimulation (Figs 3 and 4), this points to the existence of an energy sensing-independent pathway regulating the expression of glycolytic enzymes after β-adrenergic stimulation.
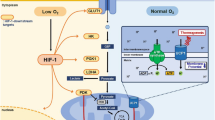
Transcriptional regulation of glycolytic genes has primarily been studied in relation to cancer. One of the best-described transcription factors controlling glycolytic gene expression in cancer cells is HIF-1α37. HIF-1α activity is mainly regulated post-transcriptionally in response to hypoxia. To this end, we observed increased HIF-1α protein levels after ISO stimulation in primary and immortalized brown adipocytes (Fig. 4). The increase in HIF-1α levels was more pronounced at protein level compared to mRNA level. Although clearly elevated after ISO stimulation, the HIF-1α protein was also detectable in untreated brown adipocytes (Figs 4–6). Hypoxia is induced by cold in BAT of lean mice, as observed by pimonidazole staining, and the cold-induced hypoxia is dependent on mitochondrial uncoupling, since it is blunted in Ucp1 knockout mice35. Accordingly, we observed a blunted HIF-1α protein accumulation after ISO stimulation of Ucp1 knockdown brown adipocytes (Fig. 4). In addition, we observed that β-adrenergic stimulation stabilized HIF-1α in a manner dependent on mitochondrial ROS production (Fig. 4). Whether this ROS production is a result of mitochondrial uncoupling and/or hypoxia is not clear. However, since FCCP-induced mitochondrial uncoupling decreases ROS production in mitochondria isolated from brown adipocytes38, 39 it is unlikely that uncoupling by itself induces ROS production in response to β-adrenergic stimulation. Treatment with MitoQ is able to decrease hypoxia-induced HIF-1α stabilization but not proteasome inhibitor-induced HIF-1α stabilization25. This might indicate that the β-adrenergically induced ROS production is hypoxia-driven. Figure 8 summarizes our hypothesis on how β-adrenergic stimulation induces HIF-1α stabilization through mitochondrial uncoupling and ROS production.
A proposed model for HIF-1α-dependent regulation of glycolysis in brown adipocytes. Illustrated is a model of how cold and β-adrenergic stimulation cause HIF-1α stabilization in brown adipocytes through mitochondrial uncoupling and reactive oxygen species (ROS) production. HIF-1α in turn induces the expression of Glut1, Hk2, Pfkl, Tpi1, Pgk1, Pkm2 and Ldha, increasing glycolytic flux capacity. 1) β-Adrenergic stimulation induces the production of mitochondrial ROS in brown adipocytes24. 2) Cold-stimulated mitochondrial uncoupling induces hypoxia in BAT41. 3) FCCP-induced mitochondrial uncoupling decreases mitochondrial ROS production in brown adipocytes42, 43. 4) Hypoxia-induced HIF-1α stabilization through increased mitochondrial ROS production25.
We observed a reduced basal expression of Glut1, Glut4, Ldha, Pfkl, Tpi1 and Pgk1 after Hif-1α knockdown in both immortalized and primary brown adipocytes (Figs 5 and 6). The expression of these genes, except for Glut4, has been described to be under control of HIF-1α in embryonic stem cells and cancer cell lines40,41,42,43. The effect of Hif-1α knockdown on basal glycolytic gene expression is consistent with HIF-1α protein being present in brown adipocytes under these conditions (Figs 4–6). Furthermore, we observed a diminished expression of Glut1, Ldha, Hk2, Pfkl, Tpi1 and Pgk1 after β-adrenergic stimulation in Hif-1α-depleted adipocytes (Figs 5 and 6). Overall, HIF-1α seems to play an important role for both the basal and the β-adrenergically induced expression of glycolytic enzymes in brown adipocytes (Fig. 8).
A key ability of brown adipocytes is to produce heat through adaptive thermogenesis. If glucose is an important substrate for this process, glycolytic flux might be rate-limiting for the thermogenic potential. We observed decreased glucose uptake, lactate secretion, glycolysis and glycolytic capacity in Hif-1α knockdown cells (Fig. 7). Furthermore, knockdown of Hif-1α reduced the fold increase in ECAR and OCR after β-adrenergic stimulation in medium containing glucose as the only supplemented energy source (Fig. 7). Mitochondrial ROS scavenging also inhibited ECAR and OCR after β-adrenergic stimulation, indicating the importance of this upstream pathway (Fig. 7). This is in accordance with previous observations of decreased thermogenic potential in vivo in response to MitoQ treatment24.
The decreased glycolytic capacity after knockdown of HIF-1α in brown adipocytes is in agreement with other studies demonstrating glucose intolerance in vivo in response to decreased HIF-1α activity20, 21. On the other hand, several papers have shown increased insulin sensitivity in response to decreased HIF-1α activity14,15,16,17. The reason for this discrepancy in the literature is unclear. However, it should be noted that we are the first to study the role of HIF-1α on β-adrenergically stimulated glucose consumption. The mechanism by which HIF-1α regulates β-adrenergically and insulin stimulated glucose uptake differs, since hypoxia reduces phosphorylation and thereby activation of the insulin receptor16. The decrease in β-adrenergically stimulated ECAR and OCR after HIF-1α knockdown can likely be ascribed to the lower expression of glycolytic enzymes, as we did not observe a decreased expression of mitochondrial genes by knockdown of Hif-1α (Fig. 7) as have previously been reported21. Our findings show that HIF-1α positively impacts glucose metabolism in brown adipocytes. Accordingly, prolyl hydroxylase domain-containing protein 2 negatively affects glycolytic gene expression and glycolysis in white adipose tissue20. Mice with an adipocyte-specific knockout of Hif-1α are resistant to high fat diet-induced obesity, which correlated with increased ability of visceral WAT to oxidize palmitate15. Since HIF-1α can elicit opposite effects on adipocyte fatty acid and glucose oxidation, an intriguing possibility is that HIF-1α contributes to the substrate choice of activated brown adipocytes. More studies are required to decipher the exact function of HIF-1α in adipocyte metabolism in general and brown adipocyte metabolism in particular.
Methods
Materials
Collagenase type II (#C6885), Dulbecco’s Modified Eagle’s Medium (DMEM) (#D5030 and #D6429), dimethyl sulfoxide, glucose, 3-isobutyl-1-methylxanthine (IBMX), dexamethasone, isoproterenol (ISO), penicillin-streptomycin, pyruvate, MISSION siRNAs, sodium ascorbate and TRI Reagent were obtained from Sigma-Aldrich. Insulin was from Roche or kindly provided by Novo Nordisk A/S. Glucose-6-phosphate dehydrogenase, hexokinase, L-lactate dehydrogenase, ATP and NADP+ and NAD+ were from Roche. DMEM (#52100), fetal bovine serum (FBS), Lipofectamine RNAiMAX, 10% newborn calf serum, L-glutamine and Opti-MEM I Reduced Serum Medium were obtained from Life Technologies. Rosiglitazone was from Cayman Chemical. SensiFAST SYBR Lo-ROX Kit was from Bioline. HEPES was from Lonza, and carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) was from Seahorse Bioscience. MitoQ was kindly provided by Dr. Mike Murphy44. Primary antibodies were against HIF-1α (#14179, Cell Signaling Technology), UCP1 (#Ab10983, Abcam) and transcription factor II B (TFIIB) (#sc-225, Santa Cruz Biotechnology). The secondary antibody was horse radish peroxidase-conjugated goat anti-rabbit (#P0448, Dako).
Animals
Adipose tissues were obtained from 10-week-old male C57BL/6JBomTac mice (Taconic). Mice were single-caged at 22 °C and fed standard chow diet. For the cold/thermoneutral experiments, mice were housed at 4 °C for a period of 3 h up to 8 days, or at 30 °C (thermoneutrality) for 8 days (n = 6 in all groups, except in for the control group in which n = 12). Mouse experiments were approved by the Danish Animal Experimental Inspectorate. Care and handling of mice were in accordance with recommendations laid down by local authorities.
Culture and differentiation of cell lines
The WT-1 cell line established by immortalization of brown pre-adipocytes from newborn mice with SV40 large T antigen was kindly provided by Dr. C. Ronald Kahn23. WT-1 cells were propagated in DMEM (Life Technologies) containing 10% FBS, 62.5 µg/ml penicillin and 100 µg/ml streptomycin. Two days after reaching confluence (designated day 0), differentiation was induced by addition of fresh propagation medium supplemented with 1 μM dexamethasone, 0.5 mM IBMX, 5 μg/ml insulin (Roche), and 0.5 μM rosiglitazone. At day 2, the medium was changed to medium with 5 μg/ml insulin and 0.5 μM rosiglitazone. From day 4, cells were cultured in propagation medium. The human brown pre-adipocyte cell model will be described elsewhere (Markussen et al., in review). Briefly, the cell model was generated by immortalization of the stromal vascular fraction of a deep neck adipose tissue biopsy with telomerase reverse transcriptase. Cell cultures were kept at 37 °C in a humidified atmosphere with 5% CO2. Cells were cultured in 6-well plates, except in siRNA experiments (see below). For the hypoxia experiments, cells were placed at 37 °C in a humidified atmosphere with 1% O2 and 5% CO2.
Isolation and culture of primary adipocytes
Primary brown pre-adipocytes from interscapular, cervical and axillary BAT from 3–4 weeks old male NMRI mice (Taconic) were isolated and cultured as described45. The cell pellet was resuspended in culture medium and plated in 48-well or 6-well plates for ISO stimulation or siRNA transfections, respectively. For the ISO stimulation experiments, the medium was refreshed at days 1, 4, 6. For the siRNA transfection experiments, the medium was refreshed at days 1, 3, 4, 6 after isolation. Cultures were incubated in a humidified atmosphere of 8% CO2 at 37 °C.
siRNA transfection
Mature adipocytes (on day 6 for WT-1 cells and day 8 for primary cultures) were reverse transfected with MISSION siRNA using Lipofectamine RNAiMAX diluted in Opti-MEM I Reduced Serum Medium as described45. The final concentrations of Lipofectamine and siRNA were 5 μl/ml and 50 nM, respectively, for WT-1 cells replated into 96-well and Seahorse plates, and 9 μl/ml and 90 nM, respectively, for primary adipocytes replated into 48-well plates. The siRNAs used targeting Hif-1α were SASI_Mm01_00070473 (siRNA 1) and SASI_Mm01_00070475 (siRNA 2) and the siRNA used for targeting Ucp1 was SASI_Mm01_0006760045. The MISSION® siRNA Universal Negative Control #1 (SIC001) was used as control in all siRNA experiments.
Seahorse measurements
Real-time measurements of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were performed using the Seahorse XF96 Extracellular Flux Analyzer (Agilent Technologies). The cell culture medium was changed 1 h before the first measurement to DMEM (Sigma-Aldrich, #D5030) supplemented with 25 mM glucose with 10% fetal bovine serum (Fig. 3B), 2% BSA and 2 mM L-glutamine (Fig. 7C) or 5 mM glucose (Fig. 7D–K) and adjusted to pH 7.4. OCR and ECAR were measured under basal conditions and after injection of 0.1 µM ISO or 1 µM FCCP (Fig. 3), 10 mM glucose, 5 µM oligomycin and 50 mM 2-deoxyglucose (Fig. 7C) or 1 µM ISO (Fig. 7D–K). The Seahorse XF Glycolysis Stress Test was performed according to the manufacturer’s instructions (Agilent Technologies).
Preparation of medium samples and quantitative measurements of glucose and lactate
Medium samples were prepared for quantitative measurements of glucose and lactate by mixing an equal amount of harvested medium from cultured adipocytes and cold 1.4 M perchloric acid (PCA). After vortexing and centrifuging (10 min, 20,000 g), the supernatant was transferred to clean tubes and pH adjusted to 7 by potassium hydroxide (KOH)/Hepes (2 M/0.1 M). The samples were then vortexed and centrifuged (10 min, 20,000 g) before the supernatant was transferred to clean tubes. The dilution factor of the medium was noted. Medium glucose and lactate were measured in cuvettes. Determination of medium glucose were performed by mixing 10 μl acid-precipitated medium, 10 µl glucose-6-phosphate dehydrogenase and 1000 µl Tris-MgCl2 buffer (0.2 M Tris, 2 mM MgCl2, 0.25 mg NADP+, 1.63 mg ATP, pH 7.5). Colorimetric measurements were then performed with an absorbance of 340 nm. After the initial measurement, 10 µl hexokinase was added and the mixture was incubated for 5–15 minutes before being measured again.
Measurements of medium lactate were performed by mixing 10 µl acid-precipitated medium with 1000 µl glycine-hydrazine buffer (38 mg glycine, 200 µl hydrazine 80%, 1.99 mg NAD+, pH 9). Colorimetric measurements were then performed with an absorbance of 340 nm. After the initial measurement, 10 µl L-lactate dehydrogenase was added and the mixture was incubated for 5–15 minutes before being measured again. For each sample, Equation 1 was used to calculate the glucose or lactate concentration:
Gene expression analysis
Total RNA was purified using TRI Reagent. Reverse transcription-quantitative PCR (RT-qPCR) was performed as previously described46, except that the SensiFAST SYBR Lo-ROX Kit was used. Primers used were: CoxII fwd-AATTGCTCTCCCCTCTCTACG, rev-GTAGCTTCAGTATCATTGGTGC; Cs fwd-CTCTTGGGAGCCAAGAACTC, rev-GCCTGCTCCTTAGGTATCAG; Cyc1 fwd-CTACCCATGGTCTCATCGTG, rev-GGAAGAGCACACCTGCTTGT; Glut1 fwd-GGACCCTGCACCTCATTG, rev-GCCACGATGCTCAGATAGG; Glut4 fwd-ATCATCCGGAACCTGGAGG, rev-CGGTCAGGCGCTTTAGACTC; Hif-1α fwd-TCCATTTTCAACTCAGGACAC, rev-GGCAGTGATGGTAGGTTTCT; Hk1 fwd-AGCATGGAGTCTGAGGTCTA, rev-CAGTACGGCCTCGTCTATTT; Hk2 fwd-AGAGAACAAGGGCGAGGAG, rev-GGAAGCGGACATCACAATC; Ldha fwd-TAATGAAGGACTTGGCGGAT, rev-TTGGAGTTCGCAGTTACACA; Pfkl fwd-GCCTATCTCATCCAGCTACG, rev-CTTGCTACTCAGGATTCGGT; Pfkp fwd-AAGCTATCGGTGTCCTGACC, rev-TCCCACCCACTTGCAGAAT; Pgc-1α fwd-AGCCGTGACCACTGACAACGAG, rev-GCTGCATGGTTCTGAGTGCTAAG; Pgk1 fwd-GAGCCTCACTGTCCAAACTA, rev-CTTTAGCGCCTCCCAAGATA; Pkm1 fwd-CTGCTGTTTGAAGAGCTTGTG, rev-GAGTCACGGCAATGATAGGA; Pkm2 fwd-TGCTGCAGTGGGGCCATTAT, rev-GAGTCACGGCAATGATAGGA; Tbp 47 fwd-ACCCTTCACCAATGACTCCTATG, rev-ATGATGACTGCAGCAAATCGC; Tfam fwd-CAAGTCAGCTGATGGGTATGG, rev-TTTCCCTGAGCCGAATCATCC; Tpi1 fwd-TATGGAGGTTCTGTGACTGGA, rev-CGGTGGGAGCAGTTACTAAA; Ucp1 fwd-AGCCGGCTTAATGACTGGAG, rev-TCTGTAGGCTGCCCAATGAAC.
Whole cell extracts and immunoblotting
Cells stimulated with ISO were harvested at day 8 after initiation of differentiation (WT-1), eight days after isolation (primary brown adipocytes) or at day 12 of differentiation (human brown adipocytes), whereas cells transfected with Hif-1α siRNA were harvested four days after transfection. Preparation of whole-cell extracts and immunoblotting were done as described47.
Statistical analyses
All data are presented as mean or mean of means + standard error of the mean (SEM). The cell culture data shown are the mean of three to five independent experiments. For gene expression studies in cell cultures, two to three wells were harvested at each time point and/or treatment in each experiment. All statistical tests of gene expression were performed on log-transformed data and as repeated measurements. Data in Figs 1, 3, 4A,F and H and 7H, were analyzed for statistical significance (P < 0.05) using one-way analysis of variance with post hoc testing of the means with Dunnett’s or Tukey’s correction for multiple comparisons. Data in Figs 2, 4B,C, 7C,E,G,I and K were analyzed for statistical significance (P < 0.05) using Student’s t-test. The results in Figs 5, 6 and 7A,B were analyzed by two-way analysis of variance with Tukey correction for multiple comparisons.
References
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiological reviews 84, 277–359, doi:10.1152/physrev.00015.2003 (2004).
Kajimura, S. & Saito, M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annual review of physiology 76, 225–249, doi:10.1146/annurev-physiol-021113-170252 (2014).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nature medicine 17, 200–205, doi:10.1038/nm.2297 (2011).
Orava, J. et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell metabolism 14, 272–279, doi:10.1016/j.cmet.2011.06.012 (2011).
Dallner, O. S., Chernogubova, E., Brolinson, K. A. & Bengtsson, T. Beta3-adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology 147, 5730–5739, doi:10.1210/en.2006-0242 (2006).
Nelson, D. L. & Cox, M. M. Lehninger principles of biochemistry. 4th edn (Freeman, 2005).
Marin-Hernandez, A. et al. Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. The FEBS journal 273, 1975–1988, doi:10.1111/j.1742-4658.2006.05214.x (2006).
Hao, Q. et al. Transcriptome profiling of brown adipose tissue during cold exposure reveals extensive regulation of glucose metabolism. American journal of physiology. Endocrinology and metabolism 308, E380–392, doi:10.1152/ajpendo.00277.2014 (2015).
Sobrino, F., Gualberto, A. & Pintado, E. Regulation of fructose 2,6-bisphosphate levels in cold-acclimated brown adipose tissue of rat. FEBS letters 229, 91–94 (1988).
Cooney, G. J. & Newsholme, E. A. The maximum capacity of glycolysis in brown adipose tissue and its relationship to control of the blood glucose concentration. FEBS letters 148, 198–200 (1982).
Watanabe, J., Kanamura, S., Tokunaga, H., Sakaida, M. & Kanai, K. Significance of increase in glucose 6-phosphatase activity in brown adipose cells of cold-exposed and starved mice. The Anatomical record 219, 39–44, doi:10.1002/ar.1092190108 (1987).
Marin-Hernandez, A., Gallardo-Perez, J. C., Ralph, S. J., Rodriguez-Enriquez, S. & Moreno-Sanchez, R. HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini reviews in medicinal chemistry 9, 1084–1101 (2009).
Kuschel, A., Simon, P. & Tug, S. Functional regulation of HIF-1alpha under normoxia–is there more than post-translational regulation? Journal of cellular physiology 227, 514–524, doi:10.1002/jcp.22798 (2012).
Jiang, C. et al. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60, 2484–2495, doi:10.2337/db11-0174 (2011).
Krishnan, J. et al. Dietary obesity-associated Hif1alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes & development 26, 259–270, doi:10.1101/gad.180406.111 (2012).
Regazzetti, C. et al. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes 58, 95–103, doi:10.2337/db08-0457 (2009).
Sun, K., Halberg, N., Khan, M., Magalang, U. J. & Scherer, P. E. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Molecular and cellular biology 33, 904–917, doi:10.1128/MCB.00951-12 (2013).
Jiang, C. et al. Hypoxia-inducible factor 1alpha regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. J Biol Chem 288, 3844–3857, doi:10.1074/jbc.M112.426338 (2013).
Jun, J. C. et al. Adipose HIF-1alpha causes obesity by suppressing brown adipose tissue thermogenesis. Journal of molecular medicine. doi:10.1007/s00109-016-1480-6 (2016).
Matsuura, H. et al. Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation 127, 2078–2087, doi:10.1161/CIRCULATIONAHA.113.001742 (2013).
Zhang, X. et al. Adipose tissue-specific inhibition of hypoxia-inducible factor 1{alpha} induces obesity and glucose intolerance by impeding energy expenditure in mice. The Journal of biological chemistry 285, 32869–32877, doi:10.1074/jbc.M110.135509 (2010).
Zhong, L. et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140, 280–293, doi:10.1016/j.cell.2009.12.041 (2010).
Tseng, Y. H., Kriauciunas, K. M., Kokkotou, E. & Kahn, C. R. Differential roles of insulin receptor substrates in brown adipocyte differentiation. Molecular and cellular biology 24, 1918–1929 (2004).
Chouchani, E. T. et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116, doi:10.1038/nature17399 (2016).
Sanjuan-Pla, A. et al. A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1alpha. FEBS letters 579, 2669–2674, doi:10.1016/j.febslet.2005.03.088 (2005).
Matsushita, M. et al. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. International journal of obesity 38, 812–817, doi:10.1038/ijo.2013.206 (2014).
Liu, X. et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell research 23, 851–854, doi:10.1038/cr.2013.64 (2013).
Shimizu, Y. et al. Effects of noradrenaline on the cell-surface glucose transporters in cultured brown adipocytes: novel mechanism for selective activation of GLUT1 glucose transporters. Biochem J 330(Pt 1), 397–403 (1998).
Inokuma, K. et al. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes 54, 1385–1391 (2005).
Yu, X. X., Lewin, D. A., Forrest, W. & Adams, S. H. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 16, 155–168, doi:10.1096/fj.01-0568com (2002).
Gasparetti, A. L. et al. Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicus. The Journal of physiology 552, 149–162, doi:10.1113/jphysiol.2003.050369 (2003).
Ashizawa, K., Willingham, M. C., Liang, C. M. & Cheng, S. Y. In vivo regulation of monomer-tetramer conversion of pyruvate kinase subtype M2 by glucose is mediated via fructose 1,6-bisphosphate. The Journal of biological chemistry 266, 16842–16846 (1991).
Si, Y., Shi, H. & Lee, K. Metabolic flux analysis of mitochondrial uncoupling in 3T3-L1 adipocytes. PloS one 4, e7000, doi:10.1371/journal.pone.0007000 (2009).
Hankir, M. K. et al. Dissociation between brown adipose tissue 18F-FDG uptake and thermogenesis in uncoupling protein 1 deficient mice. Journal of nuclear medicine: official publication, Society of Nuclear Medicine, 10.2967/jnumed.116.186460 (2017).
Xue, Y. et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell metabolism 9, 99–109, doi:10.1016/j.cmet.2008.11.009 (2009).
Ma, S. W. & Foster, D. O. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Canadian journal of physiology and pharmacology 64, 609–614 (1986).
Chen, X., Qian, Y. & Wu, S. The Warburg effect: evolving interpretations of an established concept. Free Radic Biol Med 79, 253–263, doi:10.1016/j.freeradbiomed.2014.08.027 (2015).
Shabalina, I. G. et al. ROS production in brown adipose tissue mitochondria: the question of UCP1-dependence. Biochim Biophys Acta 1837, 2017–2030, doi:10.1016/j.bbabio.2014.04.005 (2014).
Dlaskova, A., Clarke, K. J. & Porter, R. K. The role of UCP 1 in production of reactive oxygen species by mitochondria isolated from brown adipose tissue. Biochim Biophys Acta 1797, 1470–1476, doi:10.1016/j.bbabio.2010.04.008 (2010).
Iyer, N. V. et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes & development 12, 149–162 (1998).
Chen, L., Qiu, J. H., Zhang, L. L. & Luo, X. D. Adrenomedullin promotes human endothelial cell proliferation via HIF-1alpha. Molecular and cellular biochemistry 365, 263–273, doi:10.1007/s11010-012-1267-1 (2012).
Gess, B., Hofbauer, K. H., Deutzmann, R. & Kurtz, A. Hypoxia up-regulates triosephosphate isomerase expression via a HIF-dependent pathway. Pflugers Archiv: European journal of physiology 448, 175–180, doi:10.1007/s00424-004-1241-1 (2004).
He, G., Jiang, Y., Zhang, B. & Wu, G. The effect of HIF-1alpha on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pacific journal of clinical nutrition 23, 174–180, doi:10.6133/apjcn.2014.23.1.14 (2014).
Kelso, G. F. et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. The Journal of biological chemistry 276, 4588–4596, doi:10.1074/jbc.M009093200 (2001).
Isidor, M. S. et al. An siRNA-based method for efficient silencing of gene expression in mature brown adipocytes. Adipocyte 5, 175–185, doi:10.1080/21623945.2015.1111972 (2016).
Murholm, M., Dixen, K. & Hansen, J. B. Ras signalling regulates differentiation and UCP1 expression in models of brown adipogenesis. Biochim Biophys Acta 1800, 619–627, doi:10.1016/j.bbagen.2010.03.008 (2010).
Hansen, J. B. et al. Activation of peroxisome proliferator-activated receptor gamma bypasses the function of the retinoblastoma protein in adipocyte differentiation. The Journal of biological chemistry 274, 2386–2393 (1999).
Acknowledgements
We thank Lillian H.L. Hansen and Amanda Cheung for technical assistance and Mike Murphy, C. Ronald Kahn and Novo Nordisk A/S for the kind gift of materials. This work was supported by grants to J.B.H. from The Novo Nordisk Foundation and the EU FP7 project DIABAT (HEALTH-F2-2011-278373), and grants to A.L.B. and J.B.H. from The Aase and Ejnar Danielsen Foundation.
Author information
Authors and Affiliations
Contributions
A.L.B., M.S.I., S.W. and J.B.H. conceived and designed the experiments. A.L.B., M.S.I., S.W., N.S., M.M., E.S.A., S.B.P. and B.Q. performed the experiments. A.L.B., M.S.I., S.W., N.S., C.W., B.Q. and J.B.H. analysed and interpreted the data. A.L.B. and M.S.I. prepared the figures. A.L.B., M.S.I. and J.B.H. wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Basse, A.L., Isidor, M.S., Winther, S. et al. Regulation of glycolysis in brown adipocytes by HIF-1α. Sci Rep 7, 4052 (2017). https://doi.org/10.1038/s41598-017-04246-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04246-y
This article is cited by
-
Sensing the oxygen and temperature in the adipose tissues – who’s sensing what?
Experimental & Molecular Medicine (2023)
-
Regulatory modules of human thermogenic adipocytes: functional genomics of large cohort and Meta-analysis derived marker-genes
BMC Genomics (2021)
-
NBR1 is a critical step in the repression of thermogenesis of p62-deficient adipocytes through PPARγ
Nature Communications (2021)
-
H2O2-preconditioned human adipose-derived stem cells (HC016) increase their resistance to oxidative stress by overexpressing Nrf2 and bioenergetic adaptation
Stem Cell Research & Therapy (2020)
-
The role of hypoxia-inducible factors in metabolic diseases
Nature Reviews Endocrinology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.