Abstract
Dopamine transporter (DAT) is the target of cocaine and HIV-1 transactivator of transcription (Tat) protein. Identifying allosteric modulatory molecules with potential attenuation of cocaine and Tat binding to DAT are of great scientific and clinical interest. We demonstrated that tyrosine 470 and 88 act as functional recognition residues in human DAT (hDAT) for Tat-induced inhibition of DA transport and transporter conformational transitions. Here we investigated the allosteric modulatory effects of two allosteric ligands, SRI-20041 and SRI-30827 on cocaine binding on wild type (WT) hDAT, Y470 H and Y88 F mutants. Effect of SRI-30827 on Tat-induced inhibition of [3H]WIN35,428 binding was also determined. Compared to a competitive DAT inhibitor indatraline, both SRI-compounds displayed a similar decrease (30%) in IC50 for inhibition of [3H]DA uptake by cocaine in WT hDAT. The addition of SRI-20041 or SRI-30827 following cocaine slowed the dissociation rate of [3H]WIN35,428 binding in WT hDAT relative to cocaine alone. Moreover, Y470H and Y88F hDAT potentiate the inhibitory effect of cocaine on DA uptake and attenuate the effects of SRI-compounds on cocaine-mediated dissociation rate. SRI-30827 attenuated Tat-induced inhibition of [3H]WIN35,428 binding. These observations demonstrate that tyrosine 470 and 88 are critical for allosteric modulatory effects of SRI-compounds on the interaction of cocaine with hDAT.
Similar content being viewed by others
Introduction
Despite the widespread use of efficacious antiretroviral therapies to control peripheral human immunodeficiency virus (HIV) infection and improve the life of HIV patients, HIV-associated neurocognitive disorders (HAND) remain highly prevalent and represent a significant health problem1. It is commonly accepted that viral replication and proteins within the central nervous system (CNS) play a central role in the development of HAND2 particularly since most Highly Active Antiretroviral Therapy (HAART) medications do not cross the blood-brain barrier, while infected macrophages carrying the virus can3. Dopamine (DA) is essential for a variety of brain activities involved in attention, learning, memory4, 5, and motivation6, 7. Converging lines of clinical observation, supported by imaging8, 9, neuropsychological performance testing10, 11, and postmortem examinations12, have implicated DA dysregulation with the abnormal neurocognitive function observed in HAND13, 14. DA-rich brain regions are highly susceptible to the effects of both HIV infection and substance use. In the early stage of HIV infection, increased levels of DA and decreased DA turnover are found in the cerebrospinal fluid of therapy-naïve HIV patients in asymptomatic infection15, which may contribute to decreased levels of DA in DA-rich brain regions in the advanced stages of HIV infection11, 16, 17. Importantly, HIV-induced elevated levels of extracellular DA in CNS can stimulate viral replication in human macrophages within DA-rich brain regions2, 18, 19, further resulting in viral protein release, which has been implicated in the pathophysiology of HAND20. Cocaine abuse has been shown to increase the incidence of HAND and exacerbate the severity of HAND by enhancing viral replication21,22,23,24,25,26,27. Currently, there are no promising therapeutic approaches for cocaine addiction and HIV infection associated comorbidities28. Therefore, there is a pressing need to define the molecular mechanism(s) by which the impaired dopaminergic system by HIV-1 infection affects the progression of HAND in concurrent cocaine abusers.
The presynaptic dopamine transporter (DAT) plays an essential role in dopamine homeostasis and maintaining stable synaptic dopaminergic tone involved in attention, learning, memory4, 5, and motivation6, 7. Cocaine acts as a non-translocated inhibitor and exhibits non-selective binding to the DAT, serotonin transporter and norepinephrine transporter. However, the strong psychoactive behavioral responses and addictive effects of cocaine are mediated almost exclusively by its interaction with the DAT29, 30. DAT is a primary target for cocaine binding, which has been shown to overlap DA uptake site31. In addition to competitive inhibitors and substrates of DAT, there is growing interest in allosteric modulation of DAT. Allosteric sites on human DAT (hDAT) may represent novel drug targets that display neutral cooperativity with the classical DA uptake site. There are a number of advantages in using allosteric modulators of DAT as preferred therapeutic agents over classic competitor of the DA uptake site with minimal effects on the basal DA transmission but decreasing the cocaine’s action on DAT. For example, it has been shown that allosteric modulators of DAT such as the SRI-compounds act as partial antagonists of DA uptake without the full inhibitory profile that is typical of classic competitors of DAT32,33,34. In rat synaptosomes, SRI-compounds diminish cocaine’s ability to inhibit DA uptake35, however, their effect on the interaction between cocaine and hDAT is still unknown. Further, it is uncertain whether the SRI-compounds suppressive effect on cocaine inhibition of DA uptake is mediated through their interaction with DAT, since these compounds also partially inhibit both serotonin and norepinephrine transporters36, 37.
HIV-1 viral proteins are associated with the persistence of HIV-related neuropathology and subsequent neurocognitive deficits38,39,40,41. Among viral proteins, Tat protein plays a crucial role in the neurotoxicity and cognitive impairment evident in neuroAIDS42, 43. DAT activity is strikingly reduced in HIV-1-infected cocaine-using patients, correlating with the severity of HIV-1 associated cognitive deficits8, 9. We have demonstrated that Tat directly binds to DAT44, 45. Exposure to Tat alone results in an inhibition of DA transport and promotes the internalization of DAT44, 46, 47. Interplay of Tat and cocaine augments synaptic DA levels and Tat release by inhibiting DAT activity45, 48, which may contribute to the progression of HAND underlying the cognitive deficits in HIV-1 positive cocaine-using individuals8, 9. Similarly, conditioned expression of Tat in the mouse brain further potentiates cocaine rewarding in vivo 49. Together, these results suggest a synergistic effect of cocaine and Tat on DA transmission contributing to cognitive dysfunction and elevating the cocaine additive effects.
Our previous studies showed that Tat displays an allosteric modulatory effect on DAT function35, 45. Through integrated computational modeling prediction and experimental validation, we explored structural regions of Tat-hDAT model to identify potential binding sites for Tat50. The structure of human DAT was homology modeled based on the X-ray crystal structure of drosophila DAT (dDAT)51 in our previous studies47, 50, 52. The sequence identity between hDAT and dDAT is 46%, which is considered to be sufficient for constructing a satisfactory homology model53, 54. We identified that DAT tyrosine 470 and 88 replaced by histidine (Y470H) or phenylalanine (Y88F) retained the normal surface DAT expression and inhibited Tat-induced inhibition of DA transport44, 47. Further, mutating these two residues prevented zinc-induced conformational transporter transitions44, 47, which may suggest that these two residues are critical for Tat allosteric modulation of DAT. In this study we first evaluated the effects of SRI-20041 and SRI-30827 (see Fig. 1), novel allosteric modulators of DAT32, on cocaine-mediated inhibitory effect on hDAT function. Further, we assessed whether the tyrosine 470 and 88 are critical for the allosteric modulation of hDAT by the SRI-compounds in modulating cocaine’s interaction with DAT. Finally, we determined whether SRI-compounds attenuates Tat-induced inhibition of DAT binding sites. Understanding the allosteric sites of DAT may uncover new therapeutic avenues for treating both cocaine addiction and cocaine-accelerated HAND in HIV-1 positive individuals.
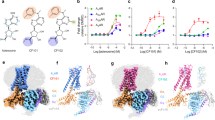
Molecular modeling of binding sites of SRI-20041 and SRI-30827 on DAT. (A) The cartoon presentation of the homology model of hDAT66 (based on the crystal structure of dDAT, PDB ID: 4M48) with SRI-20041 docked to allosteric binding site on the extracellular vestibule. Key residues Tyr470 and Tyr88 are shown in stick style. SRI-20041 and cocaine are shown in green-colored and red-colored solid-sticks, respectively. (B) The cartoon presentation of the homology model of hDAT with SRI-30827 docked to the allosteric binding site on the extracellular vestibule. SRI-30827 and cocaine are shown in green-colored and red-colored solid-sticks, respectively; (C) and (D) SRI-20041 and SRI-30827 were docked to the Tat-hDAT model66, respectively. Tat protein is shown in orange-colored cartoon (E) structure of the two allosteric ligands, SRI-20041 and SRI-30827.
Results
Mutations of Y470 and Y88 attenuate effects of SRI-20041 on cocaine-induced inhibition of DAT activity
To determine whether Y470 and Y88 are responsible for the SRI-20041-mediated allosteric modulation of hDAT, we first assessed inhibition of [3H]DA uptake by cocaine in the presence of SRI-20041 or indatraline. As shown in Table 1, Two-way ANOVA on the apparent affinity (IC50) for cocaine inhibiting [3H]DA uptake revealed significant main effects of drug (F(2,56) = 13.71, p < 0.001) and mutation (F(3,56) = 37.53, p < 0.001), respectively. Post hoc analysis indicated that cocaine IC50 values were significantly decreased in Y470H-hDAT (196 ± 23 nM, LSD: p < 0.001) and Y88F-hDAT (157 ± 10 nM, p < 0.001) but not in Y470F-hDAT (281 ± 16 nM), compared to the WT-hDAT (324 ± 19 nM). These results are consistent with our previous studies44, 47, suggesting that the mutations of Y470 and Y88 increase cocaine-mediated inhibition of DA uptake. The application of SRI-20041 (12.8 µM), an allosteric modulator, significantly increased the cocaine IC50 values of [3H]DA uptake ~35% in WT-hDAT (324 ± 19 to 431 ± 32 nM, p < 0.05) and ~40% in Y470F-hDAT (281 ± 16 to 394 ± 34 nM, p < 0.05), respectively. In contrast, SRI-20041 did not alter the inhibition of [3H]DA uptake by cocaine in either Y470H-hDAT (203 ± 28 nM) or Y88F-hDAT (156 ± 18 nM), indicating that mutations of Y470 and Y88 attenuate the allosteric modulatory effect of SRI-20041 on DA uptake. Indatraline (10 nM), a control drug for competitive inhibition of DA uptake, significantly increased IC50 values of cocaine from 324 ± 19 to 424 ± 23 nM in WT-hDAT (p < 0.05), from 196 ± 23 to 301 ± 38 nM in Y470H-hDAT (p < 0.05), from 281 ± 16 to 371 ± 32 nM in Y470F-hDAT (p < 0.05), and from 157 ± 10 to 256 ± 31 nM in Y88F-hDAT (p < 0.01), respectively. These results suggest that the mutations of Y470 and Y88 do not alter cocaine-induced competitive inhibition of DA uptake.
We next evaluated whether these mutations influence the effect of SRI-20041 on cocaine-induced inhibition of [3H]WIN35,428 binding site. As illustrated in Table 2, two-way ANOVA revealed that there were significant main effects of drug (F(2,18) = 5.16, p < 0.05), mutation (F(1,18) = 55.93, p < 0.001), and drug × mutation interaction (F(2,18) = 6.95, p < 0.01). The IC50 value of cocaine in WT-hDAT (173 ± 26 nM) was decreased by 274% in Y88F-hDAT (63 ± 5 nM, t (6) = 4.20, p < 0.01). The addition of SRI-20041 (12.8 but not 1.28 µM) produced a 64% decrease in the IC50 value of cocaine with a marginal significant effect (cocaine alone: 173 ± 26 nM; SRI-20041 + cocaine: 256 ± 18 nM, p = 0.058), indicating SRI-20041 dose-dependently alters cocaine-induced inhibition of [3H]WIN35,428 binding. However, SRI-20041 had no effect on the IC50 value of cocaine in Y88F-hDAT. Since the binding of [3H]WIN35,428 in Y470H-hDAT was dramatically decreased (~90%)44, the profile of [3H]WIN35,428 in Y470H-hDAT was not included in the current study.
Mutations of Y470 and Y88 attenuate effects of SRI-20041 on cocaine-induced dissociation of [3H]WIN35,428 binding
The dissociation assay has been typically used for determining allosteric modulation of ligands32, 37. To verify the impact of Y470 and Y88 residues on SRI-20041-induced allosteric modulation of DAT function, the effects of SRI-20041 and cocaine on the dissociation of [3H]WIN35,428 binding were examined. As reported in Table 3 and Fig. 2, the dissociation of [3H]WIN35,428 binding with cocaine (1 µM) proceeded in a monotonic manner and was well described by a single component dissociation model in WT and its mutants, which is consistent with our previous report35. Goodness-of-fit analyses of individual experiments revealed R2s ranging from 0.988 ± 0.005 to 0.775 ± 0.056 among all experimental conditions (Supplementary Table 1a). Two-way ANOVA revealed that there were significant main effects of drug (F(1,40) = 14.20, p < 0.01) and mutation (F(2,18) = 6.30, p < 0.01). The dissociation rate in WT-hDAT (K−1 = 0.161 ± 0.032 min−1) was similar to that in Y470F-hDAT (K−1 = 0.161 ± 0.027 min−1) and not significantly changed in Y88F-hDAT (K−1 = 0.272 ± 0.060 min−1, LSD: ps > 0.05). Compared to WT, although Y470H-hDAT displayed a decrease in the dissociation rate, it did not reach the significant level (K−1 = 0.076 ± 0.007 min−1, p = 0.085). The addition of SRI-20041 following the addition of cocaine significantly slowed the dissociation rate in WT-hDAT (K−1 = 0.033 ± 0.009 min−1, t (10) = 3.98, p < 0.01) and in Y470F (K−1 = 0.043 ± 0.024 min−1, t (9) = 3.25, p < 0.01). However, the effect of SRI-20041 on cocaine-induced dissociation of [3H]WIN35,428 binding was attenuated in Y470H-hDAT (K−1 = 0.062 ± 0.019 min−1, t (8) = 0.67, p = 0.52) and Y88F-hDAT (K−1 = 0.144 ± 0.030 min−1, t (13) = 1.81, p = 0.09), respectively.
Effects of SRI-20041 and cocaine on the dissociation of [3H]WIN 35,428 in WT hDAT and mutants. PC12 cells transfected with WT hDAT (A) or Y470H-hDAT (B), Y470F-hDAT (C), and Y88F-hDAT (D) were incubated with [3H]WIN 35,428 (final concentration, 5 nM) on ice for 2 h. After the 2 h incubation, the incubation reagent was replaced with fresh assay buffer. At the zero time point, cocaine (1 µM) was added to Condition-1 and Condition-2 (also see Table 3). Ten minutes later, SRI-20041 (10 µM) was added to Condition-2. Cells were lysed at indicated time points. For the data analysis, the 100% control point (no drug) was time 0 for Condition-1 and time 10 min point for Condition-2. Each data point is the mean ± S.E.M. (n = 5–8).
Mutations of Y470 and Y88 attenuate effects of SRI-30827 on cocaine-induced inhibition of DA uptake
SRI-30827 has been recently characterized as another novel DAT allosteric modulator with a chemical structure similar to that of SRI-20041 but with higher potency for inhibition of DA uptake (0.5 nM) in rat synaptosomes36. We further determined the effect of SRI-30827 on cocaine-induced inhibition of [3H]DA uptake in WT and mutants. As shown in Table 4, there was a significant main effect of mutation (F(2,25) = 49.01, p < 0.001). The cocaine-inhibited [3H]DA uptake with IC50 value (359 ± 30 nM) was significantly decreased in Y470H-hDAT (131 ± 35 nM, LSD: p < 0.001) and Y88F-hDAT (160 ± 27 nM, p < 0.001), respectively. The addition of SRI-30827 (50 nM) following addition of cocaine (1 µM) significantly increased the cocaine IC50 value to 458 ± 22 nM, t (9) = 2.60, p < 0.05) in WT-hDAT. However, the allosteric modulatory effect of SRI-30827 on cocaine-mediated inhibition of DA uptake was attenuated in Y470H-hDAT (141 ± 35 nM) and Y88F-hDAT (161 ± 34 nM), respectively, relative to their respective controls (cocaine alone).
Mutations of Y470 and Y88 attenuate effects of SRI-30827 on cocaine-induced dissociation of [3H]WIN35,428 binding
As reported in Table 5 and Fig. 3, the dissociation of [3H]WIN35,428 binding with cocaine (1 µM) proceeded in a monotonic manner and was well described by a single component dissociation model in WT and its mutants (the range of R2: 0.975 ± 0.011 to 0.676 ± 0.159, Supplementary Table 1b). Two-way ANOVA revealed a significant main effect of drug (F(1,26) = 8.57, p < 0.001) and a marginal main effect on mutation (F(2,26) = 3.19, p = 0.058). The dissociation rate in WT-hDAT (K−1 = 0.148 ± 0.012 min−1) was not significantly changed in Y88F-hDAT (K−1 = 0.134 ± 0.018 min−1, LSD: p > 0.05). However, Y470H-hDAT displayed a decrease in the dissociation rate (K−1 = 0.068 ± 0.015 min−1, p < 0.001) compared to WT, indicating that mutation of Y470 alters cocaine-induced dissociation of [3H]WIN35,428 binding. The application of SRI-30827 following the addition of cocaine significantly slowed the dissociation of rate in WT-hDAT (K−1 = 0.054 ± 0.027 min−1, t (11) = 6.46, p < 0.001). However, the effect of SRI-30827 on cocaine-induced dissociation of [3H]WIN35,428 binding was attenuated in Y470H-hDAT (K−1 = 0.061 ± 0.027 min−1, t (7) = 0.23, p = 0.82) and Y88F-hDAT (K−1 = 0.095 ± 0.014 min−1, t (8) = 1.69, p = 0.13), respectively, relative to their respective controls (cocaine alone).
Effects of SRI-30827 and cocaine on the dissociation of [3H]WIN 35,428 in WT hDAT and mutants. PC12 cells transfected with WT hDAT (A) or Y470H-hDAT (B), and Y88F-hDAT (C) were incubated with [3H]WIN 35,428 (final concentration, 5 nM) on ice for 2 h. After the 2 h incubation, the incubation reagent was replaced with fresh assay buffer. At the zero time point, cocaine (1 µM) was added to Condition-1 and Condition-2 (also see Table 5). Ten minutes later, SRI-30827 (50 nM) was added to Condition-2. Cells were lysed at indicated time points. For the data analysis, the 100% control point (no drug) was time 0 for Condition-1 and time 10 min point for Condition-2. Each data point is the mean ± S.E.M. (n = 4–7).
Effect of SRI-30827on Tat-induced inhibition of [3H]WIN35,428 binding in WT hDAT
We have demonstrated that Tat protein produces inhibitory effects on DAT function and expression. To determine whether the Tat-induced inhibition of DAT binding sites is mediated through an allosteric modulation manner, the ability of SRI-30827 to attenuate the inhibitory effect of Tat was determined (Fig. 4).
Effect of SRI-30827 (50 nM) on Tat-induced inhibition of [3H]WIN35.428 binding in WT hDAT. CHO cells transfected with WT hDAT were incubated with [3H]WIN 35,428 (final concentration, 5 nM) with or without recombinant Tat1–86 (rTat1–86) (40 nM, final concentration) on ice for 2 h. Nonspecific uptake was determined in the presence of 30 µM final concentration of cocaine. Data for rTat1–86 and SRI-30827 + rTat1–86 are expressed as the percentage of their respective controls in the absence of rTat1–86 (15675 ± 2982 DPM) and SRI-30827 (14041 ± 3212 DPM) n = 5. *p < 0.05 compared with control value.
Two-way ANOVA revealed that the main effect of Tat on the specific [3H]WIN35,428 binding was significant (F(1,20) = 7.62, p < 0.05), whereas the main effect of SRI-30827 was not significant (F(1,20) = 3.42, p = 0.08). Moreover, a significant SRI-30827 × Tat interaction (F(1,20) = 4.08, p < 0.05) was observed. Compared with control, Tat (40 nM) induced a decrease (14 ± 3.3%) in the specific [3H]WIN35,428 binding in WT hDAT (t(5) = 4.2, p < 0.01, unpaired Student’s t test). SRI-30827 itself did not alter the specific [3H]WIN35,428 binding (p > 0.05); however, SRI-30827/Tat showed no inhibitory effect on the specific [3H]WIN35,428 binding compared to SRI-30827 alone, indicating that SRI-30827 attenuates Tat-induced decrease in DAT binding sites.
Discussion
The current study reports the allosteric modulatory effects of SRI-compounds on cocaine and Tat binding to hDAT. This study is an extension of our previous work demonstrating that mutations of Y470 and Y88 of hDAT are critical for Tat-induced inhibition of DA transport and conformational transporter transitions44, 47, 50. There are three major findings from the current study. First, using indatraline to assess competitive DAT inhibition, we found that the SRI-compounds and indatraline produce a similar increase in the IC50 value of inhibition of [3H]DA uptake by cocaine in WT hDAT; however, the SRI-compounds-induced change in the IC50 of cocaine is attenuated in Y470H and Y88F. Second, using SRI-compounds to assess allosteric modulation of DAT function, the addition of either SRI-20041 or SRI-30827 following the addition of cocaine significantly slowed the dissociation rate of [3H]WIN35,428 binding compared to cocaine alone, which was totally and partially blocked in Y470H and Y88F, respectively. Third, SRI-30827 attenuates Tat-induced inhibition of [3H]WIN35,827 binding in WT hDAT. The significance of the current observations is two-fold: 1) this is the first report on the key residues responsible for the allosteric modulatory effects on cocaine and Tat binding to hDAT, and 2) the Y470 and Y88 may be related to the allosteric modulatory effects for the SRI-compounds and the Tat binding to hDAT. These findings provide a novel mechanistic basis for developing compounds that specifically attenuate cocaine and Tat binding site(s) in hDAT to normalize DA transmission to physiological levels in HIV-infected cocaine-using patients.
HIV-1 Tat protein and cocaine synergistically disrupt normal physiological dopaminergic transmission by inhibiting DAT. Attenuating inhibitory effect of Tat and cocaine on DA transport is important for preventing the DAT-mediated dysfunction of DA system in HIV infected patients with cocaine abuse. Cocaine binding sites are overlapped with DA uptake sites31, which makes it impossible to generate a competitive inhibitor of cocaine binding that treats cocaine addiction without itself inhibiting DA uptake. However, if inhibition of DA uptake by cocaine is the result of an allosteric mechanism, it would be possible, at least in theory, to generate an allosteric modulator for treatment of cocaine addiction that might attenuate cocaine binding without affecting DA transport. In general, drugs that interact with DAT are typically classified into two categories: 1) inhibitors, such as cocaine and GBR12909, and 2) substrates, such as DA and amphetamine. However, there is growing interest in allosteric modulation of DAT. Conformational transitions via substrate- and ligand-binding sites on DAT are responsible for allosteric modulation of DAT55. The conformational changes in the DA transport process involve three known conformational states of hDAT (occluded, outward-open and inward-open)31, 56, 57. Cocaine preferentially stabilizes the DAT in the outward-open state, resulting in reduction of DA uptake by directly blocking DA uptake site58, 59. Thus, characterization of the binding sites in DAT responsible for cocaine-induced conformational changes will provide a mechanistic basis to identify targets on the DAT for developing novel compounds that reduce cocaine’s potency via their allosteric modulation of DA transport process. The present study shows that SRI-20041 and SRI-30827 allosterically modulate inhibition of [3H]DA uptake by cocaine. Moreover, DAT dissociation assays show that the addition of SRI-20041 or SRI-30827 after cocaine significantly slows the dissociation rate. This is consistent with previous reports from our laboratory35 and by others32, 36, suggesting that these two SRI-compounds modulate cocaine binding to DAT through an allosteric modulation mechanism. Notably, the previous studies were conducted in rat striatal synaptosomes while the current study is the first to determine the allosteric modulatory effects of SRI-compounds in PC12 cells expressing hDAT. Given the important role of DAT in addictive effects of cocaine29, 30, determining the effects of SRI-compounds in cocaine-mediated behavior change is an interesting topic for future investigations.
The most intriguing observation is that mutants Y470H and Y88F do not appear to interact with cocaine but lead to an attenuation of allosteric modulation of SRI-compounds on cocaine-mediated inhibition of DA transport and cocaine-induced dissociation of [3H]WIN35,428 binding. Our computational modeling predicts that both cocaine and Tat interact with hDAT in the outward-open conformational state, resulting in a synergistic inhibitory effect on DA transport50. Interestingly, Y470H and Y88F displayed a higher efficacy in inhibiting DA uptake and DAT binding by cocaine compared with WT hDAT44, 47. In addition, the previous results show that Y470H- and Y88F-mutations altered zinc-induced regulation of DA uptake and WIN35,438 binding44, 47. One possibility is that Y470H- and Y88F-mediated transporter conformational transitions may contribute to these changes in cocaine-mediated inhibition of DA uptake and dissociation in these mutants. Earlier studies have shown that Phenylalanine472 and Leucine475 that are in proximity of Y88 and Y470 are critical for the interaction of cocaine with DAT31, 60, indicating a complex interaction of cocaine and Tat with DAT. The complex interaction of cocaine and Tat may change the sensitivity to cocaine. This may contribute to the clinical and preclinical observations showing an acceleration of HAND in HIV-infected patients who abuse cocaine8, 9, 13 and the neurocognitive/behavioral deficits observed in HIV-1 transgenic animals49, 61. Therefore, these results suggest that Y470H and Y88F play a critical role in the synergistic effects of cocaine and Tat on DAT.
This study provides novel mechanistic insight that Y470 and Y88 residues may influence the allosteric modulation sites on hDAT for both SRI-20041 and SRI-30827. We have demonstrated that Tat-induced inhibition of DAT is mediated by allosteric binding site(s) on DAT, not the interaction with the DA uptake site35, 45, 50. Tat molecule binds to DAT through intermolecular electrostatic attractions and complementary hydrophobic interactions50. In particular, eliminating the cation-π interaction between Y470 in hDAT and M1 in Tat by mutating Y470 into a residue such as histidine or alanine would significantly weaken the binding between hDAT and Tat47. Further, in light of the Tat-DAT binding structure, mutation of Y88 to a residue without hydrogen-bonding capacity would also weaken the Tat-DAT binding47. These computational predictions are validated by experimental data showing that Tat-induced inhibition of DAT is attenuated in Y470H and Y88F44, 47. Furthermore, Y470H but not Y470F attenuates Tat-induced inhibition on DAT because mutating Y470 to phenylalanine (Y470F) does not affect the cation-π interaction47. In the current study, we also found that SRI-compounds-induced decrease in cocaine IC50 was attenuated in Y470H but not in Y470F, further supporting the important role of Y470 in the Tat-DAT interaction. By using a hDAT homology model to dock Tat into the transporter and MD simulations to probe the conformational state of hDAT bound to Tat, we found that Tat can only bind to the outward-open structure with favorable binding energies50. Therefore, we predicted that Tat binding would block the entry pathway of the dopamine substrate, thereby inhibiting dopamine clearance from the presynaptic cleft. Since the conformational changes in DA transport process involve conversions between the outward- and inward-open conformations57, we then conducted docking studies using homology models of hDAT at the different conformational states and found that the two SRI-compounds could only fit into the outward-open hDAT model (Fig. 1). Further, both SRI-20041 and SRI-30827 interact with DAT extracellular loop 6 (EL6) that contacts directly with Tat and can partially inhibit DAT uptake function with a similar chemical structure36. According to our previous modeling results, in the outward-open hDAT conformation, Y470 extends to the extracellular region where it interacts directly with Tat residues50. In the SRI-compound docked models, SRI-compounds may potentially influence the conformation of residues Tyr470 and Tyr88 with EL6 region, and thus likely modulate the binding of Tat on hDAT via an allosteric modulatory mechanism. Hence, although SRI-compounds may not interact directly with either Tyr470 or Tyr88 for competing with Tat binding, they can weaken the Tat DAT binding by changing the DAT conformation allosterically. This has been verified by our experimental data showing that SRI-30827 attenuates Tat-induced inhibition of DAT binding sites. Taken together, considering both Y470 and Y88 are associated with hDAT-Tat interactions, developing compounds directly targeting the specific binding sites on hDAT for Tat could be a viable approach for treatment of Tat-induced dopamine dysfunction. Alternatively, developing DAT-based allosteric modulators interacting with the specific residues that are structurally distinct from Tat binding sites would be another possible therapeutic approach.
In conclusion, we have identified that Y470 and Y88 may act as allosteric modulatory sites on hDAT responsible for the effects of cocaine binding displayed by the two allosteric ligands, SRI-20041 and SRI-30827. Given that mutation of these two residues (Y470H and Y88F) attenuates Tat-induced inhibition of DAT function44, 47, our current study also demonstrates an allosteric modulatory effect of SRI-30827 on attenuation of Tat-induced inhibition of DAT binding sites. These findings presented herein raise the exciting possibility of potential therapeutic intervention for HIV infected patients with concurrent cocaine abuse. Proof of this concept could emerge from efforts directed toward discovery and development of candidate in vivo probe molecules with the desired allosteric modulation profiles coupled with favorable drug-like attributes. The effectiveness of an early intervention for HAND to preserve neurocognitive functions in HIV-infected individuals may ultimately depend on a treatment approach that combines compound(s) that specifically attenuate Tat binding site(s) in DAT with antiretroviral therapy, without affecting the normal function of DAT.
Methods
Materials
PC 12 cells (ATCC® CRL-1721TM) were obtained from ATCC (Manassas, VA). 3,4-[7-3H]DA (28 Ci/mmol) and [N-methyl-3H]WIN 35,428 (82.62 Ci/mmol) were purchased from Perkin Elmer (Boston, MA). The novel DAT allosteric modulators, SRI-20041 and SRI-30827, were synthesized at Southern Research Institute (Birmingham, AL). Cocaine hydrochloride and other fine chemicals/reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Molecular Modeling
As shown in Fig. 1, molecular docking was performed to determine the structures of hDAT binding with SRI-compounds by using the AutoDock Vina62 based on the constructed hDAT-Tat complex from our previous work50. Both the substrate-binding site and extracellular region of hDAT were defined as the search space for the docking. For each SRI-compound, 20 independent docking processes were performed in order to explore all possible binding conformations in such a large search space. The best protein-ligand binding conformation for each SRI-compound was selected according to the best binding score given by the AutoDock Vina. Further, the atomic charges for SRI-compound molecule were determined as restrained electrostatic potential (RESP)-fitted charges based on the first-principles electronic structure calculations at the B3LYP/6-31 G* level by using the Gaussian03 program63. For each SRI-compound, the selected binding conformation was combined with the constructed hDAT-Tat structure with tLeap of the AMBER12 package64. Then the SRI-hDAT-Tat complex was optimized with 100,000 steps of the deepest decent energy-minimization and 100,000 steps of the conjugate gradient energy-minimization by using the Sander module of the AMBER 12 package64.
Construction of plasmids
Point mutations of hDAT at Y470 (tyrosine to histidine, Y470H-hDAT; tyrosine to phenylalanine, Y470F-hDAT) and Y88 (tyrosine to phenylalanine, Y88F-hDAT) were selected based on the structural and mechanistic insights obtained from computational modeling and simulation and previous studies47, 50. The Y470H-hDAT is expected to eliminate both hydrogen bond and cation-π interactions in the native structure of the transporter. Both Y470F-hDAT and Y88F-hDAT are expected to preclude the formation of hydrogen bond. All mutations were generated according to WT-hDAT sequence (NCBI, cDNA clone MGC: 164608, IMAGE: 40146999) by QuikChangeTM site-directed mutagenesis kit (Agilent Tech, Santa Clara, CA) with synthetic cDNA encoding hDAT subcloned into pcDNA3.1 + (a gift provided by Dr. Haley E Melikian, University of Massachusetts) as the template. The sequences of mutated constructs were further confirmed through DNA sequencing at University of South Carolina EnGenCore facility. Plasmid DNA were propagated and purified using a plasmid isolation kit (Qiagen, Valencia, CA).
Cell culture and DNA transfection
PC12 cells were maintained in the incubator (37 °C, 5% CO2) with DMEM medium supplemented with horse serum (15%) and bovine calf serum (2.5%), glutamine (2 mM), and penicillin-streptomycin (100 U/ml). Chinese hamster ovary cells (CHO cells, ATCC, CRL-61) were maintained in F12 medium with 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin). For hDATs transfection, cells were seeded into poly-D-lysine-coated 24 well plates at a density of 1 × 105 cells/well. After 24 h, cells were transfected with WT or mutant DAT plasmids using Lipofectamine 2000 (Life Tech, Carlsbad, CA) based on the manufacturer’s instruction. Cells were used for the experiments after 24 h of transfection.
Competitive inhibition [3H]DA uptake assay
Considering that PC12 cells endogenously expressing norepinephrine transporter (NET) and DA is transported by DAT and NET, in a pilot study, we first performed kinetic analysis of [3H] DA uptake in PC12 cells with no transfection in the presence of desipramine (1 µM, final concentration) to prevent DA uptake into norepinephrine containing nerve terminals based on a previously published method65. Results showed that no specific [3H] DA uptake was found with a range of DA concentrations (1 nM-5 µM, data not shown). [3H] DA uptake assay in PC12 cells transfected with WT or mutated hDATs was performed 24 hr after transfection based on our previous studies35, 44. Briefly, assays were performed in duplicate in a final volume of 500 µl. Cells were washed twice with Krebs-Ringer-HEPES (KRH) buffer (concentration in mM: 125 NaCl, 5 KCl, 1.5 MgSO4, 1.25 CaCl2, 1.5 KH2PO4, 10 D-glucose, 25 HEPES, 0.1 EDTA, 0.1 pargyline, and 0.1 L-ascorbic acid; pH 7.4). After washing, cells in each well were incubated with KRH buffer with one of ten concentrations of cocaine (1 nM-1 mM) and a fixed concentration of SRI-20041 (12.8 µM), SRI-30827 (50 nM) or indatraline (10 nM) for 10 min at room temperature. [3H]DA (final concentration: 0.1 µM) was added for additional 8 min. In parallel, the non-specific [3H]DA uptake was determined in the presence of nomifensine (10 µM). The doses of SRI-compounds and indatraline were based on previous publications32, 35. Uptake reaction was terminated by three more washes of ice-cold KRH buffer. Cells were then lysed in 1% SDS and the remaining [3H]DA radioactivity in each well was counted by liquid scintillation spectrometry.
[3H]WIN 35,428 binding assay
The competitive binding experiments were performed in duplicate in a final volume of 250 µl. Twenty four hours after transfection with WT or mutant hDATs, PC12 cells were washed once with sucrose-phosphate assay buffer (concentration in mM: 2.1 NaH2PO4, 7.3 Na2HPO47H2O, and 320 sucrose). Cells were then incubated with one of eleven concentrations of cocaine (1 nM-1 mM) and fixed concentrations of SRI-20041 (12.8 µM or 1.28 µM) or SRI-30827 (50 nM) in the presence of [3H]WIN 35,428 (5 nM) on ice for 2 hr in duplicate. After incubation, the intact cells in each well were washed twice with ice-cold assay buffer, lysed by 1% SDS for 1 hr and subjected to liquid scintillation spectrometry measurement. For the binding dissociation assay, experiments were conducted in a final volume of 500 µl according to our previous publication35. The intact cells were incubated with a fixed concentration of [3H]WIN 35,428 (5 nM) on ice for 2 hr followed by a wash of ice-cold assay buffer. Non-specific [3H]WIN 35,428 binding was determined by addition of β-CFT naphthalenedisulfonate monohydrate (1 µM). In condition 1 (cocaine only), at the zero time point, the binding dissociation was initiated by the application of a single concentration of cocaine (1 µM). In condition 2 (cocaine + SRI-compounds), 10 min after the addition of cocaine, SRI-20041 (10 µM) or SRI-30827 (50 nM) was then added to minimize any [3H]WIN35,428 re-association. Ten, 20, 30, 40, and 60 min later, duplicate wells of cells were washed twice with assay buffer, lysed, and counted by liquid scintillation spectrometry. For data analyses, 0 min (no drugs treatment) and 10 min after the application of cocaine were set as 100% to normalize samples at each time point in condition 1 and 2, respectively, based on previous publications32, 35.
Our previous study shows that Tat inhibits [3H]WIN35,428 binding, which is consistent with the ability of Tat inhibiting DA uptake45. To determine the effect of SRI-30827 on Tat-induced inhibition of DAT binding sites, the specific [3H]WIN35,824 binding (5 nM, final concentration) in WT hDAT was measured in the presence or absence of SRI-30827 (50 nM, final concentration) or recombinant Tat1–86 (40 nM, final concentration, ImmunoDX, Woburn, MA) on ice for 2 h. This concentration of SRI-30827 was chosen because our pilot study showing SRI-30827 at this concentration did not alter basal [3H]WIN35,824 binding and also this concentration was used for the dissociation assay. Nonspecific [3H]WIN35,824 binding was determined in the presence of 30 µM cocaine.
Data analyses and Statistics
Descriptive statistics and graphical analyses were used as appropriate. Results are presented as mean ± SEM, and n represents the number of independent experiments for each experiment group. IC50 values for cocaine inhibition of [3H]DA uptake and [3H]WIN35,428 binding were calculated from inhibition curves by nonlinear regression analysis with a one-site model of variable slope. For the dissociation of [3H]WIN35,428 binding induced by cocaine, the dissociation rate (K−1) was determined by the specific [3H]WIN35,428 binding through non-nonlinear regression analysis using a single component dissociation model. Both kinetic parameters (cocaine IC50 and K−1) were calculated by Garphad Prism version 5.0 (GarphPad Software Inc., San Diego, CA). For experiments involving comparisons between unpaired samples, separate ANOVAs followed by appropriate post hoc tests [Fisher’s least significant difference (LSD) or Student’s unpaired t test] were used. All statistical analyses were performed using IBM SPSS Statistics version 24. p < 0.05 was the minimum criterion for statistical significance.
References
Heaton, R. K. et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75, 2087–2096 (2010).
Gaskill, P. J. et al. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol 175, 1148–1159 (2009).
Buckner, C. M., Luers, A. J., Calderon, T. M., Eugenin, E. A. & Berman, J. W. Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. J Neuroimmune Pharmacol 1, 160–181 (2006).
Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog Neurobiol 67, 53–83 (2002).
Cools, R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev 30, 1–23 (2006).
Tye, K. M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013).
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012).
Wang, G. J. et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 127, 2452–2458 (2004).
Chang, L. et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage 42, 869–878 (2008).
Meade, C. S., Lowen, S. B., MacLean, R. R., Key, M. D. & Lukas, S. E. fMRI brain activation during a delay discounting task in HIV-positive adults with and without cocaine dependence. Psychiatry Res 192, 167–175 (2011).
Kumar, A. M., Ownby, R. L., Waldrop-Valverde, D., Fernandez, B. & Kumar, M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol 17, 26–40 (2011).
Gelman, B. B. et al. Prefrontal Dopaminergic and Enkephalinergic Synaptic Accommodation in HIV-associated Neurocognitive Disorders and Encephalitis. J Neuroimmune Pharmacol 7, 686–700 (2012).
Berger, J. R. & Arendt, G. HIV dementia: the role of the basal ganglia and dopaminergic systems. Journal of psychopharmacology 14, 214–221 (2000).
Purohit, V., Rapaka, R. & Shurtleff, D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Molecular neurobiology 44, 102–110 (2011).
Scheller, C. et al. Increased dopaminergic neurotransmission in therapy-naive asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. J Neural Transm 117, 699–705 (2010).
Sardar, A. M., Czudek, C. & Reynolds, G. P. Dopamine deficits in the brain: the neurochemical basis of parkinsonian symptoms in AIDS. Neuroreport 7, 910–912 (1996).
Kumar, A. M. et al. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol 15, 257–274 (2009).
Gaskill, P. J., Yano, H. H., Kalpana, G. V., Javitch, J. A. & Berman, J. W. Dopamine receptor activation increases HIV entry into primary human macrophages. PLoS One 9, e108232 (2014).
Gaskill, P. J., Calderon, T. M., Coley, J. S. & Berman, J. W. Drug induced increases in CNS dopamine alter monocyte, macrophage and T cell functions: implications for HAND. J Neuroimmune Pharmacol 8, 621–642 (2013).
Li, W., Li, G., Steiner, J. & Nath, A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res 16, 205–220 (2009).
Norman, L. R., Basso, M., Kumar, A. & Malow, R. Neuropsychological consequences of HIV and substance abuse: a literature review and implications for treatment and future research. Current drug abuse reviews 2, 143–156 (2009).
Larrat, E. P. & Zierler, S. Entangled epidemics: cocaine use and HIV disease. J Psychoactive Drugs 25, 207–221 (1993).
Fiala, M. et al. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine’s connection to AIDS dementia and vasculitis? Adv Exp Med Biol 437, 199–205 (1998).
Webber, M. P., Schoenbaum, E. E., Gourevitch, M. N., Buono, D. & Klein, R. S. A prospective study of HIV disease progression in female and male drug users. AIDS 13, 257–262 (1999).
Buch, S. et al. Cocaine and HIV-1 interplay: molecular mechanisms of action and addiction. J Neuroimmune Pharmacol 6, 503–515 (2011).
Nath, A., Maragos, W. F., Avison, M. J., Schmitt, F. A. & Berger, J. R. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol 7, 66–71 (2001).
Ferris, M. J., Mactutus, C. F. & Booze, R. M. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev 32, 883–909 (2008).
Shorter, D., Domingo, C. B. & Kosten, T. R. Emerging drugs for the treatment of cocaine use disorder: a review of neurobiological targets and pharmacotherapy. Expert opinion on emerging drugs 20, 15–29 (2015).
Chen, R. et al. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci USA 103, 9333–9338 (2006).
Thomsen, M., Han, D. D., Gu, H. H. & Caine, S. B. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther 331, 204–211 (2009).
Beuming, T. et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci 11, 780–789 (2008).
Pariser, J. J., Partilla, J. S., Dersch, C. M., Ananthan, S. & Rothman, R. B. Studies of the biogenic amine transporters. 12. Identification of novel partial inhibitors of amphetamine-induced dopamine release. J Pharmacol Exp Ther 326, 286–295 (2008).
Rothman, R. B., Dersch, C. M., Ananthan, S. & Partilla, J. S. Studies of the biogenic amine transporters. 13. Identification of “agonist” and “antagonist” allosteric modulators of amphetamine-induced dopamine release. J Pharmacol Exp Ther 329 (2009).
Schmidt, H. D., McGinty, J. F., West, A. E. & Sadri-Vakili, G. Epigenetics and psychostimulant addiction. Cold Spring Harbor perspectives in medicine 3, a012047 (2013).
Zhu, J., Ananthan, S., Mactutus, C. F. & Booze, R. M. Recombinant human immunodeficiency virus-1 transactivator of transcription1-86 allosterically modulates dopamine transporter activity. Synapse 65, 1251–1254 (2011).
Rothman, R. B. et al. Studies of the biogenic amine transporters 15. Identification of novel allosteric dopamine transporter ligands with nanomolar potency. J Pharmacol Exp Ther 353, 529–538 (2015).
Nandi, A., Dersch, C. M., Kulshrestha, M., Ananthan, S. & Rothman, R. B. Identification and characterization of a novel allosteric modulator (SoRI-6238) of the serotonin transporter. Synapse 53, 176–183 (2004).
Brack-Werner, R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13, 1–22 (1999).
Frankel, A. D. & Young, J. A. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem 67, 1–25 (1998).
Johnston, J. B. et al. HIV-1 Tat neurotoxicity is prevented by matrix metalloproteinase inhibitors. Ann Neurol 49, 230–241 (2001).
Power, C. et al. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol 72, 9045–9053 (1998).
Rappaport, J. et al. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. Journal of leukocyte biology 65, 458–465 (1999).
King, J. E., Eugenin, E. A., Buckner, C. M. & Berman, J. W. HIV tat and neurotoxicity. Microbes and infection/Institut Pasteur 8, 1347–1357 (2006).
Midde, N. M. et al. Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions. J Neuroimmune Pharmacol 8, 975–987 (2013).
Zhu, J., Mactutus, C. F., Wallace, D. R. & Booze, R. M. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther 329, 1071–1083 (2009).
Midde, N. M., Gomez, A. M. & Zhu, J. HIV-1 Tat Protein Decreases Dopamine Transporter Cell Surface Expression and Vesicular Monoamine Transporter-2 Function in Rat Striatal Synaptosomes. J Neuroimmune Pharmacol 7, 629–639 (2012).
Midde, N. M. et al. Mutations at tyrosine 88, lysine 92 and tyrosine 470 of human dopamine transporter result in an attenuation of HIV-1 Tat-induced inhibition of dopamine transport. J Neuroimmune Pharmacol 10, 122–135 (2015).
Ferris, M. J., Frederick-Duus, D., Fadel, J., Mactutus, C. F. & Booze, R. M. Hyperdopaminergic tone in HIV-1 protein treated rats and cocaine sensitization. J Neurochem 115, 885–896 (2010).
Paris, J. J. et al. Effects of conditional central expression of HIV-1 tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice. Neuropsychopharmacology 39, 380–388 (2014).
Yuan, Y. et al. Molecular mechanism of HIV-1 Tat interacting with human dopamine transporter. ACS chemical neuroscience 6, 658–665 (2015).
Penmatsa, A., Wang, K. H. & Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503, 85–90 (2013).
Yuan, Y., Huang, X., Zhu, J. & Zhan, C. G. Computational modeling of human dopamine transporter structures, mechanism and its interaction with HIV-1 transactivator of transcription. Future medicinal chemistry (2016).
Sali, A., Potterton, L., Yuan, F., van Vlijmen, H. & Karplus, M. Evaluation of comparative protein modeling by MODELLER. Proteins 23, 318–326 (1995).
Nayeem, A., Sitkoff, D. & Krystek, S. Jr. A comparative study of available software for high-accuracy homology modeling: from sequence alignments to structural models. Protein Sci 15, 808–824 (2006).
Shan, J., Javitch, J. A., Shi, L. & Weinstein, H. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PLoS One 6, e16350 (2011).
Kniazeff, J. et al. An intracellular interaction network regulates conformational transitions in the dopamine transporter. J Biol Chem 283, 17691–17701 (2008).
Zhao, Y. et al. Single-molecule dynamics of gating in a neurotransmitter transporter homologue. Nature 465, 188–193 (2010).
Reith, M. E., Berfield, J. L., Wang, L. C., Ferrer, J. V. & Javitch, J. A. The uptake inhibitors cocaine and benztropine differentially alter the conformation of the human dopamine transporter. J Biol Chem 276, 29012–29018 (2001).
Loland, C. J., Norregaard, L., Litman, T. & Gether, U. Generation of an activating Zn(2+) switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc Natl Acad Sci USA 99, 1683–1688 (2002).
Huang, X., Gu, H. H. & Zhan, C. G. Mechanism for cocaine blocking the transport of dopamine: insights from molecular modeling and dynamics simulations. J Phys Chem B 113, 15057–15066 (2009).
McIntosh, S., Sexton, T., Pattison, L. P., Childers, S. R. & Hemby, S. E. Increased Sensitivity to Cocaine Self-Administration in HIV-1 Transgenic Rats is Associated with Changes in Striatal Dopamine Transporter Binding. J Neuroimmune Pharmacol 10, 493–505 (2015).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Frisch, A. Gaussian 03. (Gaussian, 2004).
Case, D. A. et al. AMBER 12. University of California, San Francisco (2012).
Zhu, J., Apparsundaram, S. & Dwoskin, L. P. Nicotinic receptor activation increases [3H]dopamine uptake and cell surface expression of dopamine transporters in rat prefrontal cortex. J Pharmacol Exp Ther 328, 931–939 (2009).
Yuan, Y. et al. Molecular Mechanism of HIV-1 Tat Interacting with Human Dopamine Transporter. ACS Chem. Neurosci. 6, (658–665 (2015).
Acknowledgements
This research was supported by grants from the National Institute on Drug Abuse to Jun Zhu (R01DA035714 and R21DA041932). The synthesis of the compounds was supported by a grant from the National Institute on Drug Abuse to Subramaniam Ananthan (R33 DA029962).
Author information
Authors and Affiliations
Contributions
W.L.S. conducted most of the experiments, analyzed the results, and wrote most of the paper. P.M.Q. conducted experiments on D.A. uptake in W.T. and mutated hDAT. SA provided SRI-compounds. Y.Y. and W.Z. conducted molecular docking studies for SRI-compounds interacting with hDAT. C.G.Z. performed computational modeling for Tat and hDAT. J.Z. conceived the idea for the project and finalized the manuscript with W.L.S.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, WL., Quizon, P.M., Yuan, Y. et al. Allosteric modulatory effects of SRI-20041 and SRI-30827 on cocaine and HIV-1 Tat protein binding to human dopamine transporter. Sci Rep 7, 3694 (2017). https://doi.org/10.1038/s41598-017-03771-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03771-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.