Abstract
Chemical conditioning has been used for enhancing wastewater sludge dewaterability for many years, but the characteristics of odorous pollutants emission in sludge conditioning were still unclear. In this work, the transfer behavior of different odorous pollutants between air, liquid and solid phases under typical chemical conditioning processes for high-pressure dewatering was systematically investigated. The results indicated that that besides hydrogen sulfide (H2S) and ammonia (NH3), 21 kinds of volatile organic contaminants (VOCs) were identified and quantified by gas chromatography-mass spectrometry (GC-MS), while the concentrations and composition of odorous pollutants varied greatly for different conditioning processes. VOCs were composed by three main constituents including benzenes, halogeno benzene and hydrocarbons. According to mass balance analysis, about 50% of VOCs adsorbed within sludge extracellular polymeric substances (EPS) fraction. Since EPS was damaged and/or flocculation in different chemical conditioning processes, VOCs distributed in solid phase transformed into liquid phase and then released into air. The discrepancies in mass of odorous pollutants before and after chemical conditioning were likely to be related to chemical conversion under acidification, oxidation and precipitation in the presence of ferric ions.
Similar content being viewed by others
Introduction
With development of urbanization and growth of population, huge amount of sludge is produced in municipal wastewater treatment. Microorganisms in sludge system are highly active, inorganic salts, organic matters and microorganisms in sewage sludge might break down into a variety of small molecules and volatile gas in the wastewater and sludge treatment process. These gaseous contaminants were easy to release into air environment, thus disturbing healthy and normal lives of residents around wastewater treatment plant (WWTP)1,2,3. Due to differences in each process of wastewater treatment, they differed greatly from types to concentrations of odors4,5,6.
The chemical constituents of odorous contaminants are very complex and primarily consisted of H2S, NH3 and VOCs. VOCs in WWTP are generally divided into several categories: sulfur compounds, nitrogenous compounds, halogen and its derivatives, hydrocarbons and organic compounds containing oxygen7, 8. Hydrogen sulfide and ammonia in odor are not only able to simulate smell, but also seriously corrode equipments in WWTP, consequently shortening the equipment life9. In addition, odor has a strong irritant effect to nerve, circulatory, respiratory and endocrine system of bodies10. Generally, the concentrations of H2S and NH3 in air are much higher than that of VOCs, but some of VOCs are believed to be more hazardous than inorganic gaseous pollutants. VOCs would stimulate the body deformities, cancer and genetic mutations, which are the potential and long-term harm to human11. It’s well known that odorous pollution from WWTP is one of the seven typical urban pollutions12. Odour emissions are considered to be the main cause of disturbance noticed by the citizens living near some facilities. Wang et al.13 noted that sludge treatment unit is a most important source of odor pollution in whole the WWTP.
It was reported that EPS played an important role in sludge dewatering process14,15,16. In biological wastewater treatment, EPS are produced by the microorganisms in aerobic and anaerobic sludge when organic materials present in wastewater are consumed. EPS are comprised of protein, polysaccharide, humic acid and nucleic acid, in which proteins and polysaccharides are the majority17. EPS are considered as the key constituents affecting physicochemical and biological properties, and they are also responsible for sustaining the structural and functional integrity of aggregates. EPS accounts for 60–80% of the mass of waste activated sludge, they play important roles in the removal of pollutants from wastewater, bioflocculation, settling and dewatering of activated sludge18,19,20. According to EPS distribution outside the cells, EPS can be divided into soluble EPS (SEPS) and bound EPS (BEPS)21,22,23. BEPS has rheological double layers including the loosely bound EPS (LB-EPS) and the tightly bound EPS (TB-EPS). LB-EPS plays a decisive role for properties of activated sludge24. Organic pollutants in the wastewater were generally removed by EPS sorption through hydrophobic and electrostatic interactions.
High-pressure dewatering processes have been widely used in current China to alleviate the pressure of steadily increasing sludge production. Chemical conditioning is an indispensable step in sludge treatment for dewatering performance enhancement. Organic flocculants were always used in low-pressure dewatering process. Inorganic coagulants were commonly applied as conditioners in high-pressure dewatering process, since their hydrolysis products were to enhance sludge floc strength and reduce sludge compressibility. It was well known that EPS was highly hydrophilic, and traditional chemical flocculants were ineffective to remove the bound water trapped in sludge. Therefore, advanced treatment processes were developed to destroy sludge EPS fractions and convert the bound water within sludge flocs into free water25, 26.
Unlike organic flocculants, addition of inorganic conditioners might greatly affect sludge properties and solution chemistry conditions, subsequently resulting in emissions of odorous pollutants. Additionally, hydrophobic organic substances such as VOCs absorbed in sludge was very likely to release with EPS degradation under advanced treatment processes. However, few of studies have examined the transfer behavior of different odorous pollutants under chemical conditioning processes. In this work, the emissions characteristics and mechanisms of odorous contaminants under typical sludge conditioning processes were investigated in details. Especially, various VOCs were identified and quantified based on GC-MS. Finally, the changes in physicochemical properties of sludge system were analyzed to understand their relationships with odorous pollutants.
Results and Discussion
Effects of chemical conditioning with different methods on the characteristics of sludge
Effects of different chemical conditioning processes on sludge dewatering performance
According to the value of specific resistance to filtration (SRF), sludge can be classified into the sludge with bad dewaterability (>E + 12 – E + 13 m · kg−1), medium dewaterability ((5–9) E + 11 m · kg−1) and good dewaterability (<4 E + 11 m · kg−1)27. As described in Fig. 1(a), SRF of raw sludge was 37.21 E + 12 m/kg. Fenton treatment performed better in sludge dewatering improvement than other methods. As can be seen from Fig. 1(b), the moisture content of sludge cake (MC) with typical chemical reagents was as follows: FeCl3 + CaO > acidification > PAC > Fenton treatment. It was reported that addition of inorganic coagulants could eliminate the negative surface charge of the sludge particles by charge neutralization and interparticle bridging, resulting in particle destabilization and aggregation, they were not able to break EPS and reduce bound water in sludge flocs14. However, Fenton treatment was more effective in converting bound water into free water by destructing sludge EPS with OH · oxidation, and consequently improving solid content of sludge cake.
Influence of chemical conditioning on EPS properties
EPS are considered to be one of key constituents and affect physicochemical and biological properties of activated sludge system. They are also mainly responsible for sustaining the structural and functional integrity of the aggregates. EPS accounted for 60–80% of the mass of waste activated sludge, they play important roles in the removal of pollutants from wastewater, bioflocculation, settling and dewatering of activated sludge20, 21, 28. Figure 2 showed concentrations of SEPS were increased after treatment with different chemical conditioners. The concentrations of SEPS were 575.80 mg DOC/L, 277.40 mg DOC/L, 276.20 mg DOC/L, and 67.28 mg DOC/L under chemical conditioning with Fenton, acidification, FeCl3 + CaO and PAC respectively. As mentioned above, sludge particles were always negatively charged due to the ionization of anionic functional groups, such as carboxyl, amino and phosphate groups and so on. The presence of negative charge on the surface of particles could produce electronic repulsion and keep stability of a colloidal system. Inorganic coagulants could result in destabilization and aggregation of sludge particles by charge neutralization and bridging, and SEPS transferred from sludge bulk into solid phase through complex adsorption action of their hydrolyzed products15.
For Fenton treatment, ferrous could react with hydrogen peroxide under acidic conditions to generate highly reactive hydroxyl radicals which were able to quickly, efficiently solubilze and degrade organic matters in sludge system. Neyens et al.29 reported that Fenton reagent showed favourable cell-lysing effects and EPS dissolution, prompting water release. Acidic treatment could not only destroy EPS structure but caused protonation of anionic functional groups of EPS and aggregation of sludge system. Protein-like substances of low MC were solubilized and release into sludge bulk in acidic environment, consequently leading to increase of SEPS concentration. Additionally, under FeCl3 and lime conditioning, EPS dissolution can be attributed to the increases in proteins solubility and negative charge on the surface of sludge particles due to unprotonation of EPS at strong alkaline conditions, consequently decreasing the binding strength of EPS30.
3D-EEM spectroscopy has been used as an effective method to characterize EPS composition in wastewater treatment systems31. Each 3D-EEM spectrum is able to accurately respond the chemical compositions of EPS17, 32. Chen et al.17 had proposed a semi-quantification method using 3D-EEM base on fluorescence region integration (FRI). According to the peaks located at the excitation/emission wavelengths (Ex/Em), aromatic protein-like substances (APN), tryptophan protein-like substances (TPN), humic acids (HA) and fulvic acids (FA) were indicated respectively33. According to TOC and 3D-EEM data, the concentration of different organic substances in EPS could be obtained.
As shown in Fig. 3, the main peaks for the SEPS were located at the excitation/emission wavelengths range of 260–300/300–375 nm and 300–380/375–475 nm, corresponding to TPN and HA, respectively. It was obvious that matters were dominated by humic substances in SEPS. Zhang et al.16 reported that the dominant composition of SEPS changed with the seasons, humic substances (HS) dominated in summer and protein-like matters in winner. HA was polymer organic acid consisting of aromatic and multiple functional groups and always originated from microbial decomposition of organic matters. FA contained significant amounts of phenolic hydroxyl and carbonyl groups which would react with oxides and metal ions. From Fig. 3, the fluorescent intensities of both TPN and HA were slightly decreased under PAC conditioning, and the fluorescent intensities of both TPN and HA were obviously decreased after conditioning with Fenton treatment. It was because proteins and HA were both removed by complex adsorption with hydrolyzed products of PAC. So the concentrations of SEPS with PAC were reduced in comparison to that of raw sludge. For Fenton treatment, ferrous could react with hydrogen peroxide under acidic conditions to generate highly reactive hydroxyl radicals which were able to quickly, efficiently solubilze and degrade organic matters in sludge system. Neyens et al.29 reported that Fenton reagent showed favourable cell-lysing effects and EPS dissolution, prompting bound water release. Acidic treatment could not only destroy EPS structure but caused protonation of anionic functional groups of EPS and aggregation of sludge system. Protein-like substances of low MC were solubilized and release into sludge bulk in acidic environment, consequently leading to increase of SEPS concentration. Additionally, under FeCl3 and lime conditioning, EPS dissolution could be attributed to the increases in proteins solubility and negative charge on the surface of sludge particles due to unprotonation of EPS at strong alkaline conditions, consequently decreasing the binding strength of EPS. And there was a strong binding strength between iron ions and protein, and protein transferred from SEPS to BEPS by the coagulation effect of iron ions in high pH value30, 34.
Effects of different chemical conditioners on the odorous pollutants emissions
Inorganic gaseous pollutants
It is well known that H2S gas and NH3 gas come from biochemical reactions of inorganic salts and organic substances containing sulphur and nitrogen. Sulfate ions can be converted into H2S by sulfate-reducing bacteria (SRB) under anaerobic conditions. SRB can utilize the low molecular-weight organic matters as the carbon/energy substrates which are oxidized either completely to CO2 and/or some intermediate compounds using sulfate as a terminal electron acceptor, consequently generating sulphide (Eq. 1)35.
Moreover, there are other common microbial actions in the nitrogenous compounds (such as nitrate respiration and ammonification). Variety of bacterial and fungal use nitrate which exist in sludge and sludge liquor as the final receptor oxidized organic compounds and energy source, due to nitrate reductase (NR) is played the important role (Eq. 2). The reaction form and physiological effect are the similar to aerobic respiration. It is a far more efficient than fermentation. Following denitrification, non-nitrogen organic compounds were oxidized (Eq. 3). Many bacteria, gram-negative nonspore-bearing bacillus, can cause denitrification (such as fluorescent bacteria, stu perczel pylori). These are usually facultative aerobic bacteria, and denitrification often occurs under anaerobic condition. And some chemical autotrophic bacteria can also cause denitrification under anaerobic condition. For example, denitrifying sulfur bacteria use nitrate to oxide sulfur and reduce nitrate (Eq. 4). SRB also use nitrogen in amino acid as nitrogen source on one condition. A few SRB get nitrogen through dissimilatory reduction reaction from nitrate and nitrite. Ammonification is also called deamination. It is a degradation process of organic nitrogen compounds in the ammonification microorganisms (e.g. Bacillus, Clostridium difficile Bacillus and single spore bacteria, etc.), then NH3 release (Eq. 5). It was reported that Bacillus played a strong role on ammonification of organic nitrogen. And nitrite is also reduced to NH4 + by alienation of nitrite reduction bacterium (Eq. 6).
As described in Fig. 4(a), the amount of H2S release increased gradually with dosages of varied inorganic coagulants adding. Hydrogen sulfide in absorption solutions were determined by simplified methylene blue spectrophotometric method. It can be seen that the varied inorganic coagulants demonstrated a positive promoting role in the H2S release process. The contents of H2S released under chemical conditioning were as follows: Fenton > Acidizing > PAC > FeCl3 + CaO. As described in Fig. 4(b), the amounts of NH3 release increased gradually with dosages of inorganic coagulants. NH3 concentration in sludge liquor was determined by sodium hypochlorite-salicylic acid method. It can be seen that the contents of NH3 release were as follows: PAC > FeCl3 + CaO > Fenton > Acidizing. Moreover, NH3 emission was much lower than the H2S emission. Allen et al. concluded that emissions of NH3 during the normal operation of waste water treatment plants had not been considered as a significant source35. Battye et al.36 also reported that the sewage treatment plant was unapparent source of NH3 emissions. However, NH3 emission was significantly intensified under chemical conditioning processes.
In the Fenton conditioning, pH value was firstly adjusted to 3. It was because the conditioning effect of Fenton mainly achieved balance when the pH value was less than 4. Then Fe2+ could form FeS precipitates with sulfide (Eq. 7).
Low pH value was conducive to inhibit the formation of FeS precipitates. It prompted H2S to release from sludge into air35. Moreover, Yuan et al.37 reported that ferrous irons could significantly improve the activity of activated sludge microorganisms by accelerating electron transfer and function as enzyme activators, thus improving organic matters degrading, so NH3 and HS− were produced. Acidizing method only accelerated the forward reaction of Eq. 8, and H2S gas quickly released from sludge solution. So pH value was increased rise slightly due to emission of H2S (Figure S1). However, acidizing treatment inhibited the NH3 releasing due to formation of NH4 + through protonation, and the amount of NH3 release was lower in comparison to other chemical reagents.
Hydrolysis reaction of Al3+ formed aluminum hydroxide (Al(OH)3) deposition which was able to neutralize negative surface charge of sludge particles and promote their aggregation. As shown in the Eq. 9, S2− in solution can enhance hydrolysis reaction of Al3+, and H2S was released at the same time. Then PAC was the most commonly inorganic polymer flocculants and was characterized of hydrolysis stability. So pH value of slurry changed slightly and the concentration of H2S released was lower than acidizing and Fenton treatment. In neutral conditions, the positive reaction of Eq. 8 was weak. The concentration of H2S release was quite up to the balance (Fig. 4(a)). Besides, there was a little bit of influence by the microorganism. Al(OH)3 flocculation caused hypoxia inside of microorganism. It was because anoxic condition formed with the increasing of oxygen transfer resistance and consumption of external aerobic bacteria. So advantage microbial was nitrifying bacteria. Then NH3 emissions decreased. Burton38 had studied that the nitrogen lost to the atmosphere as ammonia was reduced under anaerobic conditions.
Fe3+ directly formed Fe2S3 precipitates with sulfide, which facilitated ionization of H+. S2− could be oxidized to S precipitate by Fe3+ (Eq. 10). Then Fe2S3 transformed into FeS. With addition of lime (CaO), pH value rised to above 10, which greatly promoted NH3 release from sludge. Liu et al.39 reported that emission of NH3 increased with increase in lime dosage.
VOCs emission
They always come from petrochemical wastewater, coking industry wastewater, pesticide wastewater, pharmaceutical wastewater, etc. Thus, VOCs was mainly composed of organic compounds containing benzene and hydrocarbons. VOCs are hydrophobic substances with low water solubility, they are inclined to adsorb on extracellular polymeric substance (EPS) through hydrophobic interactions. As described in Fig. 5, VOCs emission was evidently increased under different chemical conditioning processes. The total VOCs concentrations were 0.05 ug/m3, 36.70 ug/m3, 41.22 ug/m3, 38.24 ug/m3 and 38.30 ug/m3 for raw sludge, PAC, FeCl3 + CaO, acidizing and Fenton treatment, respectively.
As shown in the Fig. 6, there were 21 VOCs could be detected by GC-MS. Dibromochloromethane, tetrachloroethane, 1,2-dibromoethane, ethylbenzene, o-xylene, m-xylene and p-xylene were the main compounds in VOCs. And dibromochloromethane emission was the maximum under FeCl3 + CaO conditioning.
VOCs emissions were generally classified into three main compositions: benzenes, halogeno benzenes and hydrocarbons. As can be seen from Fig. 5, benzenes were 18.48 ug/m3 and 18.53 ug/m3 for Fenton and acidizing treatment, which was higher than other two conditioning processes. There was no obvious difference in the amounts of halogeno benzene released for four conditioning methods. Hydrocarbons emission in FeCl3 + CaO conditioning was higher than that under other three conditioning processes.
Mass balance of odorous pollutants
The concentrations of H2S, ammonia nitrogen (Ammonia-N) and VOCs in the condition of solid, liquor and gas were analyzed. From Fig. 7(a), the concentration of H2S in the liquor phase of raw sludge was obviously much more than it in the solid phase. The concentration of H2S in the liquor phase with PAC and FeCl3 + CaO conditioning was also more than that treated with acidizing and Fenton conditioning. Compared with raw sludge, the concentrations of H2S in the liquor phase and solid phase both drastically reduced. And the concentration of H2S in the gas phase with acidizing and Fenton treatment was higher than that conditioned with PAC and FeCl3 + CaO conditioning. As shown in Fig. 7(a), it indicated a migration process of sulfur (S) from solid to liquor, and to air lastly. In the liquor phase and solid phase, Ammonia-N was used to represent nitrogen (N) content. As described in Fig. 7(b), there was no ammonia-N detected in the solid phase. The concentration of ammonia-N in the liquor phase significantly decreased after sludge conditioning. The concentrations of NH3 emission in the gas phase were higher under PAC and FeCl3 + CaO treatment, and while NH3 concentration were higher in the liquor phase after conditioning with acidizing and Fenton. It was very likely that protonation of NH3 inhibited its release at acidic conditions. As shown in Fig. 7(c), the concentrations of VOCs in the liquor phase had no significant changes in raw sludge, PAC and Fenton conditioning. And yet the concentrations of VOCs in the solid phase greatly were reduced under FeCl3 + CaO and acidizing conditioning. It was because that VOCs was generally hydrophobic and adsorbed on slurry by EPS by hydrophobic and electrostatic interactions. Then VOCs released when EPS was destroyed. The total VOCs emission was highest under Fenton conditioning, it was minimum under acidizing treatment. It was reported that VOCs emissions were influenced by variations of temperature40, 41, oxygen42, pH43 and EPS properties33. However, pH value of sludge was significantly changed under different conditioning (see in the Figure S1). As mentioned above, pH value was one of most important factors affecting odours emission in the process of sludge treatment. The major mechanism of flocculation conditioning was EPS densification, VOCs emission was the minimum with PAC. Acidific treatment resulted in solubilization of complexes of EPS and cations29, so the concentration of SEPS increased and VOCs released at the same time. It was because the concentration of SEPS not only reflected the destruction extent of EPS with chemical reagents, but also indirectly reflected the adsorption of EPS on VOCs. Fenton treatment could effectively destroy EPS fraction through oxidation process, consequently cause emission of a large amounts of VOCs. From Fig. 7(a–c), H2S and NH3 released were mainly originated from liquor phase, while VOCs came from solid and liquid phases. Obviously, there were significant differences in total mass of odorous pollutants before and after conditioning, especially for H2S. A portion of sulfide ions were oxidized into S0/sulfate ions under Fenton treatment, while they were able to form metal sulfur precipitates with addition of ferric ions. However, the contents of element sulfur and metal sulfur precipitates in sludge particles were rather difficult to be determined, leading to discrepancy in mass balance of different odorous pollutants. In addition, Fenton treatment also might result in VOCs degraded.
Conclusions
The study investigated transfer behavior of odorous pollutants in wastewater sludge system under typical chemical conditioning processes for dewaterability enhancement. A considerable amount of research work has been carried out on the relationships between sludge dewaterability and sludge characteristics, but few of them has reported emission of odorous pollutants under chemical conditioning. This study indicated that a large amounts of odorous pollutants (H2S, NH3 and VOCs) were released a under different conditioning processes. There were significant differences in composition and concentration of odorous pollutants at various treatments. The released contents of H2S were 29.70 mg/m3, 14.05 mg/m3, 5.74 mg/m3 and 0.12 mg/m3, while that of NH3 were 1.88 mg/m3, 1.72 mg/m3, 2.75 mg/m3 and 2.40 mg/m3 under chemical conditioning with Fenton, acidification, PAC, FeCl3 + CaO, respectively. Different conditioning processes could result into VOCs emission by affecting sludge solution chemistry conditions and EPS properties. The total VOCs concentrations were 36.70 ug/m3, 41.22 ug/m3, 38.24 ug/m3 and 38.30 ug/m3 for PAC, FeCl3 + CaO, acidizing and Fenton, respectively. PAC was ineffective to solubilize sludge EPS and thus VOCs emission was the minimum among four conditioning processes. Fenton and acidific treatment could effectively destroy EPS fraction, consequently cause emission of a large amounts of VOCs. H2S and NH3 released were mainly originated from liquor phase, while VOCs came from solid and liquid phases. This work provided a scientific and technical support for odors control in the process of chemical conditioning.
Materials and Methods
Materials
Waste sludge
Sludge was obtained from sludge thickening tank in Xiaohongmen wastewater treatment plant, Beijing, China. It treats approximately 600,000 m3 of wastewater daily, and is treated by Anaerobic–Anoxic–Oxic (A/A/O). Samples were stored at 4 °C and were analyzed within 7 days after sampling. The characteristics of the sludge are listed in Table 1. As shown in the Table 1, the raw sludge was bad dewaterability because the SRF value of the raw sludge was 37.21 E + 12 m · kg−1. The moisture content of sludge was 98.69%. The value of pH was 7.01, and zeta poteitial of sludge was −15.07 mV. From the testing of TOC analyzer, the SEPS of the raw sludge was 67.29 mg DOC/L.
Chemical agents
The reagents used in this study were of analytical grade and purchased from Sinopharm Group Chemical Reagent Co., Ltd, such as FeCl3, CaO, H2SO4, H2O2 and FeSO4. PAC was produced by a local factory (Beijing global water science and technology Co. Ltd, China).
Jar test
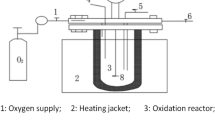
Jar tests were conducted on a programmable jar test equipment (Daiyuan Jar Test Instruments, China). Sludge samples were reacted with coagulants by using magnetic stirrer, and tester was started at rapid mixing of 200 rpm, different chemical conditioners were quickly added. Meanwhile, inorganic gaseous pollutants (H2S and NH3) and VOCs were gathered by solution absorption in the bubble absorption tube and activated carbon tube respectively. Figure 8 showed the experimental device of sludge conditioning and odorous contaminants collection. Note that the dosages of different chemical reagents used in this work were base on our previous studies.
In addition, sludge sample was settled down at 100 g for 15 min, and the supernatant (bulk solution) was collected as SEPS. Cellulose acetate (CA) membranes with a pore size of 0.45 µm were used to remove the particulates present in the sludge supernatant. The filtered fractions were used for analyzing fluorescence EEM and dissolved organic carbon (DOC).
Analytical methods
Odorous pollutants analysis
H2S was measured by methylene blue spectrophotometric method (GB/T 11742–1989). NH3 was determined according to sodium hypochlorite-salicylic acid method (GB/T 14679–1993). GC-MS (6890GC/5973MSD, U.S.A, Agilent Co.) is used for VOCs analysis.
SRF
SRF was measured with the standard Buchner funnel test using a quantitative filter paper. It can be obtained by Eq. (11):
Where P (kg m−2) denotes pressure, A (m2) is filtration area, µ (kg s m−2) is kinetic viscosity, w (kg m−3) denotes dry solid weight per unit volume sludge on the filtrate media, b is slope of filtration equation t/V = bV + a, and t (s) is time, V (m3) denotes volume of filtrate. The raw or conditioned waste sludge was poured into a Buchner funnel with a 0.45 µm filter paper to filter under a pressure of 0.6 MPa with vacuum pump. Weight of filtrate was recorded every 10 s before surface cracking was observed. The equipment was shown as Figure S2.
Soluble EPS analysis
The sample was diluted with Milli Q water until concentration of DOC was below 10 mg/L. The peak locations, peak intensities and the ratios of different peaks in EEM spectra of the EPS samples were not substantially influenced by ionic strength25. Three dimensional excitation emission matrix (3-DEEM) spectra were measured by a Hitachi F-4500 fluorescence spectrophotometer with an excitation range from 200 to 400 nm at 10 nm sampling intervals and an emission range from 220 to 550 nm at 10 nm sampling interval. The spectra were recorded at a scan rate of 12,000 nm/min, using excitation and emission slit bandwidths of 10 nm. Each scan had 37 emission and 27 excitation wavelengths.
Other analytic methods
The dissolve organic carbon (DOC) of SEPS was analyzed using TOC analyzer (Shimadzu, Kyoto, Japan). pH was measured by a pHS-3C (Shanghai, China) pH meter, which was calibrated using pH 4.01, pH 7.01 and pH 9.18 buffers. Zeta potential of sludge was analyzed using zeta potential and nano/submicron particle size Analyzer (ZetaPALS, Malvern, UK).
References
Smet, E., Van Langenhove, H. & De Bo, I. The emission of volatile compounds during the aerobic and the combined anaerobic/aerobic composting of biowaste. Atmospheric Environment 33, 1295–1303 (1999).
Kazlauskas, D. et al. Researches of H2S generation from municipal landfills and systematical evaluation of landfills pollution. Vilniaus Gedimino technikos universitetas 2005.
Giuliani, S., Zarra, T., Naddeo, V. & Belgiorno, V. A novel tool for odor emission assessment in wastewater treatment plant. Desalination and Water Treatment 55, 712–717 (2015).
Karageorgos, P. et al. Treatment of unpleasant odors in municipal wastewater treatment plants. Water Science and Technology 61, 2635–2644 (2010).
Mills, B. Review of methods of odour control. Filtration & Separation 32, 147–146 (1995).
Liu, H., Zhang, P. & Yu, C. Air pollution treatment technology of Municipal Sewage Treatment Plant. Environmental Engineering 27, 75–78 (2009).
Bertoni, D., Mazzali, P. & Vignali, A. Analisi e controlo degli odori, Pitagora 1993.
Rafson, H. J. Odor and VOC Control Handbook. McGraw-Hill, New York 1998.
Deng, E. The stench of the Present Situation and Prospect of wastewater treatment plant. Industrial safety and environmental protection 34, 45–46 (2008).
Heaney, C. D. et al. Relation between malodor, ambient hydrogen sulfide, and health in a community bordering a landfill. Environmental research 111, 847–852 (2011).
Shen, P., Chen, Z. & Zhang, D. Odor evaluation and analysis. Beijing, Chemical Industry Press, 25–40 (2005).
Han, Y., Liang, L. & Guo, G. The stench of Governance. Petrochemical safety and environmental protection technology 25, 43–46 (2009).
Wang, S., Qin, F. & Yu, Y. Deodorization design for wastewater treatment plant. Water & Wastewater Engineering 31, 20–23 (2005).
Niu, M. et al. Correlation of physicochemical properties and sludge dewaterability under chemical conditioning using inorganic coagulants. Bioresource technology 144, 337–343 (2013).
Zhang, W. et al. Understanding the impact of chemical conditioning with inorganic polymer flocculants on soluble extracellular polymeric substances in relation to the sludge dewaterability. Separation and Purification Technology 132, 430–437 (2014).
Zhang, W. et al. Understanding the evolution of stratified extracellular polymeric substances in full-scale activated sludges in relation to dewaterability. RSC Advances 5, 1282–1294 (2015).
Chen, W., Westerhoff, P., Leenheer, J. A. & Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environmental science & technology 37, 5701–5710 (2003).
Liu, X., Sheng, G. & Yu, H. Physicochemical characteristics of microbial granules. Biotechnology advances 27, 61061–1070 (2009).
Adav, S. S., Lee, D. J. & Tay, J. H. Extracellular polymeric substances and structural stability of aerobic granule. Water Research 42, 1644–1650 (2008).
Liu, Y. & Fang, H. H. Influences of extracellular polymeric substances (EPS) on flocculation, settling, and dewatering of activated sludge, 237–273 (2003).
Sheng, G., Yu, H. & Li, X. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnology advances 28, 882–894 (2010).
Murthy, S. N. & Novak, J. T. Effects of potassium ion on sludge settling dewatering and effluent properities. Water Science and Technology 379, 317–324 (1998).
Rosenberger, S. & Kraume, M. Filterability of activated sludge in membrane bioreactors. Desalination 146, 373–379 (2002).
Guan, W., Xiao, P., Zhou, X. & Ding, C. Sludge extracellular Polymeric substances (EPS) advances. Chemical Engineer 23, 35–39 (2009).
Sheng, G. P. & Yu, H. Q. Characterization of extracellular polymeric substances of aerobic and anaerobic sludge using three-dimensional excitation and emission matrix fluorescence spectroscopy. Water Research 40, 1233–1239 (2006).
Qi, Y., Thapa, K. B. & Hoadley, A. F. Application of filtration aids for improving sludge dewatering properties–a review. Chemical Engineering Journal 171, 373–384 (2011).
Barker, D. J. & Stuckey, D. C. A review of soluble microbial products (SMP) in wastewater treatment systems. Water research 33, 3063–3082 (1997).
Mao, I. et al. Critical components of odors in evaluating the performance of food waste composting plants. Science of the Total Environment 370, 323–329 (2006).
Neyens, E., Baeyens, J. & Dewil, R. Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering. Journal of hazardous materials 106, 83–92 (2004).
Zhang, W. et al. Variations in distribution and composition of extracellular polymeric substances (EPS) of biological sludge under potassium ferrate conditioning: Effects of pH and ferrate dosage. Biochemical Engineering Journal 106, 37–47 (2016).
Ouyang, E. M., Wang, W., Long, N. & Li, H. Three-dimensional excitation emission matrix fluorescence spectroscopic characterization of loosely bound and tightly bound extracellular polymeric substances of sludge. Spectroscopy and Spectral Analysis 29, 1313–1318 (2009).
Hudson, N. et al. Can fluorescence spectrometry be used as a surrogate for the biochemical oxygen demand (BOD) test in water quality assessment? An example from South West England. Science of the Total Environment 391, 149–158 (2008).
Gao, H., Zhang, W. & Song, Z. H2S emission in sludge conditioning with different inorganic salt coagulants and its relationships with sludge properties. RSC Advances 6, 83060–83068 (2016).
Yu, G., He, P., Shao, L. & He, P. Stratification structure of sludge flocs with implications to dewaterability. Environmental science & technology 42, 7944–7949 (2008).
Allen, A. G., Harrison, R. M. & Wake, M. T. A meso-scale study of the behaviour of atmospheric ammonia and ammonium. Atmospheric Environment (1967) 22, 1347–1353 (1988).
Battye, R., Battye, W., Overcash, C. & Fudge, S. Development and selection of ammonia emission factors. EPA contract 68-D3, 0034 (1994).
Yuan, L. & Bi, X. Effect of ferric salt on microbial DHA and ETS activity in activated sludge. Environmental Engineering 6, 97–99 (2010).
Burton, C. H. A review of the strategies in the aerobic treatment of pig slurry: purpose, theory and method. Journal of Agricultural Engineering Research 53, 249–272 (1992).
Liu, W. et al. Characteristics of ammonia emission during thermal drying of lime sludge for co-combustion in cement kilns. Environmental technology 36, 226–236 (2015).
Wilber, C. & Murray, C. Odor source evaluation. Biocycle 31, 68–72 (1990).
Williams, T. O. Odors and VOC emissions control method. Biocycle 36, 49–56 (1995).
Fukumoto, Y., Osada, T., Hanajima, D. & Haga, K. Patterns and quantities of NH3, N2O and CH4 emissions during swine manure composting without forced aeration—effect of compost pile scale. Bioresource Technology 89, 109–114 (2003).
Atchley, S. H. & Clark, J. B. Variability of temperature, pH, and moisture in an aerobic composting process. Applied and environmental microbiology 38, 1040–1044 (1979).
Acknowledgements
This study was financially supported by National Natural Science Foundation of China (Nos 21277130, 51478445, 51338010 and 51678546), National Natural Science Foundation of Hubei province in China (ZRMS2016000811) Chinese Universities Scientific Fund (CUG160824) and China Postdoctoral Science Foundation (2016M590733).
Author information
Authors and Affiliations
Contributions
Hongyu Gao designed and supervised the experiments; Zhenzhen Song, Lian Yang and Mengdi Cao performed some of the experiments; Xiaofang Yang prepared Figures S1 and S2; Dongsheng Wang and Guiying Liao provided the equipment analysis methods and critically revised the article for important intellectual content; Hongyu Gao and Weijun Zhang wrote the manuscript text.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, H., Zhang, W., Song, Z. et al. Transfer behavior of odorous pollutants in wastewater sludge system under typical chemical conditioning processes for dewaterability enhancement. Sci Rep 7, 3417 (2017). https://doi.org/10.1038/s41598-017-03727-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03727-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.