Abstract
The evolution of family living is underpinned by conflict and cooperation between family members. While family groups can be maintained by reducing conflict between parents and offspring, interactions between siblings may play an equally important role. Here, we compared the level of aggressive interactions between siblings to that between parents and their offspring in the family living skink Liopholis whitii. Aggressive interactions occurred much more frequently between siblings and between fathers and offspring than between mothers and their offspring. These results suggest that ecological and social conditions that reduce conflict between siblings and between males and offspring will be fundamental in the evolutionary maintenance and diversification of family living in these lizards.
Similar content being viewed by others
Introduction
The evolution of family living is mediated by reduction in within-group conflict. Research in this context has largely focused on parent-offspring conflict and specifically the factors that affect the costs and benefits of prolonged parental investment for both parents and offspring1, 2. However, conflict between siblings may be just as important, or more so, than conflict between parents and offspring for mediating family life3,4,5. Indeed, conditions that reduce conflict or facilitate cooperation between siblings can stabilise family life, allowing the emergence of larger family groups6. Conversely, conditions that increase conflict between siblings can result in the dissolution of family living7, 8. Yet, the relative roles of parent-offspring vs sibling-sibling conflict for the initial origins of family life are not well understood.
Here we compare the levels of parent-offspring and sibling-sibling aggression (as a proxy for conflict) in the social skink Liopholis whitii. Liopholis whitii live in small family groups characterised by a long-term adult pair bond and prolonged associations between parents and offspring9, 10. These prolonged associations involve semi-independent offspring delaying dispersal and parents tolerating offspring within their core home ranges, sometimes for up to several years9, 10. The prolonged association with parents provides several benefits to offspring, including access to resources and protection from infanticidal conspecifics11. Importantly, these parent-offspring associations are facultative and there is considerable variation in their strength; from no association between parents and offspring to associations between parents and multiple cohorts of offspring9, 10. Furthermore, while there are no costs of associating with offspring for parents11, offspring appear to pay a considerable cost of associating with their siblings via increased competition for food and shelter (G. While unpublished data, see also ref. 7). This suggests that 1) aggression between siblings should be high compared to parent-offspring aggression, and 2) that variation in the extent of sibling conflict may explain variation in social complexity in this species. Here we report results from a test of the first of the prediction that aggression between siblings is higher than between parents and offspring.
Methods
Collection and housing of the study animals
Liopholis whitii is a medium sized (75–100 mm snout-vent length (SVL)) viviparous skink that occurs throughout south-eastern Australia12, 13. We captured 103 adult lizards (95 females and 8 males) for three separate experiments in 2014, 2015 and 2016. For each of these experiments, lizards were caught from populations on the east coast of Tasmania, Australia (approximately 42°57′S, 157° 88′E). In all cases, lizards were captured in the field and transported in cool, damp cloth bags back to the University of Tasmania. At the University, lizards were weighed (±1 mg), measured for SVL and total length (±0.5 mm) and sexed via hemipene eversion. Each lizard was uniquely toe-clipped to enable individual identification. No individual lizard was sampled for interaction data more than once (either within or between years). Lizards were then housed individually in plastic terraria (30 × 60 × 40 cm) and kept under a 25 W basking light set to an 8:16 hour cycle with overhead lights set on a 10:14 hour light/dark cycle (to replicate light conditions on the east coast of Tasmania, where the lizards were caught). Each terrarium had a basking rock underneath the basking light, with a wooden shelter at the opposite end. Lizards were provided with water daily and food three times a week (Tenebrio larvae and fruit puree mixed with protein powder).
At the end of gestation (mid-Jan), female containers were checked twice daily for the birth of offspring. Upon birth, the date of birth, weight (±1 mg), SVL and total length (±0.5 mm) of offspring were recorded. Offspring were then toe clipped for permanent identification. A coloured ‘bee tag’ (Pender Beekeeping Supplies, Cardiff, Australia) was attached at a point on the dorsal side of offspring using non-toxic glue to allow for instant identification of individuals from video footage (see below). We could not sex the offspring at birth since female offspring retain their hemipenes until sexual maturity and males and females are otherwise morphologically identical. Thus, all data collection on offspring aggression below was collected independent of information on offspring sex.
Interaction trials: collection of video footage
We filmed mothers and their offspring in their terrariums for a 2-hour period on the day following the completion of the birth of all of a mother’s offspring (determined via abdominal palpation of the female). All interaction trials started at 0900 hrs (following the 1 hr acclimation to the camera being placed above the terrarium and turned on) and filming was conducted using a GoPro Hero4 camera (California, U.S.A). In the 2016 experiment, we also collected video footage of father-offspring interactions for a limited number of families. Father-offspring interaction trials were run the day after the mother and offspring had been filmed together. Specifically, we removed the mother from the home terrarium on the evening prior to filming the father-offspring trials and replaced her with the father (adult males and females were otherwise always housed separately). Filming then began on the following day at 0900 hrs, i.e., 24 hrs after the mother-offspring interaction trial. Overall, each parent was only used once to provide a measure of either mother or father-offspring aggression, and each offspring provided a measure of sibling aggression and was present for both estimates of parent-offspring aggression. The non-independence arising from using the same litter to provide a measure of both sibling and parent-offspring aggression was accounted for statistically (detailed below). After these trials, animals were either released back into their natural population or into semi-natural enclosures as part of on-going projects.
Scoring interaction data from video footage
For all videos, we scored interaction data from the second hour of footage (following the 1 hr of acclimation). Specifically, we recorded the number of times individuals bit and chased one another as our measures of aggressive behaviour (see Supplementary Videos 1 and 2 for footage of both behaviours). As these two variables were highly correlated (Spearman’s rank correlation = 0.79, p < 0.01), we chose to select one variable for our analysis of differences between parent-offspring and sibling aggression. We decided to analyse the number of chases per hour as our measure of aggression over number of bites per hour. This choice was made for two reasons. First, bites are harder to accurately record than chases. Bites occur quickly as a single motion whereas a chase is composed of a series of small events; specifically, an individual switching from being still or crawling to running directly at a second individual who flees in response; this is distinct from any other type of interaction we have observed in L. whitii and therefore it is virtually impossible to record a false positive for chasing. Second, and more importantly, we believe chasing is more representative of aggressive behaviour for this species. For example, while a bite may not illicit any further indication of aggression, for a chase to occur, it is necessary for a given individual to actively switch from one behavior (such as basking or feeding) to instead direct energy into the task of aggressing another individual. Further, chases are typically relentless, with a single chase event ending only once the victim hides underneath a shelter. Regardless, when running analyses with frequency of bites as our conflict measure, our results and interpretations were identical.
Across the three experiments we recorded aggressive interactions between mothers and offspring from 95 one hour videos, between siblings from 89 one hour videos (the sample size for sibling interactions was lower than that for mother-offspring interactions as some mothers gave birth to only one offspring), and between fathers and offspring for 8 one hour videos. Thus, our sample sizes for each component of the study are, n = 95 for mother-offspring interactions, n = 89 for sibling-sibling interactions, and n = 8 for father-offspring interactions. For the father-offspring videos we only used data from the father-offspring interactions, as data on sibling interactions had already been collected as part of the mother-offspring recording. However, there were no differences in the levels of sibling aggression when siblings were with their mother vs. their father (z ratio = 0.67, p = 0.91) suggesting no effects of order or sex of the parent present on the levels of sibling aggression.
Ethics statement
All experimental protocols were carried out in accordance with the Australian code of practice for the care and use of animals for scientific purposes as approved by the University of Tasmania Animal Ethics Committee (project numbers: A15058, A14380 and A14602).
Data analysis
Differences in levels of sibling and parent-offspring aggression were modelled using a Generalized Linear Mixed Model (GLMM) fit by maximum likelihood. This was run in R version 3.3.0 (R development core team 2016) using the ‘glmmADMB’ package14 with the negative binomial family specified to account for overdispersion. Family interaction (sibling-sibling, mother-offspring, or father-offspring) was entered as a fixed effect, clutch size was entered as a covariate, and experiment year (2014, 2015, or 2016) and litter identity were included as non-nested random effects. Litter identity was included to control for non-independence arising from having a litter provide measurements of both sibling and parent-offspring conflict. Post-hoc comparisons were undertaken using the Tukey’s HSD method for p-value adjustment (implemented through the ‘lsmeans’ package15). We did not sample males in the 2014 and 2015 experiments and for the 2016 experiment there were extremely low levels of mixed paternity (only two litters16). Therefore, we unfortunately could not conduct any formal tests of the role that relatedness may play in mediating conflict (see discussion).
Results and Discussion
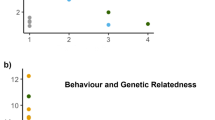
Family dyads differed significantly in the extent of aggressive behaviour (Wald’s χ2 (2) = 24.33, p < 0.01). Chasing occurred between siblings in 57.3% of interaction trials, fathers chased offspring in 75% of trials whereas mothers only chased offspring in 3.2% of trials. Post hoc analyses revealed that both sibling-sibling and father-offspring aggression were significantly higher than mother-offspring aggression (z ratio = 8.06, p < 0.01 and z ratio = 4.41, p < 0.01 respectively; Fig. 1) but did not differ significantly from each other (z ratio = −0.64, p = 0.79; Fig. 1).
These results suggest that sibling aggression is a key component of Liopholis whitii family life. This is consistent with other family living species, particularly birds and mammals, where high levels of aggression between siblings characterises the post birth/hatching environment (reviewed in ref. 17). Importantly, these interactions can play an important role in mediating the composition of the family groups via effects on offspring growth and survival17. Unlike many birds and mammals, Liopholis whitii offspring are semi-independent at birth, so the most likely consequence of sibling aggression is that it influences the extent to which individuals disperse out of the natal home range as well as the potential identity of dispersers. Such sibling aggression could therefore be a key mechanism determining the considerable variation in family structure observed in L. whitii populations9, 10. For example, high levels of sibling aggression could explain why only one offspring usually remains within the parental home range despite average litter sizes of 2–3, as observed in some populations of L. whitii 9. In contrast, low levels of sibling conflict may facilitate the emergence of larger family groups that consist of several offspring remaining within the parental home range or family groups in which multiple cohorts of offspring remain, as has also been observed in a number of L. whitii populations9, 10, as well as a number of other Egernia species18, 19.
Sibling aggression is unlikely to be the sole mediator of family dynamics. Indeed, we found that aggressive interactions between fathers and their offspring occurred as frequently as between siblings. Male tolerance of offspring within their natal home range is therefore also likely to be an important mediator of family dynamics within this system. However, the small sample sizes for male-offspring tolerance suggests that this result should be interpreted with caution. In contrast, we found little evidence of mothers exhibiting aggression towards their offspring. Specifically, we found evidence of mothers being aggressive towards their offspring in only 3 of the 95 trials. This may be because Liopholis whitii do not exhibit post-hatching provisioning, in contrast to many birds and mammals, and thus the cost of tolerating offspring for females is low11. Nevertheless, mothers may still shape sibling conflict because birth of offspring is often spread out across several days (analogous to hatching asynchrony), which influences the outcome of aggressive interactions between siblings20. Hatching asynchrony is important in this respect in birds17 and deserves further attention in Egernia lizards.
These results suggest the maintenance of family living in White’s skink, and perhaps the evolutionary diversity of social organisation in the Egernia lizards more broadly, will be dictated by factors that mediate conflicts between siblings and between fathers and offspring. Such conflicts will be influenced primarily by patterns of relatedness between individuals and the costs and benefits of tolerating other individuals21. Within-group relatedness will largely be dictated by patterns of female polyandry whereas the costs and benefits of tolerating other individuals will be a function of the environment, in particular resource availability and predation risk. Both of these factors are likely to be important in L. whitii. First, we have previously shown considerable levels of polyandry (~30%) within natural populations of L.whitii and that this influences the composition of the family group by promoting enhanced dispersal of extra-pair offspring9. Second, experimental manipulations of resource availability have been shown to mediate offspring dispersal and the level and nature of parent-offspring associations11, 22, 23. While the behavioural mechanisms mediating these facultative responses have not been directly studied, our results here indicate that social interactions with kin are involved. Studies that manipulate levels of female polyandry and examine the consequences for within group conflict could tease apart the causal relationship between polyandry, within group conflict and the evolutionary diversification of social complexity as inferred by recent, large-scale, comparative analyses1, 6.
In summary, our results suggest that stable family living in this and other species of lizard rely on reducing conflict between siblings and between fathers and offspring. Since these conflicts depend on both female polyandry and ecological opportunity, studies that manipulate these factors will generate insights into the processes responsible for the maintenance and diversification of family living both within and between lizard species.
References
Griffin, A. S., Alonzo, S. H. & Cornwallis, C. K. Why do cuckolded males provide paternal care? Plos Biol. 11, 10.1371/journal.pbio.1001520 (2013).
Covas, R. & Griesser, M. Life history and the evolution of family living in birds. Proc. R. Soc. Lond. B 274, 1349–1357, doi:10.1098/rspb.2007.0117 (2007).
Falk, J., Wong, J. W. Y., Kolliker, M. & Meunier, J. Sibling cooperation in earwig families provides insights into the early evolution of social life. Am. Nat. 183, 547–557, doi:10.1086/675364 (2014).
Ruch, J., Herberstein, M. E. & Schneider, J. M. Offspring dynamics affect food provisioning, growth and mortality in a brood-caring spider. Proc. R. Soc. Lond. B. 281, 10.1098/rspb.2013.2180 (2014).
Ruch, J., Herberstein, M. E. & Schneider, J. M. Families hunt more successfully: effect of group composition on hunting and communal feeding. Anim. Behav. 91, 171–178, doi:10.1016/j.anbehav.2014.03.013 (2014).
Cornwallis, C. K., West, S. A., Davis, K. E. & Griffin, A. S. Promiscuity and the evolutionary transition to complex societies. Nature 466, 969–U991, doi:10.1038/nature09335 (2010).
West, S. A., Pen, I. & Griffin, A. S. Cooperation and competition between relatives. Science 296, 72–75, doi:10.1126/science.1065507 (2002).
Platt, T. G. & Bever, J. D. Kin competition and the evolution of cooperation. Trends Ecol. Evol. 24, 370–377, doi:10.1016/j.tree.2009.02.009 (2009).
While, G. M., Uller, T. & Wapstra, E. Within-population variation in social strategies characterize the social and mating system of an Australian lizard, Egernia whitii. Aust. Ecol 34, 938–949, doi:10.1111/j.1442-9993.2009.02002.x (2009).
Chapple, D. G. & Keogh, J. S. Group structure and stability in social aggregations of white’s skink. Egernia whitii. Ethology 112, 247–257, doi:10.1111/j.1439-0310.2006.01153.x (2006).
Botterill-James, T. et al. Habitat structure influences parent-offspring association in a social lizard. Front. Ecol. and Evol. 4, 10.3389/fevo.2016.00096 (2016).
Wilson, S. & Swan, G. Complete guide to reptiles of Australia. 4th edn, (New Holland, 2013).
Chapple, D. G. Ecology, life-history, and behavior in the Australian Scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetol. Monogr. 17, 145–180 (2003).
Fournier, D. A. et al. AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Method. Softw. 27, 233–249 (2012).
Lenth, R. V. Least-squares means: the R Package lsmeans. J. Stat. Softw. 69, 1–33, doi:10.18637/jss.v069.i01 (2016).
Botterill-James, T. et al. Experimental manipulation suggests effect of polyandry but not mate familiarity on within-pair aggression in the social skink. Liopholis whitii. Behav. Ecol. Sociobiol. 71, 71, doi:10.1007/s00265-017-2302-8 (2017).
Mock, D. W. & Parker, G. A. The Evolution of Sibling Rivalry. (Oxford University Press, 1997).
Gardner, M. G. et al. Group living in squamate reptiles: a review of evidence for stable aggregations. Biol. Rev. 91, 925–936, doi:10.1111/brv.12201 (2016)
Whiting, M. J. & While, G. M. Sociality in Lizards. In Rubenstein, D. R. & Abbot, P. (eds), Comparative Social Evolution. Pp 390–426, (Cambridge University Press) (2017).
While, G. M. & Wapstra, E. Are there benefits to being born asynchronously: an experimental test in a social lizard. Behav. Ecol. 19, 208–216, doi:10.1093/beheco/arm124 (2008).
Hamilton, W. D. The genetical evolution of social behaviour. I. J. Theo. Biol. 7, 1–16, doi:10.1016/0022-5193(64)90038-4 (1964).
Halliwell, B. et al. Habitat saturation promotes delayed dispersal in a social reptile. Behav. Ecol. 28, 515–522, doi:10.1093/beheco/arw181 (2017).
Halliwell, B., Uller, T., Wapstra, E. & While, G. M. Resource distribution mediates social and mating behavior in a family living lizard. Behav. Ecol. 28, 145–153, doi:10.1093/beheco/arw134 (2017).
Acknowledgements
The work was funded by the Australian Research Council (DP150102900 awarded to G.M.W. and T.U.) and Holsworth Wildlife Research Grants (to T.B.J. and B.H.). T.B.J. and B.H. were supported by University of Tasmania Postgraduate Scholarships, T.U. was supported by the Royal Society and the Knut and Alice Wallenberg Foundations, G.M.W. is supported by the Australian Research Council (DE150100336).
Author information
Authors and Affiliations
Contributions
All authors conceived of and designed the study. T.B.J., B.H., J.S. and S.M. caught field animals and recorded video footage. T.B.J. recorded interaction data from the video footage and conducted statistical analyses with input from G.W. and B.H., T.B.J., G.W. and T.U. drafted the manuscript with input from all other authors. All authors gave approval for publication and agree to be held accountable for the content therein.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Botterill-James, T., Halliwell, B., McKeown, S. et al. Family aggression in a social lizard. Sci Rep 7, 3502 (2017). https://doi.org/10.1038/s41598-017-03531-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03531-0
This article is cited by
-
Competitive asymmetries, birthing asynchrony and sibling rivalry in a social lizard
Behavioral Ecology and Sociobiology (2024)
-
Low food availability during gestation enhances offspring post-natal growth, but reduces survival, in a viviparous lizard
Oecologia (2019)
-
Developmental asynchrony and antagonism of sex determination pathways in a lizard with temperature-induced sex reversal
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.