Abstract
HAART is very effective in suppressing HIV-1 replication in patients. However, patients staying on long-term HAART still develop various HIV-associated neurological disorders, even when the viral load is low. The underlying pathogenic mechanisms are largely unknown. Emerging evidence implicated that persistent neuroinflammation plays an important role in NeuroAIDS. Although residual virus or viral proteins are commonly thought as the causal factors, we are interested in the alternative possibility that HAART critically contributes to the neuroinflammation in the central nervous system (CNS). To test this hypothesis, we have determined the effect of NRTIs on the expression of proinflammatory cytokines in the various CNS regions. Mice (C57Bl/6) were administered with AZT (Zidovudine 100 mg/kg/day), 3TC (Lamivudine 50 mg/kg/day) or D4T (Stavudine 10 mg/kg/day) for 5 days, and cortices, hippocampi and spinal cords were collected for immunoblotting. Our results showed that NRTI administration up-regulated cytokines, including IL-1β, TNF-α and IL-6 in various CNS regions. In addition, we found that NRTIs also up-regulated Wnt5a protein. Importantly, BOX5 attenuated NRTI-induced cytokine up-regulation. These results together suggest that NRTIs up-regulate proinflammatory cytokines via a Wnt5a signaling-dependent mechanism. Our findings may help understand the potential pathogenic mechanisms of HAART-associated NeuroAIDS and design effective adjuvants.
Similar content being viewed by others
Introduction
Human immunodeficiency virus-1 (HIV-1) was identified as the etiologic pathogen for acquired immunodeficiency syndrome (AIDS) over three decades ago1. About 35 million people have died of HIV-1 infection, and there are around 36 million people living with HIV2. Although there is still no cure for HIV-1 infection, the highly active antiretroviral therapy (HAART, a.k.a. combined antiretroviral therapy, cART) has been proved to be a very effective therapy for inhibiting the viral replication, significantly decrease HIV-associated mortality and morbidities, and become the standard treatment for HIV patients3.
Despite its efficiency in suppressing HIV-1 viral load to a very low level, long-term HAART is associated with various detrimental effects. Among the critical HAART side-effects are the damages in the nervous system4, 5. Convergent evidence suggests that the prevalence of HIV-associated neurological disorders (HAND) in HIV patients on HAART remains high6, 7. HAND in post-HAART era significantly affect the quality of life of HIV patients and may directly contribute to them on-adherence to treatment. However, the potential mechanism by which HAART contributes to HAND is still poorly understood, and interventions are not available.
Neurotoxicity is a suggested mechanism by which HAART could contribute to HAND. Progressive neuron loss was reported in HIV patients on HAART8. Antiretroviral drugs also led to neuronal damage and death in animal models9. Neurotoxicity appears to associate with major types of antiretroviral drugs in HAART, including nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitor (NNRTI) and protease inhibitors (PI)10,11,12,13.NRTIs are the backbone in current HAART, and ample evidence indicates NRTI-associated neurotoxicity in both peripheral nervous system (PNS) and CNS14,15,16,17, is probably contributed by their mitochondrial toxicity18,19,20.
Chronic neuroinflammation is implicated in various neurological diseases, including HAND8, 21,22,23. A consistent finding in the postmortem biopsies of HIV patients is neuroinflammation, as indicated by the presence of activated microglia and up-regulated pro-inflammatory cytokines24.HIV infection and toxic viral proteins such as gp120 and Tat are commonly thought as the cause of neuroinflammation in HIV patients. Indeed, the activity of gp120 and Tat in inducing neuroinflammation has been demonstrated in cultured glial cells25,26,27 and animal models28,29,30,31. However, the potential contribution of HAART drugs to the manifestation of persistent neuroinflammation has not been conclusively tested. Because HIV patients usually stay on long-term HAART, this question is clinically relevant.
In this study, we test the hypothesis that long-term administration of NRTIs to mice induces neuroinflammation. We measured the expression level of IL-1β, TNF-α and IL-6 in different CNS regions from mice that were administered with AZT (Zidovudine 100 mg/kg/day), 3TC (Lamivudine 50 mg/kg/day) or D4T (Stavudine 10 mg/kg/day) for 5 days by western blotting. Our results showed that NRTIs up-regulated the cytokines in CNS, and that Wnt5a signaling played a critical role in NRTIs-induced cytokine up-regulation.
Result
NRTIs up-regulate the expression of inflammatory cytokines in the CNS
Persistent neuroinflammation is considered to contribute to the development of HAND32,33,34. As HAART is the currently common treatment to suppress HIV replication in patients, we wanted to determine the potential effect of NRTIs, the essential components in HAART, on neuroinflammation in the CNS. Mice (C57Bl/6, males, 6–8 weeks) were subcutaneously injected with AZT (100 mg/kg/day), 3TC (50 mg/kg/day) or D4T (10 mg/kg/day) for 2, 5, 10, or 14 days and CNS tissues including cortices, hippocampi and spinal cords, were collected at the end of NRTIs treatment. Western blotting analysis was performed to determine the expression levels of IL-1β, TNF-α and IL-6. Preliminary experiments indicated an increase of the cytokines was already evident at day 5 after NRTIs administration. Thus, we focused our analysis on this time point to save animals.
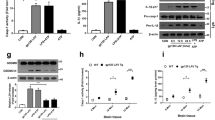
As shown in Fig. 1, IL-6, TNF-α and IL-1β in cerebral cortices, hippocampi and spinal cords were significantly increased after NRTIs treatment (Fig. 1). Among the NRTIs,3TC (50 mg/kg/day) showed the most evident effect on cerebral cytokines (IL-1β: 2.18 folds, p < 0.01; TNF-α: 3.22 folds, p < 0.01;IL-6: 2.56 folds, p < 0.01) (Fig. 1a). AZT (100 mg/kg/day), 3TC (50 mg/kg/day) and D4T (10 mg/kg/day) also caused different magnitudes of cytokine up-regulation in hippocampi and spinal cords (Fig. 1b,c). These results suggest that NRTIs induce pro-inflammatory cytokines up-regulation in different regions of the CNS.
NRTIs up-regulate Wnt5a in the CNS
Wnt5a is emerging as a major inflammatory regulator35,36,37 and is up-regulated in the spinal cord of ‘pain-positive’ HIV patients on HAART38. We determined if AZT, 3TC and D4T administration (5 days) up-regulated Wnt5a protein in the cerebral cortex (Fig. 2a), the hippocampus (Fig. 2b) and the spinal cord (Fig. 2c). In the cerebral cortex, Wnt5a was significantly increased in the 3TC group (2.13 fold; p < 0.01). All three NRTIs induced significant Wnt5a increase in the hippocampus (AZT: 2.06 folds, p < 0.01; 3TC: 2.61 folds, p < 0.01; D4T: 1.88 folds, p < 0.001) and the spinal cord (AZT: 3.55 folds, p < 0.01; 3TC: 4.40 folds, p < 0.001;D4T: 3.42 folds, p < 0.01). We also performed immunohistochemistry staining of Wnt5a in the spinal cord and observed that evident increase of Wnt5a after AZT, 3TC or D4T administration (5 days) (Fig. 2d). These datas confirm that Wnt5a is up-regulated in the CNS by NRTIs.
NRTIs up-regulate Wnt5a in the CNS. Protein levels of Wnt5a in cortex (a), hippocampus (b) and spinal cords (c) treated with NRTIs for 5 days. Datas presented in graphs are means ± SEM from 5 mice per group *p < 0.05,**p < 0.01, ***p < 0.001 vs vehicle. (d) immunohistochemistry staining of Wnt5a in spinal cords from mice treated with NRTIs.
Wnt5a antagonists attenuate NRTIs-induced cytokine up-regulation in the CNS
Wnt5a was reported to regulate HIV-1 gp120-induced cytokine expression in the spinal cord39. To investigate the potential role of Wnt5a in NRTI-induced cytokines in the CNS, we determined the effect of Wnt5a antagonist BOX5. BOX5 (10 μg/day)40 was administered via the mouse nasal cavity at 3 hours after each NRTI injection. The cerebral cortex, hippocampus and spinal cord were collected for Western blotting after 5 days of drugs administration. We observed that BOX5 administration significantly attenuated NRTIs-induced up-regulation of inflammatory cytokines in the CNS regions (Fig. 3). These results suggest that Wnt5a at least partly mediated the cytokine up-regulation induced by NRTIs. Interestingly, BOX5 treatment also significantly reduced the expression of Wnt5a in the CNS (Fig. 4). This observation indicates that Wnt5a signaling plays a key role in the Wnt5a up-regulation induced by NRTIs.
Wnt5a antagonists attenuate NRTIs-induced cytokine up-regulation in the CNS. Protein levels of cytokine in cortex (a), hippocampus (b) and spinal cord (c) treated with NRTIs and BOX5 for 5 days. Datas presented in graphs are means ± SEM from 5 mice per group *p < 0.05, **p < 0.01,***p < 0.001vs vehicle.
Discussion
In this study, we have tested the hypothesis that NRTIs cause neuroinflammation in the CNS. It is well documented that NRTIs can cause neurotoxicity41, 42, especially in the peripheral nervous system11, 43, 44, but comprehensive investigation on the effect of NRTIs on CNS neuroinflammatory has not been reported. We systematically measure the expression of three cytokines, including IL-1β, TNF-α and IL-6, after the administration of three NRTIs (AZT, 3TC, D4T) in three CNS regions (cortex, hippocampus and spinal cord).Our results show that all three tested NRTIs induce cytokine up-regulation in general, despite that drug-specific effects are observed for different NRTIs. Although it is commonly thought that residual virus and/or viral protein are the causal factors for the commonly observed persistent neuroinflammation in the CNS, our findings indicate that we need to at least include the antiretroviral regimens containing NRTIs as potential critical etiological factor underlying the neuroinflammation in the CNS of HIV patients on HAART. Although some “old’ NRTIs (e.g. AZT and d4T) that are no longer recommended for the first-line treatment are included in the study, this insight may have a general clinically-relevant implication, and is consistent with the reported up-regulation of inflammatory cytokines by other HAART components such as NNRTI and PI in the CNS45,46,47.
Cytokines such as IL-1β, TNF-α and IL-6 are implicated in inflammatory responses associated with various CNS damages48,49,50,51. Thus, NRTI-induced proinflammatory cytokines revealed in this study may directly contribute to the development of neuroAIDS/HAND52,53,54,55,56. In support of this notion, Zheng et al. found that TNF-α is involved in neuropathic pain induced by 2′,3′-dideoxycytidine (ddC) in rats57. This new understanding may add significant insights into the pathogenesis of neuroAIDS from the perspective of antiretroviral therapy. Flower et al. reported an anti-inflammatory activity of NRTIs (e.g. AZT and d4T) in primary human retinal pigment epithelium cells or Raji/TK+ cells58. It will be interesting to examine if NRTIs have pro- and anti-inflammatory effects in different biological systems.
Our results further suggest a Wnt5a-mediated mechanism by which NRTIs up-regulate cytokines in the CNS. Specifically, we show that Wnt5a is also up-regulated by NRTIs (Fig. 2a,b,c), and that a specific antagonist of Wnt5a, BOX5, attenuates the NRTIs -induced cytokine up-regulation (Fig. 3).Although Wnt5a has been suggested to regulate inflammation59,60,61, including neuroinflammation62, 63, this study reports for the first time an important role of Wnt5a signaling in the regulation of NRTI-evoked CNS neuroinflammation.
Taken together, our findings suggest that HAART may contribute to neuroAIDS pathogenesis by inducing neuroinflammation in the CNS.Wnt5a signaling probably playscritical role in this pathogenic process by regulating NRTIs-induced cytokines, which could directly contribute to neuroAIDS-related neurodenegeration (Fig. 5).This mechanistic understanding suggests controlling NRTI-induced neuroinflammation as a potential strategy to reduce the risk of neuroAIDS.
Materials and Methods
Animals and tissue dissection
All animals (C57BL/6 mice, male, 6–8 weeks) were purchased from Shanghai Ling Chang Biological Technology Co., Ltd. All procedures used in the study were approved by the Zhejiang Sci-tech University Experimental Animal Ethics Committee, and all experimental procedures were carried out in accordance with relevant guidelines and regulations. Specific experimental procedures are described below.
Cerebral cortex and hippocampus
Mice were euthanized with inhaled anesthesia ether, and heads were quickly removed and put into phosphate buffer saline (PBS) precooled on ice. The skulls of completely soaked heads were opened and the brains were removed to clean dishes. Cerebral cortices and hippocampi were carefully dissected and frozen immediately inliquid nitrogen. Frozen tissues were stored in a −80 °C refrigerator.Spinal cord: The whole spines of euthanized mice were exposed by cutting along the spine skin on the back. After exposure, the spine was cut near the tail cavity. The spinal cord was flashed out with precooled PBS. Collected spinal cords were frozen in liquid nitrogen and stored at −80 °C freezer.
NRTI Drugs
NRTI drugs: zidovudine (AZT), lamivudine (3TC) and stavudine (D4T) were purchased from Sigma (Sigma-Aldrich). NRTIs were dissolved in PBS to a final concentration of AZT (8 mg/ ml), 3TC (4 mg/ml) and D4T (0.8 mg/ml) and stored at −20 °C.
Subcutaneous (SC) injection
NRTIs were administered by SC injection. First, the injection site was disinfected with 75% alcohol. Skins of the back of the mouse was gently raised for slowly injecting the drug (0.25 ml/20 g) into the subcutaneous space. After injection, the injection site was pressed gently with alcohol wipes for a moment to prevent drug leakage. Injection volume:0.25 ml/20 g/day.
Nasal drug administration
Mice were administered with BOX5 (dissolved in PBS to the concentration of 0.5 μg/μL) by nasal dripping as previously described64, at 3 hours after NRTI injection. Briefly, a mouse was placed into a fixator tube (a centrifuge tube about the size of the mouse so that the animal can easily claw in). A small hole was made in the bottom of the fixator to expose the nostrils. One person held the animal with the left hand, and gently fixed the mouse head with the right hand to properly expose the nostrils. Another person applied the drug to the nostrils with a 10 μL pipette. Left and right nostrils of mouse were separately administered with BOX5 (10 μL). Drug solution drops were applied to the nostril slowly, usually with one drop every few seconds, and the next drop was applied only after the previous one was completely absorbed. After completing drug application to one nostril, the same procedure was performed to the other nostril. The time interval was about 1 minute. The animal was released from the fixation device after the completion of the whole procedure. Injection volume:10 μL each nostril per day.
Western blotting and antibodies
Collected tissues were homogenized in cell lysis buffer (Beyotime Institute of Biotechnology), with protease inhibitors (Biotool).The homogenates were centrifugated for 10 min (12,000 g), and protein concentration in the supernatant was measured using the BCA Protein Assay Kit (BIOMIGA). Equal amounts of total protein (40–60 μg) were loaded and separated on 12% SDS-PAGE by electrophoresis (120 V, 90–120 min). Proteins on SDS-PAGE were blotted onto PVDF membranes (100 V, 90–120 min). The membranes were blocked with 5% skim milk dissolved in TBST (20mMTris-HCl,150mMNaCl,0.05%Tween-20) solution for 2 h, and incubated sequentially with primary antibodies in TBST against IL-6 (1:10000, abcamab7737), IL-1β (1:2000, R&D Systems, AF-401-NA), TNF-α (1:1000, abcamab1793), Wnt5a (1:2500, abcamab72583), α-tubulin (1:10000, Proteintech,66031-1-lg) or GAPDH (1:10000, Santa Cruz sc-25778) for 2 h.After washes with TBST3 times for 10 minutes each and the membranes were incubated with goat anti-rabbit IgG (1:30000, abcamab97051) or goat anti-mouse IgG (1:30000 abcamab97023) secondary antibody conjugated with horseradish peroxidase (HRP) in TBST, After washes with TBST3 times for 10 minutes each and the protein bands were visualized by ECL (Advansta). Tubulin and GAPDH were used as loading controls.
Immunohistochemistry
Immunohistochemistry was performed as described65. Briefly, paraffin sections were collected and deparaffinized. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide in methanol. The sections were incubated in EDTA (pH 8.0) buffer at 95–100 °C for 20 min for antigen repair. The pre-treated sections were incubated with primary antibody at room temperature (25 °C) for 0.5–1 h, followed by incubation with secondary antibody for 30 min and staining with DAB (Gene Technology). The slides were lightly counterstained with hematoxylin, rinsed with distilled water, dehydrated in ethanol and mounted.
References
Barrésinoussi, F. et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220, 868–871 (1983).
HIV/AIDS global situation and trends. http://www.who.int/gho/hiv/en (2015).
Ghosh, A. K., Osswald, H. L. & Prato, G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J. Med Chem. 59, 5172–5208, doi:10.1021/acs.jmedchem.5b01697 (2016).
James, C. W., McNelis, K. C., Matalia, M. D., Cohen, D. M. & Szabo, S. Central nervous system toxicity and amprenavir oral solution. Ann Pharmacother. 36, 174 (2002).
Vivithanaporn, P., Asahchop, E. L., Acharjee, S., Baker, G. B. & Power, C. HIV protease inhibitors disrupt astrocytic glutamate transporter function and neurobehavioral performance. AIDS. 30, 543–552, doi:10.1097/QAD.0000000000000955 (2016).
Heaton, R. K. et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 75, 2087–2096, doi:10.1212/Wnl.0b013e318200d727 (2010).
Simioni, S. et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. Aids. 24, 1243–1250, doi:10.1097/QAD.0b013e3283354a7b (2010).
Gongvatana, A. et al. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. Journal of NeuroVirology. 19, 209–218 (2013).
Akay, C. et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. Journal of Neurovirology. 20, 39–53, doi:10.1007/s13365-013-0227-1 (2014).
van Oosterhout, J. J. et al. Stavudine toxicity in adult longer-term ART patients in Blantyre, Malawi. PLoS One 7, e42029, doi:10.1371/journal.pone.0042029 (2012).
Abers, M. S., Shandera, W. X. & Kass, J. S. Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs. 28, 131–145, doi:10.1007/s40263-013-0132-4 (2014).
Xu, H. et al. Lamivudine/telbivudine-associated neuromyopathy: neurogenic damage, mitochondrial dysfunction and mitochondrial DNA depletion. J Clin Pathol. 67, 999–1005, doi:10.1136/jclinpath-2013-202069 (2014).
Pettersen, J. A. et al. Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor-mediated neurotoxicity. Ann Neurol. 59, 816–824, doi:10.1002/ana.20816 (2006).
Schindzielorz, A., Pike, I., Daniels, M., Pacelli, L. & Smaldone, L. Rates and risk factors for adverse events associated with didanosine in the expanded access program. Clin Infect Dis. 19, 1076–1083 (1994).
Cepeda, J. A. & Wilks, D. Excess peripheral neuropathy in patients treated with hydroxyurea plus didanosine and stavudine for HIV infection. AIDS. 14, 332–333 (2000).
Kelleher, T., Cross, A. & Dunkle, L. Relation of peripheral neuropathy to HIV treatment in four randomized clinical trials including didanosine. Clinical Therapeutics. 21, 1182–1192 (1999).
Blanche, S. et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 354, 1084–1089 (1999).
Moodley, A., Bhola, S., Omar, F. & Mogambery, J. Antiretroviral therapy-induced Leber’s hereditary optic neuropathy. Southern African Journal of Hiv Medicine 15, 69–71, doi:10.7196/Sajhivmed.1056 (2014).
Koczor, C. A. et al. AZT-induced mitochondrial toxicity: an epigenetic paradigm for dysregulation of gene expression through mitochondrial oxidative stress. Physiological Genomics. 47, 447–454, doi:10.1152/physiolgenomics.00045.2015 (2015).
Kohler, J. J., Hosseini, S. H. & Lewis, W. Mitochondrial DNA impairment in nucleoside reverse transcriptase inhibitor-associated cardiomyopathy. Chemical Research in Toxicology. 21, 990–996, doi:10.1021/tx8000219 (2008).
Shah, A. et al. Neurotoxicity in the Post-HAART Era: Caution for the Antiretroviral Therapeutics. Neurotox Res 30, 677–697, doi:10.1007/s12640-016-9646-0 (2016).
Zheng, W. et al. IL-10 mediated by herpes simplex virus vector reduces neuropathic pain induced by HIV gp120 combined with ddC in rats. Mol Pain. 10, 49, doi:10.1186/1744-8069-10-49 (2014).
Yuan, S. B. et al. A Wnt5a signaling pathway in the pathogenesis of HIV-1 gp120-induced pain. Pain. 156, 1311–1319, doi:10.1097/j.pain.0000000000000177 (2015).
Shi, Y., Gelman, B. B., Lisinicchia, J. G. & Tang, S. J. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J. Neurosci. 32, 10833–10840, doi:10.1523/JNEUROSCI.5628-11.2012 (2012).
Shah, A. & Kumar, A. HIV-1 gp120-mediated increases in IL-8 production in astrocytes are mediated through the NF-kappa B pathway and can be silenced by gp120-specific siRNA. Journal of Neuroinflammation 7, doi:Artn 9610.1186/1742-2094-7-96 (2010).
Shah, A. et al. HIV-1 gp120 induces expression of IL-6 through a nuclear factor-kappa B-dependent mechanism: suppression by gp120 specific small interfering RNA. PLoS One. 6, e21261, doi:10.1371/journal.pone.0021261 (2011).
Nookala, A. R. & Kumar, A. Molecular mechanisms involved in HIV-1 Tat-mediated induction of IL-6 and IL-8 in astrocytes. J. Neuroinflammation 11, 214, doi:10.1186/s12974-014-0214-3 (2014).
Jones, L. D., Jackson, J. W. & Maggirwar, S. B. Modeling HIV-1 Induced Neuroinflammation in Mice: Role of Platelets in Mediating Blood-Brain Barrier Dysfunction. PLoS One. 11, e0151702, doi:10.1371/journal.pone.0151702 (2016).
Fields, J. A. et al. HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol Dis. 86, 154–169, doi:10.1016/j.nbd.2015.11.015 (2016).
Louboutin, J. P., Reyes, B. A., Agrawal, L., Van Bockstaele, E. J. & Strayer, D. S. HIV-1 gp120-induced neuroinflammation: relationship to neuron loss and protection by rSV40-delivered antioxidant enzymes. Exp Neurol. 221, 231–245, doi:10.1016/j.expneurol.2009.11.004 (2010).
Louboutin, J. P., Agrawal, L., Reyes, B. A., Van Bockstaele, E. J. & Strayer, D. S. Oxidative Stress Is Associated with Neuroinflammation in Animal Models of HIV-1 Tat Neurotoxicity. Antioxidants (Basel). 3, 414–438, doi:10.3390/antiox3020414 (2014).
Vartak-Sharma, N., Nooka, S. & Ghorpade, A. Astrocyte elevated gene-1 (AEG-1) and the A(E)Ging HIV/AIDS-HAND. Prog Neurobiol, doi:10.1016/j.pneurobio.2016.03.006 (2016).
Gougeon, M. L. Alarmins and central nervous system inflammation in HIV-associated neurological disorders. J Intern Med, 10.1111/joim.12570 (2016).
Hong, S. & Banks, W. A. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 45, 1–12, doi:10.1016/j.bbi.2014.10.008 (2015).
Pereira, C., Schaer, D. J., Bachli, E. B., Kurrer, M. O. & Schoedon, G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arteriosclerosis Thrombosis and Vascular Biology. 28, 504–510, doi:10.1161/Atvbaha.107.157438 (2008).
Bhatt, P. M. & Malgor, R. Wnt5a: A player in the pathogenesis of atherosclerosis and other inflammatory disorders. Atherosclerosis. 237, 155–162, doi:10.1016/j.atherosclerosis.2014.08.027 (2014).
Li, B. et al. WNT5A signaling contributes to Abeta-induced neuroinflammation and neurotoxicity. PLoS One. 6, e22920, doi:10.1371/journal.pone.0022920 (2011).
Shi, Y., Shu, J., Gelman, B. B., Lisinicchia, J. G. & Tang, S. J. Wnt signaling in the pathogenesis of human HIV-associated pain syndromes. J Neuroimmune Pharmacol 8, 956–964, doi:10.1007/s11481-013-9474-4 (2013).
Li, B. et al. Wingless-type mammary tumor virus integration site family, member 5A (Wnt5a) regulates human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120)-induced expression of pro-inflammatory cytokines via the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) signaling pathways. J. Biol Chem. 288, 13610–13619, doi:10.1074/jbc.M112.381046 (2013).
Yuan, S., Shi, Y. & Tang, S. J. Wnt signaling in the pathogenesis of multiple sclerosis-associated chronic pain. J Neuroimmune Pharmacol 7, 904–913, doi:10.1007/s11481-012-9370-3 (2012).
Abers, M. S., Shandera, W. X. & Kass, J. S. Neurological and Psychiatric Adverse Effects of Antiretroviral Drugs. Cns Drugs. 28, 131–145, doi:10.1007/s40263-013-0132-4 (2014).
Warren, G. et al. Amphiphilic Cationic Nanogels as Brain-Targeted Carriers for Activated Nucleoside Reverse Transcriptase Inhibitors. Journal of Neuroimmune Pharmacology. 10, 88–101, doi:10.1007/s11481-014-9576-7 (2015).
Arenas-Pinto, A. et al. Peripheral neuropathy in HIV patients in sub-Saharan Africa failing first-line therapy and the response to second-line ART in the EARNEST trial. Journal of Neurovirology. 22, 104–113, doi:10.1007/s13365-015-0374-7 (2016).
Micheli, J. E. et al. Genetic Predictors of Nucleoside Reverse Transcriptase Inhibitor (Nrti) and Hiv Induced Sensory Peripheral Neuropathy. Clinical Pharmacology & Therapeutics. 93, S11–S12 (2013).
O’Mahony, S. M., Myint, A. M., Steinbusch, H. & Leonard, B. E. Efavirenz induces depressive-like behaviour, increased stress response and changes in the immune response in rats. Neuroimmunomodulation. 12, 293–298, doi:10.1159/000087107 (2005).
Streck, E. L. et al. Effects of the HIV treatment drugs nevirapine and efavirenz on brain creatine kinase activity. Metab Brain Dis. 23, 485–492, doi:10.1007/s11011-008-9109-2 (2008).
Zhang, X. et al. Reduction of the HIV protease inhibitor-induced ER stress and inflammatory response by raltegravir in macrophages. PLoS One. 9, e90856, doi:10.1371/journal.pone.0090856 (2014).
Vezzani, A. & Viviani, B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 96, 70–82, doi:10.1016/j.neuropharm.2014.10.027 (2015).
Montesinos, J., Alfonso-Loeches, S. & Guerri, C. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcoholism-Clinical and Experimental Research. 40, 2260–2270, doi:10.1111/acer.13208 (2016).
Abboud, A. et al. Inflammation Following Traumatic Brain Injury in Humans: Insights from Data-Driven and Mechanistic Models into Survival and Death. Frontiers in Pharmacology 7, doi:Artn 34210.3389/Fphar.2016.00342 (2016).
Peferoen, L. A. N. et al. Ageing and recurrent episodes of neuroinflammation promote progressive experimental autoimmune encephalomyelitis in Biozzi ABH mice. Immunology. 149, 146–156, doi:10.1111/imm.12644 (2016).
Gelman, B. B. Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Current Hiv/Aids Reports 12, 272–279, doi:10.1007/s11904-015-0266-8 (2015).
Soontornniyomkij, V. et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. Aids. 28, 1297–1306, doi:10.1097/Qad.0000000000000262 (2014).
Gelman, B. B. et al. Neurovirological Correlation With HIV-Associated Neurocognitive Disorders and Encephalitis in a HAART-Era Cohort. Jaids-Journal of Acquired Immune Deficiency Syndromes. 62, 487–495, doi:10.1097/QAI.0b013e31827f1bdb (2013).
Bade, A. N. et al. Manganese-Enhanced Magnetic Resonance Imaging Reflects Brain Pathology During Progressive HIV-1 Infection of Humanized Mice. Molecular Neurobiology. 53, 3286–3297, doi:10.1007/s12035-015-9258-3 (2016).
Dahal, S., Chitti, S. V. P., Nair, M. P. N. & Saxena, S. K. Interactive effects of cocaine on HIV infection: implication in HIV-associated neurocognitive disorder and neuroAIDS. Frontiers in Microbiology. 6, doi:Artn 93110.3389/Fmicb.2015.00931 (2015).
Zheng, X. X. et al. TNF alpha is involved in neuropathic pain induced by nucleoside reverse transcriptase inhibitor in rats. Brain Behavior and Immunity 25, 1668–1676, doi:10.1016/j.bbi.2011.06.010 (2011).
Fowler, B. J. et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science. 346, 1000–1003 (2014).
George, S. J. Wnt pathway - A new role in regulation of inflammation. Arteriosclerosis Thrombosis and Vascular Biology 28, 400–402, doi:10.1161/Atvbaha.107.160952 (2008).
Nakamura, K. et al. Secreted Frizzled-related Protein 5 Diminishes Cardiac Inflammation and Protects the Heart from Ischemia/Reperfusion Injury. Journal of Biological Chemistry. 291, 2566–2575, doi:10.1074/jbc.M115.693937 (2016).
Zhao, Y. et al. Up-Regulated Expression of WNT5a Increases Inflammation and Oxidative Stress via PI3K/AKT/NF-kappa B Signaling in the Granulosa Cells of PCOS Patients. Journal of Clinical Endocrinology & Metabolism. 100, 201–211, doi:10.1210/jc.2014-2419 (2015).
Halleskog, C. & Schulte, G. WNT-3A and WNT-5A counteract lipopolysaccharide-induced pro-inflammatory changes in mouse primary microglia. Journal of Neurochemistry. 125, 803–808, doi:10.1111/jnc.12250 (2013).
Li, B. et al. Wingless-type Mammary Tumor Virus Integration Site Family, Member 5A (Wnt5a) Regulates Human Immunodeficiency Virus Type 1 (HIV-1) Envelope Glycoprotein 120 (gp120)-induced Expression of Pro-Inflammatory Cytokines via the Ca2+/Calmodulin-dependent Protein Kinase II (CaMKII) and c-Jun N-terminal Kinase (JNK) Signaling Pathways. Journal of Biological Chemistry. 288, 13610–13619, doi:10.1074/jbc.M112.381046 (2013).
Wang, X. et al. Intranasal administration of Exendin-4 antagonizes Abeta31-35-induced disruption of circadian rhythm and impairment of learning and memory. Aging Clin Exp Res 28, 1259–1266, doi:10.1007/s40520-016-0548-z (2016).
Rafalo, A. et al. The level of the zinc homeostasis regulating proteins in the brain of rats subjected to olfactory bulbectomy model of depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry 72, 36–48, doi:10.1016/j.pnpbp.2016.08.009 (2017).
Acknowledgements
This work was supported by Zhejiang Provincial Natural Science Foundation (No. LY13H090015) to JS, and NIH grants R01NS095747, R01NS079166 and R01DA036165 to SJT.
Author information
Authors and Affiliations
Contributions
J.H.S., W.P.Z. and S.J.T. conceived and designed the study. T.W., M.X.G. and J.Z. performed the experiments.M.X.G., T.W., J.H.S., W.P.Z. and S.J.T. discussed and analyzed the results. T.W. and S.J.T.wrote the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, T., Zhang, J., Geng, M. et al. Nucleoside reverse transcriptase inhibitors (NRTIs) induce proinflammatory cytokines in the CNS via Wnt5a signaling. Sci Rep 7, 4117 (2017). https://doi.org/10.1038/s41598-017-03446-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03446-w
This article is cited by
-
Associations of Wnt5a expression with liver injury in chronic hepatitis B virus infection
BMC Infectious Diseases (2023)
-
Progress in Pathological and Therapeutic Research of HIV-Related Neuropathic Pain
Cellular and Molecular Neurobiology (2023)
-
Characterization of a mouse neuropathic pain model caused by the highly active antiviral therapy (HAART) Stavudine
Pharmacological Reports (2021)
-
The Role of the Spinal Wnt Signaling Pathway in HIV-Related Neuropathic Pain
Cellular and Molecular Neurobiology (2020)
-
Quercetin attenuates AZT-induced neuroinflammation in the CNS
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.