Abstract
Activated platelets promote cancer progression and metastasis. Nevertheless, the prognostic value of platelet indices in melanoma had been rarely reported. The aim of this study was to investigate the predictive significance of platelet indices in melanoma. A total of 220 consecutive patients with melanoma were retrospectively enrolled between January 2009 and December 2009. The relationship between PDW and clinicopathological characteristics were analyzed. Kaplan-Meier method and Cox regression were used to evaluate the prognostic impact of PDW. Of the 220 patients, high platelet distribution width (PDW) levels were observed in 63 (28.6%) patients. Increased PDW was associated with tumor subtype (P < 0.001). Survival curves found that patients with increased PDW had significantly shorter survival time than those with normal PDW (P < 0.001). Cox regression analysis revealed that elevated PDW was an independent prognostic factor for overall survival (hazard ratio, 2.480; 95% confidence interval [CI], 1.386–4.436, P = 0.002). In conclusion, PDW is easily available in routine blood test. Our findings indicated that PDW is an independent predictor and that it may also be a potential parameter for targeted therapy in melanoma.
Similar content being viewed by others
Introduction
Malignant melanoma is an aggressive form of cancer with an increasing incidence and mortality worldwide. Despite multiple and aggressive therapeutic interventions, some patients still recur after treatment. Therefore, it is of great importance to look for appropriate and effective prognostic markers in melanoma.
Platelets play an essential role in cancer development, progression and metastasis though their direct interaction with tumor cell1. Platelet actions trigger autocrine and paracrine activation processes that cause phenotypic changes in stromal cells which contribute to the development of cancer2. Increased platelets were associated with poor prognosis in patients with a wide spectrum of malignancies, such as pancreatic cancer, gastric cancer, colorectal cancer, endometrial cancer, and ovarian cancer3,4,5,6,7. However, platelet count is determined by the balance between the rate of production and consumption of platelets. A normal platelet count could conceal the presence of highly hypercoagulative and pro-inflammatory cancer phenotypes in the presence of efficient compensatory mechanisms8.
Mean platelet volume (MPV), the most commonly used measure of platelet size, is an index of platelet activation and is available in clinical practice9. Platelet distribution width (PDW), another platelet index, indicates variation in platelet size10. Altered MPV levels were reported in gastric cancer, ovarian cancer, lung cancer, colon cancer, and breast cancer. However, the clinical implications of PDW have not been well defined. In the current study, therefore, we aimed to evaluate the prognostic roles of MPV and PDW in patients with melanoma.
Results
Between Jan, 2009 and Dec, 2009, a total of 220 patients were enrolled in this study. Among the 220 patients, 116 (52.7%) were women and 104 (47.3%) were men, and the median age was 56.3 ± 12.4 years (range 21–86). In terms of the staging system, 36 cases were categorized as stage I and stage II, 129 as stage III and stage IV.
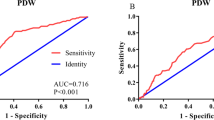
A ROC curve for OS prediction was plotted to verify the optimal cut-off value for PDW, which was 17.2 (Fig. 1). It demonstrated that PDW predicts cancer prognosis with a sensitivity of 51.1% and a specificity of 68.3% (AUC = 0.683, 95% CI: 0.618–0.744, p < 0.0001). Then, patients were divided into 2 groups: patients with PDW ≤ 17.2% and patients with PDW > 17.2%. There were 157 (71.4%) patients with PDW ≤ 17.2% and 63 (28.6%) patients with PDW > 17.2%.
The relationships between PDW and clinical characteristics were shown in Tables 1 and 2. Our study revealed that PDW was associated with tumor subtype (P < 0.001). However, no significant differences were observed between the groups with regard to age, gender, tumor location, ulceration, tumor size, lymph node metastasis, distant metastasis, and clinical stage.
With a median follow up of 60 months, 47 (21.4%) patients had death events. Patients with PDW ≤ 17.2% had a significantly better 5-year OS than patients with PDW > 17.2% (85.4% vs. 61.9%, P < 0.001). The Kaplan-Meier OS curves of the normal versus elevated PDW showed a significant separation (Fig. 2).
In univariate analysis, lymph node metastasis, PDW (categorical variable), albumin, and clinical stage were significant predictors of OS (Table 3). Age (categorical variable) (p = 0.093), lymphocytes (p = 0.071), and tumor subtype (p = 0.075) showed weak associations. Other parameters were not found to be in correlation with OS. Next, all the factors with a P value less than 0.05 in univariate analysis were included in multivariate analysis (Table 4). In multivariate analyses, we demonstrated that PDW was an independent prognostic factor in patients with melanoma. Patients with PDW > 17.2% had a hazard ratio (HR) of 2.480 [95% confidence interval (CI): 1.386–4.436, P = 0.002] for OS.
Discussion
This study showed that PDW is associated with patient’s survival and is an independent risk factor for prognosis in melanoma.
Platelets facilitate cancer progression and metastasis by inducing tumor growth, epithelial-mesenchymal transition, and invasion11. An increasingly body of evidence have identified the involvement of activated platelets in melanoma. Platelet-derived growth factor (PDGF) secreted by melanoma cell could stimulate the development of tumor stroma and new blood vessels12. Moreover, Boukerche H et al. showed platelet-melanoma cell interaction is mediated by the glycoprotein IIb-IIIa complex13. Kolber DL et al. confirmed that recombinant platelet factor 4, a known angiogenesis inhibitor, could effectively suppress tumor-induced neovascularization in mice14. In accordance with the studies above, the current study indirectly confirmed the findings using a simple platelet index. These data are also consistent with the current knowledge that anti-platelet is considered to be a part of cancer adjuvant therapy1. In addition, our study can form the basis for further mechanistic studies and ultimately aid in patient-tailored selection of therapeutic strategies.
The mechanisms to explain the association between PDW and survival are poorly understood. Bone marrow cells (including megakaryocytes) dys-function may contribute to altered PDW. PDW is a measure of platelet heterogeneity caused by heterogeneous demarcation of megakarocytes15. Recent reports demonstrated several cytokines, such as interleukin-6 (IL-6), granulocytes colony stimulating factor (G-CSF) and macrophage colony stimulating factor (M-CSF), regulate megakaryocytic maturation, platelet production and platelet size16. IL-6 promotes tumor angiogenesis, metastasis and metabolism17. Furthermore, the cytokines G-CSF and M-CSF that be secreted by tumor cells could stimulate megakaryopoiesis and subsequent thrombopoiesis in cancer18. However, the clinical value of PDW has not been studied in melanoma. Another possible mechanism is that platelets promote the hypercoagulable state in cancer19. Activated platelets create a procoagulant micro-environment that enables the tumor cells to cover themselves with platelets and evade the host immune system20.
The present study has several limitations. First, this was a single-center retrospective study and additional larger validation studies with multiethnic groups are needed to confirm our results. Second, the mechanisms underlying the involvement of PDW in melanoma remains unclear, to which further investigation should be addressed. Third, the patients were composed of Chinese. The application to other ethnic groups still needs further investigation.
In conclusion, PDW is easily available with routine blood counts. Increased PDW may serve as a marker of adverse prognosis in melanoma. Further studies are warranted to clarify the exact role of PDW in melanoma.
Patients and Methods
Study population
This study consisted of 220 consecutive melanoma cases (mean age 56.3 ± 17.4 years, range 18–86 years). Cases were admitted to the Third Affiliated Hospital, Harbin Medical University between January 2009 and December 2009. All patients undergone complete surgical resection. The pathologic diagnoses of melanoma were evaluated by pathologists from biopsy reports. None of the patients received preoperative chemotherapy or radiation therapy. Patients were excluded if they had hematological disorders, coronary artery disease, hypertension, diabetes mellitus, and medical treatment with anticoagulant, statins, and acetylic salicylic acid.
Standard demographic and clinicopathological data were collected from the patients’ records in hospital. For all the study participants, venous peripheral blood samples were collected at admission. Survival data were obtained through follow-up. Overall survival (OS) was defined as the interval from the date of diagnosis to death or last follow-up. The median follow-up time was 60 months. The platelet-to-lymphocyte ratio (PLR) was calculated as the absolute platelet count measured in ×109/L divided by the absolute lymphocyte count measured in ×109/L. The neutrophil-to-lymphocyte ratio (NLR) was calculated as the absolute neutrophil count measured in ×109/L divided by the absolute lymphocyte count measured in ×109/L.
The Institutional Ethics Review Board of the 3rd Affiliated Hospital of Harbin Medical University approved this study prior to commencement of data collection and waived the informed consent requirement because it was a retrospective study.
Statistical analysis
All statistical analyses were performed using SPSS Statistics version 22.0 (SPSS Inc., Chicago, IL, USA). The descriptive statistics are presented as means ± SD or medians (interquartile range) for continuous variables and percentages of the number for categorical variables. Inter-group differences in categorical variables were assessed for significance using the Chi-square test; differences in continuous variables were assessed using the Mann-Whitney U test or t-test. The optimal cutoff value of PDW was determined by receiver operating characteristic (ROC) curve. We used univariate analysis to narrow down the list of possible prognostic factors. Variables with P value < 0.05 in univariate analysis were brought into multivariate Cox proportional hazard model to determine their independency. Kaplan-Meier curves and log-rank test were used to compare survival differences among groups. All reported p-values are two-sided and statistical significance was assumed as p < 0.05.
References
Mezouar, S. et al. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb. Res. 139, 65–76, doi:10.1016/j.thromres.2016.01.006 (2016).
Dovizio, M., Alberti, S., Guillem-Llobat, P. & Patrignani, P. Role of platelets in inflammation and cancer: novel therapeutic strategies. Basic Clin. Pharmacol. Toxicol. 114(1), 118–127, doi:10.1111/bcpt.2013.114.issue-1 (2014).
Suzuki, K. et al. Platelets counts closely correlate with the disease-free survival interval of pancreatic cancer patients. Hepatogastroenterology 51(57), 847–853 (2004).
Long, Y, Wang, T, Gao, Q, Zhou, C. Prognostic significance of pretreatment elevated platelet count in patients with colorectal cancer: a meta-analysis. Oncotarget (2016).
Pietrzyk, L. et al. Diagnostic Power of Blood Parameters as Screening Markers in Gastric Cancer Patients. Asian Pac. J. Cancer Prev. 17(9), 4433–4437 (2016).
Ekici, H. et al. Do Leukocyte and Platelet Counts Have Benefit for \Preoperative Evaluation of Endometrial Cancer. Asian Pac. J. Cancer Prev. 16(13), 5305–5310, doi:10.7314/APJCP.2015.16.13.5305 (2015).
Qiu, J. et al. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J. Obstet. Gynaecol. Res. 38(4), 651–657, doi:10.1111/j.1447-0756.2011.01780.x (2012).
Seretis, C., Youssef, H. & Chapman, M. Hypercoagulation in colorectal cancer: what can platelet indices tell us. Platelets 26(2), 114–118, doi:10.3109/09537104.2014.894969 (2015).
Gasparyan, A. Y., Ayvazyan, L., Mikhailidis, D. P. & Kitas, G. D. Mean platelet volume: a link between thrombosis and inflammation. Curr. Pharm. Des. 17(1), 47–58, doi:10.2174/138161211795049804 (2011).
Kaito, K. et al. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br. J. Haematol. 128(5), 698–702, doi:10.1111/j.1365-2141.2004.05357.x (2005).
Meikle, C. K. et al. Cancer and Thrombosis: The Platelet Perspective. Front Cell Dev Biol 4, 147, doi:10.3389/fcell.2016.00147 (2016).
Barnhill, R. L., Xiao, M., Graves, D. & Antoniades, H. N. Expression of platelet-derived growth factor (PDGF)-A, PDGF-B and the PDGF-alpha receptor, but not the PDGF-beta receptor, in human malignant melanoma in vivo. Br. J. Dermatol. 135(6), 898–904, doi:10.1046/j.1365-2133.1996.d01-1092.x (1996).
Boukerche, H. et al. Platelet-melanoma cell interaction is mediated by the glycoprotein IIb-IIIa complex. Blood 74(2), 658–663 (1989).
Kolber, D. L., Knisely, T. L. & Maione, T. E. Inhibition of development of murine melanoma lung metastases by systemic administration of recombinant platelet factor 4. J. Natl. Cancer Inst. 87(4), 304–309, doi:10.1093/jnci/87.4.304 (1995).
Paulus, J. M. Recent advances in the story of megakaryocyte physiology. Pathol. Biol. 29(3), 133–135 (1981).
Kaushansky, K. Growth factors and hematopoietic cell fate. A new feature: controversies in hematology. Blood 92(2), 345–344 (1998).
Kumari, N., Dwarakanath, B. S., Das, A., Bhatt, A. N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. (2016).
Kowanetz, M. et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. USA 107(50), 21248–21255, doi:10.1073/pnas.1015855107 (2010).
Li, N. Platelets in cancer metastasis: To help the “villain” to do evil. Int. J. Cancer 138(9), 2078–2087, doi:10.1002/ijc.v138.9 (2016).
Franco, A. T., Corken, A. & Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 126(5), 582–588, doi:10.1182/blood-2014-08-531582 (2015).
Acknowledgements
This work was supported financially by grants from the Harbin special fund for scientific and technological innovation talents (RC2016XK004068).
Author information
Authors and Affiliations
Contributions
N.L. and Z.Y.D. participated in manuscript preparation, data analysis and editing. XY.H., and Y.N. participated in data collection and data analysis. T.M.L. and Z.P.L. participated in study design, and manuscript revision. R.T.W. and K.J.Y. participated in study design, data analysis, and manuscript preparation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, N., Diao, Z., Huang, X. et al. Increased platelet distribution width predicts poor prognosis in melanoma patients. Sci Rep 7, 2970 (2017). https://doi.org/10.1038/s41598-017-03212-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03212-y
This article is cited by
-
Prognostic value of inflammation-based indices in patients with resected hepatocellular carcinoma
BMC Cancer (2021)
-
Predictors of residual hepatic reserve and hepatic decompensation in cirrhotic patients after ablated hepatocellular carcinoma treated by DDAs or systemic therapy
Egyptian Liver Journal (2021)
-
Preoperative Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio are Associated with the Prognosis of Group 3 and Group 4 Medulloblastoma
Scientific Reports (2019)
-
Elevated preoperative platelet distribution width predicts poor prognosis in Esophageal Squamous Cell Carcinoma
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.