Abstract
Few studies have investigated the association of environmental chromium exposure and preterm birth in general population. This study was designed to investigate whether maternal chromium exposure during pregnancy is associated with reduced gestational age or risk of preterm birth using the data from Healthy Baby Cohort study conducted in Hubei, China between 2012 and 2014 (n = 7290). Chromium concentrations in maternal urine samples collected at delivery were measured with inductively coupled plasma mass spectrometry. Tertiles of chromium concentrations was negatively associated with gestational age in multivariable linear regression analyses [β (95% CI): low = reference; middle = −0.67 days (−1.14, −0.20); high = −2.30 days (−2.93, −1.67); p trend <0.01]. Logistic regression analyses also indicated that higher maternal chromium [adjusted odds ratio (OR) (95% CI): 1.55(0.99, 2.42) for the medium tertile; 1.89(1.13, 3.18) for the highest tertile; p trend <0.01] was associated with increased risk of preterm birth. The associations appeared to be more pronounced in male infants (adjusted OR (95% CI): 2.54 (1.29, 4.95) for the medium tertile; 2.92 (1.37, 6.19) for the highest tertile; p trend <0.01). Our findings suggest maternal exposure to higher chromium levels during pregnancy may potentially increase the risk of delivering preterm infants, particularly for male infants.
Similar content being viewed by others
Introduction
Preterm birth, defined as delivery before 37 weeks of completed gestation, is a leading cause of infant mortality and significant precursor to future morbidity. Every year, about 15 million babies are born prematurely—more than one in 10 of all babies born around the world1. Although medical conditions and nutritional status have greatly improved, the rate of preterm birth has not obviously declined. In the U.S., the preterm delivery rate is 12–13%; in Europe and other developed countries, reported rates are generally 5–9%2; in Africa, the rate is 11.9%3. A variety of risk factors have been linked to preterm birth including medical conditions of the mothers or fetus, genetic influences, behavioral and socioeconomic factors and iatrogenic prematurity2, and the maternal exposure to environmental contaminations has been considered as an important contributing factor.
Chromium is a transition metal that is naturally dispersed in the environment, and is primarily in two forms, trivalent (chromium 3+) and hexavalent (chromium 6+). The trivalent chromium is human required, which is directly involved in carbohydrate, fat, and protein metabolism4,5,6, while hexavalent chromium is a toxic metal and has been classified as a human carcinogen. At least 74 million people living in more than 7000 communities in the U.S. drink tap water polluted with chromium7. China is a major producer of chromium and the total atmospheric emissions of chromium have increased at annual growth rate of 8.8%8. Environmental contamination with chromium is a major threat to human health and has been increasing due to the wide range of industrial uses of chromium globally. Chromium exposure may lead to serious health problems, including oxidation–reduction, protein denaturation and abnormal enzymatic activity9, 10. The epidemiological studies reported frequent health problems in chromium exposed population such as, cancer, dermatitis, asthma, chronic bronchitis, hypertension, chromosomal aberrations, back pains, metabolic syndrome, and hemoglobin changes11,12,13.
Chromium can transfer through the placenta to developing fetuses14. In animal studies, high doses of chromium exposure during pregnancy impair embryonic development, implantation15, and leads to reduced fetal weight, retarded fetal development, skeletal defects, malformations, dead and fetus resorptions16,17,18. The current knowledge concerning the effect of chromium exposure on human health has largely been based on data from occupationally exposed people. Although some studies have demonstrated a significantly increased risk of congenital malformations, low birth weight and DNA damage with infants born to residents living near chromium contaminated areas19,20,21, there is little information on the developmental effects of early life chromium exposure in general population.
In the present study, we conducted a prospective birth cohort study to evaluate the association between maternal chromium exposure during pregnancy and the risk of preterm birth among 7290 pregnant women in Hubei province, China.
Methods
Study design and study population
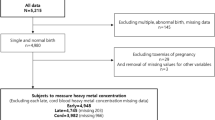
The study populations were selected from the Healthy Baby Cohort (HBC) study in China, a longitudinal birth cohort study of environmental exposures and children’s health. Pregnant women admitted to the hospital for labor have been asked to participate in the study. The eligibility criteria for participants are as follows: (1) residence in Wuhan City at the time of the recruitment period with an expectation to reside continually in this area for the foreseeable future; (2) with a single gestation and live birth; and (3) ability to comprehend the Chinese language and complete the questionnaire. Participants were invited to provide blood and urine samples and to attend face-to-face interviews. Between September 2012 and October 2014, 11,311 women were recruited at Women and Children Medical and Healthcare Center of Wuhan city in central of China. Exclusion criteria included without urine samples available for analysis (n = 3952), and those who gave birth to an infant with a birth defect (n = 57). For the women who had two times delivery in our cohort (n = 3), we chose the first delivery record. In this study, we also excluded 9 women who were smoking (n = 7) and drinking (n = 2) during pregnancy and finally chose 7290 women as our study population.
Ethics statement
The research protocol was approved by the ethics committee of the Tongji Medical College, Huazhong University of Science and Technology (No. [2012]07), and Women and Children Medical and Healthcare Center of Wuhan (No. 2012003). All participants provided written informed consent at enrollment, and all methods were performed in accordance with the relevant guidelines and regulations.
Data collection
The face-to-face interviews were conducted with the participants within three days before or after delivery by specially trained nurses in the hospital. The interviews collected a variety of information, including demographic socioeconomic characteristics (e.g., maternal age, education, occupation, household income, and self-reported weight before pregnancy) and lifestyle factors during pregnancy (e.g., smoking, passive smoking, and alcohol consumption). Information about the history of pregnancy outcomes and diseases of mothers, and information concerning the birth date, gender, gestational age at birth and birth weight of infants were retrieved from medical records. The body mass index of mothers was calculated using the self-reported weight before pregnancy and height, which was measured using a stadiometer. Preterm birth was defined as delivery before 37 completed weeks of gestation. The gestational age at delivery was calculated in completed weeks from the first day of the last menstrual period.
Urine collection and Chromium measurement
The maternal urine samples were collected during admission to the hospital as part of the preparation for delivery. Each subject provided clean and midstream spot urine in a polypropylene collection cup before delivery. The urine specimens were placed in the refrigerator immediately after collection and promptly separated into 5 mL aliquots. All of the urine samples were stored in polypropylene tubes at −20 °C until further analysis.
Prior to analysis, urine samples were thawed at room temperature, and 0.5 mL of urine was introduced in polypropylene conical centrifuge tubes. Then, 1.2% HNO3 was added to the final volume of 2.5 mL for overnight nitrification. The resulting sample was dissolved by ultrasound at 40 °C for 1 h and then analyzed for chromium by inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7900, Agilent Technologies). The operation conditions of ICP-MS were: RF power 1550 w, plasma gas flow 15.00 L/min, auxiliary gas flow 0.8 L/min, carrier gas flow 0.25 L/min, resolution (peak high 10%) 0.65~0.80 amu, improve quantity of samples 0.4 mL/min, unimodal residence time 0.1 s.
The Standard Reference Material Human Urine (SRM2670a Toxic Elements in Urine, National Institute of Standards and Technology, USA) was used as an external quality control in each batch to assess the instrument performance. The control samples were analyzed for elements after calibration and after every 20th sample, and the concentrations measured were within the certified range recommended by the manufacturer (5%). If concentrations were significantly different from the certified value of SRM2670a, the instrument was recalibrated and the previous batch of samples was reanalyzed. A 1.2% HNO3 blank was processed in each batch of samples to control for possible contamination. The limit of detection (LOD) for chromium was 0.01 µg/L. The chromium measurements were repeated three times and the average value was used for all statistical analyses. Chromium was detected in 99.2% of the urinary samples in the study. Values below the LOD (n = 62) were replaced with the LOD divided by the square root because this method is used by the CDC22 and produces reasonably nonbiased estimates23.
Urine creatinine concentrations were determined by a creatinine kit (Mindray BS-200 CREA Kit, Shenzhen Mindray Bio-medical Electronics CO., LTD). Chromium concentrations in urine (µg/L) were adjusted for creatinine in order to account for variations in urine dilution in spot urine specimens, and results were expressed as µg/g cr.
Statistical analysis
We examined the frequency distributions of maternal sociodemographic characteristics, lifestyle factors, medical and reproductive histories, and infant gender. We used χ2 tests to compare selected characteristics between preterm and term births. The distribution of creatinine-corrected, urinary chromium concentration was right-skewed by the Kolmogorov-Smirnov normality test. The Wilcoxon test was used to compare chromium concentrations between male and female infants. We used a natural log-transformation (ln-Cr) to diminish the influence of extreme values on the regression coefficients. Maternal urinary chromium was also categorized into low (≤1.09 μg/g cr), middle (1.09–3.76 μg/g cr), and high (>3.76 μg/g cr) tertiles. Multivariable linear regression was used to model the relation between continuous ln-Cr or tertile of maternal urinary chromium and each continuous gestational age (days). Then we ran logistic regression to fit the models using maternal urinary chromium concentrations as categorical variables, based on the tertile distribution of chromium concentrations in term births, and the lowest tertile was assigned as the referent group. In these models, we used preterm birth as outcome variable. Further adjustment was based on a priori selection of known risk factors for preterm birth and on results from a forward step-wise model selection procedure with inclusion in final models if they altered urinary chromium concentration effect estimates by >10%. In this study, the three variables that may represent socioeconomic status (SES) including household income (≥50,000 or <50,000 yuan per year), maternal education (more than high school, high school, less than high school) and employment during pregnancy(yes or no), were weakly correlated (Pearson correlation coefficients were r = 0.26 between education and income, and r = 0.35 between education and employment). The likelihood ratio test was used to assess model fit, and inclusion of all three SES variables (education, income, employment) into the model did not significantly improve the model fit compared to addition of each individual variable into the model separately. We selected education to adjust for SES in this study because its adjustment showed a larger impact on the ORs for the association between chromium and preterm birth than the other two SES variables.
In the final models, we adjusted for potential confounding variables including maternal age (<25, 25–29, ≥ 30 years), education (more than high school, high school, less than high school), pre-pregnancy BMI (maternal pre-pregnancy body mass index)(weight (kg)/height(m)2)(<18.5, 18.5–23, ≥ 24), parity(1 or ≥ 2), and pregnancy-induced hypertension (yes or no). Additional adjustment for passive smoking during pregnancy (yes or no), gestational diabetes mellitus (yes or no), and infant sex(male or female) did not result in material changes in the observed associations and thus were not included in the final models. Several metals that may be associated with adverse birth outcome (lead, arsenic, cadmium, vanadium and thallium) were also adjusted for in the models to control potential confounding. We also performed sensitivity analyses that excluded maternal urine samples with creatinine <0.3 g/L or >3 g/L24, and pregnancy-induced hypertension during pregnancy.
All analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 18.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were considered to be significant at an alpha level of 0.05 for a two-tailed test.
Results
Of the 7290 singleton live births, the average gestational age was 39.2 ± 1.2 weeks and 3.9% were preterm with a mean gestational age of 35.5 ± 1.2 weeks (Table 1). The mean age of all the participating mothers was 28.5 ± 3.7 years old. About half of the women were in the range from 25 to 29 years old. 67.2% of the women had more than high school educational attainment. More than half of the women had a normal pre-pregnancy BMI. More than 80% of the women were primiparous. There were 3891 male infants and 3399 female infants. Women who had preterm deliveries were more likely to be either younger than 25 years of age or older than 30 years of age, have lower educational attainment, be unemployed during pregnancy, have lower household income and be parous. Women who were diagnosed pregnancy-induced hypertension were also more likely to have preterm birth. Distributions of pre-pregnancy BMI, infant gender, passive smoking, and gestational diabetes mellitus were similar between preterm and term deliveries. The geometric mean of creatinine-adjusted chromium concentration in maternal urine was 2.26, 2.17, and 5.88 µg/g cr in all samples, term birth and preterm birth, respectively. There were no significant differences between the creatinine-adjusted chromium levels of maternal urine samples from the boys’ and girls’ mothers (p > 0.05).
Among all infants, we observed statistically significant decreases in gestational age with increasing maternal urinary chromium (both continuous ln-Cr and tertiles of urinary chromium) (Table 2). One unit increase in continuous ln-Cr approximately equals 3-fold increase in continuous chromium (μg/g cr). Thus, for about 3-fold increase in maternal urinary chromium concentrations, we observed 1.05 days decrease in gestational age in the unadjusted analysis (95%CI: −1.19, −0.91). Adjustment for potential confounders, this association approximately halved decreased [β = −0.68 day, 95%CI: (−0.88, −0.48)] and were similar for male [(β = −0.71 day, 95%CI: (−0.98, −0.44)] and female infants [(β = −0.65 day, 95%CI: (−0.95, −0.36)]. For tertiles of chromium concentrations, we observed reduced gestational age with increasing tertile of maternal urinary chromium[β (95%CI): low = reference; middle = −0.67 day (−1.14, −0.20); high = −2.30 day (−2.93, −1.67); p trend <0.01]. The inverse trend across tertiles of maternal urinary chromium and gestational age was only slightly enhanced among male infants[β (95%CI): low = reference; middle = −0.95 day (−1.60, −0.29); high = −2.50 day (−3.37, −1.62); p trend <0.01] and attenuated among the female infants [β (95% CI): low = reference; middle = −0.39 day (−1.08, −0.28); high = −2.10 day (−3.01, −1.20); p trend <0.01].
The unadjusted and adjusted ORs and 95% CIs for preterm birth according to the tertiles of chromium concentrations in maternal urine are shown in Table 3. Compared to the lowest tertile of urinary chromium concentrations, a positive significant trend was found between risk of preterm birth and levels of chromium in the adjusted analysis (OR = 1.55, 95%CI: 0.99, 2.42 for the medium tertile; OR = 1.89, 95%CI: 1.13, 3.18 for the highest tertile; p trend <0.01). When 1860 women with creatinine concentrations <0.3 g/L or >3.0 g/L were excluded, we observed the adjusted OR for preterm birth was similarly elevated (adjusted OR = 2.05, 95%CI: 1.13, 3.74 for the medium tertile; OR = 3.04, 95%CI: 1.58, 5.84 for the highest tertile; p trend <0.01). We also performed an analysis that excluded the women with pregnancy-induced hypertension, and found that the adjusted OR for preterm birth associated with chromium exposure was essentially unchanged (adjusted OR = 1.46, 95%CI: 0.91, 2.34 for the medium tertile; OR = 1.92, 95%CI: 1.12, 3.29 for the highest tertile; p trend = 0.02).
We then conducted stratified analyses by infant gender (Table 4). Among male infants, we observed increased risk with increasing tertiles of maternal urinary chromium (adjusted OR = 2.54, 95% CI: 1.29, 4.95 for the medium tertile; adjusted OR = 2.92, 95% CI: 1.37, 6.19 for the highest tertile; p trend <0.01). No clear trend in the risk of preterm birth was observed across tertiles of maternal urinary chromium among female infants (adjusted OR = 0.96, 95% CI: 0.51, 1.80 for the medium tertile; adjusted OR = 1.12, 95% CI: 0.54, 2.35 for the highest tertile; p trend = 0.72). A borderline significant interaction was observed with respect to infant sex (p for interaction = 0.06).
Discussion
In the present study, we evaluated the relationship between prenatal exposure to chromium and preterm birth in a birth cohort in Hubei China. We found an increased risk of preterm birth associated with higher maternal urinary chromium levels, and the associations appeared to be more evident in male infants. To the best of our knowledge, the present study is the first cohort study to comprehensively examine the association between maternal chromium exposure during pregnancy and the risk of preterm birth.
According to previous epidemiological studies, infants born to residents living near a hazardous waste landfill including chromium contamination have been associated with increased risk of preterm birth20. Paternal exposure to welding fumes containing chromium may increase the risk of preterm delivery25. In these studies, exposure pollutants contain a wide range of biologically active substances, not only chromium. A study from Guiyu China compared heavy metals including chromium in placenta from women lived in an electronic waste recycling area with those from a control area where no electronic waste processing occurred, and reported that there was no correlation between chromium in placenta and gestational age26. Although epidemiologic study on the association between maternal chromium exposure and the risk of preterm delivery is limited, it was confirmed in animal study that chromium increased preterm labor and decreased full-term labor27. Our findings provide evidence of a positive association between maternal chromium exposure and risk of preterm birth in general population with environmental level of chromium exposure. In addition, the adjustment for a range of characteristics, including other metals, which have been suggested to be potentially associated with adverse birth outcome in previous studies28,29,30,31,32, only slightly attenuated but did not eliminate the significant associations between maternal urinary chromium levels and gestational age, as well as the risk of preterm birth.
Chromium is a naturally occurring heavy metal that can exist in air, water, soil, and food. In the environment, chromium mainly exists in two different stable oxidation states: trivalent chromium(III) and hexavalent chromium(VI). Trivalent chromium, found in most foods and nutrient supplements, is an essential trace metal required for normal carbohydrate and lipid metabolism with very low toxicity5. But the essentiality of chromium as a nutrient is now in doubt33. Hexavalent chromium is a heavy metal and much more toxic than trivalent chromium34. The International Agency for Research on Cancer (IARC)has classified chromium(VI) as a human carcinogen through the inhalation route of exposure11. Both chromium (VI) and chromium (III) can cause adverse effects on fertility, reproduction and embryonic development18, 35,36,37. In both industrial and environmental situations chromium (III) and chromium (VI) can inter-convert, with reduction of chromium (VI) to chromium (III) generally being favored in most environmental situations38. The body has several systems for reducing chromium (VI) to chromium (III), and this chromium (VI) detoxification leads to increased levels of chromium (III)39. Chromium levels in body fluids, such as urine and serum are reliable markers of exposure to chromium in oxidation states (VI) and (III) and provide a measure of the internalized dose of chromium40. So we evaluate prenatal chromium exposure by urinary total chromium analysis.
The total chromium in biological samples is analyzed in many studies because of the biological reduction of chromium (VI) to chromium (III)41. The pregnant women in our study had higher levels of urinary chromium (median 1.01 µg/L and 1.86 μg/g cr) than those measured in pregnant women in Australia (median 0.25 µg/L and 0.44 μg/g cr)42. Our study population also had higher levels of urinary chromium than those reported in non-occupationally exposed adults from the UK (median 0.35 µg/L)43, Belgium (median 0.13 µg/L and 0.11 μg/g cr)44, Italy (median 0.10 µg/L)45, Austria (median1.08 μg/g cr)46, and the USA (median 0.12 µg/L and 0.11 μg/g cr)47. These findings suggest a regional difference in human exposure to metals. There are currently limited data on chromium exposure levels in the general population in China, and little previous study has reported the urinary chromium levels in Chinese pregnant women. The levels of urinary chromium in our study were slightly higher than those reported in a recent study of men from an infertility clinic in Hubei province48, possibly due to the high excretion of chromium as a physiological response with advancing pregnancy49. Chromium is a common pollutant introduced into natural waters mainly due to the discharge of wastewater from relative chromium industries. The emissions of chromium by the discharge of wastewater from chromium industries are highly concentrated in the provinces of the eastern, central and southern regions in China, such as Guangdong, Zhejiang, Jiangsu, Hubei and Hunan8. The issue may explain that the populations in our study and in the Zeng et al. study48 have relatively higher levels of urinary chromium than those reported in developed countries.
Although there is currently limited understanding of the potential mechanism between maternal exposure to chromium and an increased risk of preterm infants, one possible mechanism is oxidative stress caused by chromium50. Oxidative stress is described as an imbalance in the production of reactive oxygen species (ROS) and the ability of antioxidant (AOX) defenses to scavenge them51. Chromium triggers oxidative stress in the cell through increasing lipid oxidation52. Increased oxidative stress may play an important role in preterm birth53. In animal studies, chromium VI induced developmental toxicity of placenta through downregulating cell survival proteins and increasing cell death by spatiotemporal modulation of apoptotic signaling54. The imbalance between ROS and AOXs induced by chromium VI may be one of the mechanisms which potentially affect placental growth and fetal development and thus increased risk of preterm birth55. In the stratified analysis, we found that the associations may vary by infant gender in our study, as the significant association between maternal urinary chromium levels and risk of delivering preterm birth was stronger in male infants than in female. One possible explanation would be that chromium has the effect of environmental estrogen and may interfere with the production of estrogen56. And the androgen precursors involved in the production of estrogen may be increased in males57 and may facilitate preterm labour58. It is plausible that the risk of preterm birth associated with chromium exposure could differ by infant sex.
A strength of our study is the large sample size (n = 7290). In addition, interviews conducted with all participants allowed us to adjust for other potential risk factors for preterm birth, such as educational level, household income and passive smoking. Birth outcomes and maternal complications during pregnancy were obtained from medical records, which minimized potential disease misclassification.
There are some limitations to this study. First, maternal urinary chromium levels were only measured at one spot time before delivery and may not be perfect surrogates for prenatal chromium levels. However, the half-life of chromium in urine in occupationally exposed people has been reported to be 129 months59, and urinary chromium as a non-invasive biomarker, is considered to be a relatively reliable indicator to reflect chronic exposure. Second, we did not measure the placenta chromium levels, which may not directly reflect accumulation of chromium in the fetus. Third, we did not measure each form of chromium. Chromium (VI) is much more toxic than chromium (III). In the present study, whether the correlation between chromium exposure and preterm birth could be attributable to relatively high percent chromium (VI) dose still needs future studies to validate.
In conclusion, our study found a significantly positive association between maternal urinary chromium levels and the risk of preterm birth. These findings suggest that exposure to chromium in pregnant women may be an important risk factor in the etiology of preterm birth. Appropriate public health measures need to be implemented to reduce preterm birth related to developmental exposure to environmental pollutants, including chromium.
References
Howson, C., Kinney, M. & Lawn, J. Born too soon: the global action report on preterm birth. Geneva : World Health Organization (2012).
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. The Lancet 371, 75–84, doi:10.1016/S0140-6736(08)60074-4 (2008).
Beck, S. et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization 88, 31–38 (2010).
Lukaski, H. C. Chromium as a supplement. Annual review of nutrition 19, 279–302, doi:10.1146/annurev.nutr.19.1.279 (1999).
Mertz, W. Chromium in human nutrition: a review. J Nutr 123, 626–633 (1993).
Mertz, W. Chromium occurrence and function in biological systems. Physiological reviews 49, 163–239 (1969).
Sutton, R. & Group, E. W. Chromium-6 in US tap water. (Environmental Working Group, 2010).
Cheng, H., Zhou, T., Li, Q., Lu, L. & Lin, C. Anthropogenic chromium emissions in china from 1990 to 2009. PloS one 9, e87753, doi:10.1371/journal.pone.0087753 (2014).
Anderson, R. A. Essentiality of chromium in humans. Science of The Total Environment 86, 75–81, doi:10.1016/0048-9697(89)90196-4 (1989).
Moukarzel, A. A. et al. Excessive chromium intake in children receiving total parenteral nutrition. The Lancet 339, 385–388, doi:10.1016/0140-6736(92)90078-H (1992).
Cancer, I. A. f. R. o. & Cancer, I. A. f. R. o. Chromium, nickel and welding. IARC monographs on the evaluation of carcinogenic risks to humans 49 (1990).
Kornhauser, C. et al. Possible adverse effect of chromium in occupational exposure of tannery workers. Industrial Health 40, 207–213 (2002).
Mikoczy, Z. & Hagmar, L. Cancer incidence in the Swedish leather tanning industry: updated findings 1958–99. Occupational and environmental medicine 62, 461–464 (2005).
Ziaee, H., Daniel, J., Datta, A., Blunt, S. & McMinn, D. Transplacental transfer of cobalt and chromium in patients with metal-on-metal hip arthroplasty A controlled study. Journal of Bone & Joint Surgery, British Volume 89, 301–305 (2007).
Kanojia, R. K., Junaid, M. & Murthy, R. C. Embryo and fetotoxicity of hexavalent chromium: a long-term study. Toxicology letters 95, 165–172 (1998).
Junaid, M., Murthy, R. & Saxena, D. Chromium fetotoxicity in mice during late pregnancy. Vet Hum Toxicol 37, 320–323 (1995).
Trivedi, B., Saxena, D. K., Murthy, R. C. & Chandra, S. V. Embryotoxicity and fetotoxicity of orally administered hexavalent chromium in mice. Reproductive Toxicology 3, 275–278 (1989).
Marouani, N. et al. Embryotoxicity and fetotoxicity following intraperitoneal administrations of hexavalent chromium to pregnant rats. Zygote 19, 229–235 (2011).
Eizaguirre-García, D., Rodríguez-Andrés, C. & Watt, G. Congenital anomalies in Glasgow between 1982 and 1989 and chromium waste. Journal of Public Health 22, 54–58, doi:10.1093/pubmed/22.1.54 (2000).
Berry, M. & Bove, F. Birth weight reduction associated with residence near a hazardous waste landfill. Environmental health perspectives 105, 856–861 (1997).
Li, Y. et al. The hazard of chromium exposure to neonates in Guiyu of China. Science of The Total Environment 403, 99–104, doi:10.1016/j.scitotenv.2008.05.033 (2008).
Nhanes, I. Fourth national report on human exposure to environmental chemicals. Department of Health and Human Services Centers for Disease Control and Prevention. Atlanta, Georgia (2009).
Hornung, R. W. & Reed, L. D. Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene 5, 46–51 (1990).
Organization, W. H. Biological monitoring of chemical exposure in the workplace: guidelines. (1996).
Quansah, R. & Jaakkola, J. J. Paternal and maternal exposure to welding fumes and metal dusts or fumes and adverse pregnancy outcomes. Int Arch Occup Environ Health 82, 529–537 (2009).
Guo, Y. et al. Monitoring of lead, cadmium, chromium and nickel in placenta from an e-waste recycling town in China. Science of the total environment 408, 3113–3117 (2010).
Banu, S. K. et al. Gestational Exposure to Chromium (VI) Impairs Pregnancy Outcome and Fetal Development. Biology of Reproduction 83, 367–367 (2010).
Hu, X. et al. Distributions of heavy metals in maternal and cord blood and the association with infant birth weight in China. The Journal of reproductive medicine 60, 21–29 (2015).
Bell, M. L. et al. Prenatal exposure to fine particulate matter and birth weight: variations by particulate constituents and sources. Epidemiology (Cambridge, Mass.) 21, 884–891, doi:10.1097/EDE.0b013e3181f2f405 (2010).
McMichael, A. J., Vimpani, G. V., Robertson, E. F., Baghurst, P. A. & Clark, P. D. The Port Pirie cohort study: maternal blood lead and pregnancy outcome. Journal of epidemiology and community health 40, 18–25 (1986).
Al-Saleh, I., Shinwari, N., Mashhour, A. & Rabah, A. Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. International journal of hygiene and environmental health 217, 205–218, doi:10.1016/j.ijheh.2013.04.009 (2014).
Shi, X. et al. Geospatial association between adverse birth outcomes and arsenic in groundwater in New Hampshire, USA. Environmental geochemistry and health 37, 333–351, doi:10.1007/s10653-014-9651-2 (2015).
Eastmond, D. A., MacGregor, J. T. & Slesinski, R. S. Trivalent chromium: assessing the genotoxic risk of an essential trace element and widely used human and animal nutritional supplement. Critical reviews in toxicology 38, 173–190 (2008).
Barceloux, D. G. Chromium. Journal of toxicology. Clinical toxicology 37, 173–194 (1999).
Elbetieha, A. & Al-Hamood, M. H. Long-term exposure of male and female mice to trivalent and hexavalent chromium compounds: effect on fertility. Toxicology 116, 39–47, doi:10.1016/S0300-483X(96)03516-0 (1997).
Sivakumar, K. K. et al. Prenatal exposure to chromium induces early reproductive senescence by increasing germ cell apoptosis and advancing germ cell cyst breakdown in the F1 offspring. Developmental biology 388, 22–34, doi:10.1016/j.ydbio.2014.02.003 (2014).
Junaid, M., Murthy, R. C. & Saxena, D. K. In Vet Hum Toxicol Vol. 37 320-323 (1995).
Kimbrough, D. E., Cohen, Y., Winer, A. M., Creelman, L. & Mabuni, C. A Critical Assessment of Chromium in the Environment. Critical Reviews in Environmental Science and Technology 29, 1–46, doi:10.1080/10643389991259164 (1999).
Valko, M., Morris, H. & Cronin, M. T. Metals, toxicity and oxidative stress. Current medicinal chemistry 12, 1161–1208 (2005).
Kakkar, P. & Jaffery, F. N. Biological markers for metal toxicity. Environ Toxicol Pharmacol 19, 335–349, doi:10.1016/j.etap.2004.09.003 (2005).
Wilbur, S. et al. Toxicological Profile for Chromium. (2012).
Callan, A. C. et al. Maternal exposure to metals–concentrations and predictors of exposure. Environmental research 126, 111–117, doi:10.1016/j.envres.2013.07.004 (2013).
Morton, J., Tan, E., Leese, E. & Cocker, J. Determination of 61 elements in urine samples collected from a non-occupationally exposed UK adult population. Toxicology letters 231, 179–193, doi:10.1016/j.toxlet.2014.08.019 (2014).
Hoet, P., Jacquerye, C., Deumer, G., Lison, D. & Haufroid, V. Reference values and upper reference limits for 26 trace elements in the urine of adults living in Belgium. Clinical chemistry and laboratory medicine: CCLM/FESCC 51, 839–849, doi:10.1515/cclm-2012-0688 (2013).
Soleo, L. et al. [Health risk assessment of exposure to metals in the workers of the steel foundry and in the general population of Taranto (Italy)]. Giornale italiano di medicina del lavoro ed ergonomia 34, 381–391 (2012).
Zeiner, M., Ovari, M., Zaray, G. & Steffan, I. Selected urinary metal reference concentrations of the Viennese population - urinary metal reference values (Vienna). Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements (GMS) 20, 240–244, doi:10.1016/j.jtemb.2006.07.001 (2006).
Paschal, D. C. et al. Trace metals in urine of United States residents: reference range concentrations. Environmental research 76, 53–59 (1998).
Zeng, Q. et al. Urinary metal concentrations in relation to semen quality: a cross-sectional study in China. Environmental science & technology 49, 5052–5059, doi:10.1021/es5053478 (2015).
Saner, G. Urinary chromium excretion during pregnancy and its relationship with intravenous glucose loading. The American journal of clinical nutrition 34, 1676–1679 (1981).
Samuel, J. B. et al. Persistent hexavalent chromium exposure impaired the pubertal development and ovarian histoarchitecture in wistar rat offspring. Environmental Toxicology 29, 814–828, doi:10.1002/tox.21810 (2014).
Sies, H. Oxidative stress: oxidants and antioxidants. Experimental physiology 82, 291–295 (1997).
Khan, F. H., Ambreen, K., Fatima, G. & Kumar, S. Assessment of health risks with reference to oxidative stress and DNA damage in chromium exposed population. The Science of the total environment 430, 68–74, doi:10.1016/j.scitotenv.2012.04.063 (2012).
Buonocore, G. et al. Oxidative Stress in Preterm Neonates at Birth and on the Seventh Day of Life. Pediatr Res 52, 46–49 (2002).
Banu, S. K. et al. Chromium VI - Induced developmental toxicity of placenta is mediated through spatiotemporal dysregulation of cell survival and apoptotic proteins. Reproductive toxicology (Elmsford, N.Y.). doi:10.1016/j.reprotox.2016.07.006 (2016).
Banu, S. K. et al. Exposure to CrVI during Early Pregnancy Increases Oxidative Stress and Disrupts the Expression of Antioxidant Proteins in Placental Compartments. Toxicol Sci 155, 497–511, doi:10.1093/toxsci/kfw231 (2017).
Choe, S.-Y. et al. Evaluation of estrogenicity of major heavy metals. Science of The Total Environment 312, 15–21, doi:10.1016/S0048-9697(03)00190-6 (2003).
Winter, J. The ontogeny of the pituitary–gonadal axis and sexual differentiation in the human fetus. Textbook of Fetal Physiology. In: G., Thorburn, editor. New York: Oxford Medical Publications, 341 (1994).
Romero, R., Scoccia, B., Mazor, M., Wu, Y. K. & Benveniste, R. Evidence for a local change in the progesterone/estrogen ratio in human parturition at term. American journal of obstetrics and gynecology 159, 657–660 (1988).
Petersen, R., Thomsen, J. F., Jørgensen, N. K. & Mikkelsen, S. Half life of chromium in serum and urine in a former plasma cutter of stainless steel. Occupational and environmental medicine 57, 140–142 (2000).
Acknowledgements
This work was supported by the National key Research and Development Plan (2016YFC0206203, 2016YFC0206700), the National Natural Science Foundation of China (21437002, 81372959, 81402649, and 91643207), and the Fundamental Research Funds for the Central Universities, HUST (2016YXZD043).
Author information
Authors and Affiliations
Contributions
Xinyun Pan, Jie Hu, Wei Xia and Bin Zhang wrote the main manuscript text. Wenyu Liu, Chuncao Zhang, Jie Yang, and Chen Hu did the chromium measurements and prepared the Table 1. Aifen Zhou, Zhong Chen, Jiangxia Cao, and Yiming Zhang prepared the Tables 2, 3, and 4. Zheng Huang, Bin Lv, Ranran Song, Jianduan Zhang, Shunqing Xu, and Yuanyuan Li designed the study and revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, X., Hu, J., Xia, W. et al. Prenatal chromium exposure and risk of preterm birth: a cohort study in Hubei, China. Sci Rep 7, 3048 (2017). https://doi.org/10.1038/s41598-017-03106-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03106-z
This article is cited by
-
Toxic metal mixtures in private well water and increased risk for preterm birth in North Carolina
Environmental Health (2023)
-
Content of selected heavy metals in the umbilical cord blood and anthropometric data of mothers and newborns in Poland: preliminary data
Scientific Reports (2023)
-
Effect of maternal thallium exposure in early pregnancy on the risk of preterm birth
Environmental Science and Pollution Research (2022)
-
Cyclic drying and wetting tests on combined remediation of chromium-contaminated soil by calcium polysulfide, synthetic zeolite and cement
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.