Abstract
Eu-doped calcium aluminate was synthesized via the low-cost self-propagating combustion synthesis (SPCS) technique, whose phase constitutions were identified as Ca9Al6O18 and Ca3Al2O6. The Ca2+ ions in Ca9Al6O18 rather than Ca3Al2O6 phase were replaced by Eu3+ ions. The product exhibits the superior luminescent property and photocatalytic activity, which may find potential applications in the display devices and environment treatments.
Similar content being viewed by others
Introduction
In recent years, the energy conversion and environmental issues have attracted more extensive attention. Therefore, exploiting the green functional materials and utilizing non-polluting renewable energy are gradually becoming main topics. The functional materials doped with rare earth ions have been widely used in aviation, construction, electronics, optical conversion device and bio-molecular probe fields because of their advantages of low-cost, pollution-free, and nontoxic1,2,3,4,5, for instance, the rare earth permanent magnetic materials6, the rare earth superconducting materials7, the rare earth hydrogen storage material8 and so on.
Aluminates are a kind of low-cost, stable, anti-radiation and eco-friendly host of the rare earth luminescence materials9,10,11,12,13,14,15. However, one hand, the synthesis temperature of aluminates is usually very high and reaches up to 1200–1600 °C when using the traditional high-temperature calcination method by oxides (e.g. CaO and Al2O3) as starting material in the industry. On the other hand, the obtained product is also usually composed of mixed phases of multiple aluminates such as CaAl2O4, CaAl4O7, CaAl12O19, Ca3Al2O6, Ca12Al14O13 and so on, the mixed phase identification of which is hardly been discussed in the past researches. Recently, the SPCS technique was widely applied to synthesize inorganic materials, which can effectively reduce the reaction temperature and shorten the reaction time. For example, Rafiaei et al. prepared Gd and Eu ions doped Y2O3 materials, respectively, which exhibited superior luminescent emission property16, 17.
In this study, a functional material of Eu-doped calcium aluminate was synthesized by the SPCS technique, which realized the obviously fast reaction at low temperature with low energy consumption compared with the traditional high-temperature calcination method. The product presents not only the superior luminescent property but also high-efficiency photocatalytic activity, which casts a potential application in the display devices and environment treatments. The phase constitution is studied by X-ray Diffraction (XRD) pattern, Fourier Translation Infrared (FT-IR) spectrum and transmission electron microscopy (TEM) etc. approaches, as well as the luminescent and photocatalytic mechanisms are also discussed in detail.
Results and Discussion
Figure 1(a) shows the XRD pattern of the as-prepared Eu-doped calcium aluminate, which agrees well with the standard spectrum of both Ca9Al6O18 (PDF #70-0839) and Ca3Al2O6 (PDF #38-1429). No other miscellaneous diffraction peaks are observed, indicating that the as-prepared sample is completely converted to Ca3Al2O6 or/and Ca9Al6O18 crystalline phases without generating other types of calcium aluminates. In addition, these strong, narrow and sharp diffraction peaks reveal that the sample has a high degree of crystallinity. It is carefully analyzed that the angles of two strong peaks at 2θ = 33.26° and 2θ = 47.72°are slightly increased compared with the standard spectrum, which demonstrates that the doped crystal lattices are slightly contracted owing to the effect of some Eu3+ replaced Ca2+ ions, considering the ionic diameter of Ca2+ and Eu3+ are 0.099 and 0.095 nm, respectively. Besides, the metallic element contents in the sample were detected by inductive coupling plasma emission spectrograph (ICP-AES). The results show that the atom ratio of Al, Ca and Eu was 3.9: 6.6: 0.02, which was close to theoretical content in the sample.
The FT-IR spectrum of Eu-doped calcium aluminate sample is also performed. It was reported that the absorption band of AlO4 and AlO6 in condensed matter locates in the range of 900–700 cm−1 and 680–500 cm−1, respectively18. As we can see from the spectrum, the strong absorption band at 900–600 cm−1 in Fig. 1(b) can be attributed to the AlO4 vibration, which coincides with the corner-sharing tetrahedron AlO4 structure in the Ca3Al2O6 or/and Ca9Al6O18 crystals. Two groups of absorption at 600–400 cm−1 are derived from characteristic vibration of Al-O bond19. It proves that the lattice structures of Ca3Al2O6 or/and Ca9Al6O18 crystals have not changed but with slight distortion, and the Ca2+ ions rather than Al3+ are replaced by Eu3+. In addition, the absorption bands at 1500 cm−1 and 3450 cm−1 occurs due to the vibration from CO3 2− and OH− groups, respectively, because of CO2 and H2O in the air. The morphology observed in SEM, as shown in Fig. 1(c), indicates significant aggregate and the particle shape is generally irregular due to the high-temperature calcination. Fig. 1(d) confirmed the porous feature. This may be caused by the urea combustion which leading to the gases releasing and then inhibiting the formation of dense bulk samples in the SPCS process.
Figure 2 gives the TEM analysis results of the as-prepared Eu-doped calcium aluminate sample. Fig. 2(a) and (b) are bright field (BF) and high-angle annular dark field (HAADF) images, respectively, from the same aggregation. It is obvious that there are two phases with different contrast, which intersperse with each other. The selected area electron diffraction patterns (EDPs) obtained by careful tilting are shown in Fig. 2(d,e and f). These EDPs can be assigned as either Ca3Al2O6 or Ca9Al6O18 phase. It is very difficult to distinguish the isolated Ca3Al2O6 or Ca9Al6O18 phase due to their interspersion. The strongest spots in most EDPs can be assigned as both the two phases, only with the differences of the indices, as shown in the image of Fig. 2(d,e and f). Further TEM study reveals part of the aggregated particles are pure Ca9Al6O18 phase. This can be proved by the EDP, as shown in Fig. 2(g), which is from the adjacent area. The intensities of the strongest spots in Fig. 2(g), e.g. (000) and (040), are approximately the same. While in Fig. 2(e) the intensities of those spots, like (000) and (040), is not the same, and the stronger spots, like (020), are also from both the two phases as the strongest spots do. But the weaker spots are only from Ca9Al6O18 phase. The HRTEM image in Fig. 2(c), which is corresponding to the EDP in Fig. 2(f), shows the interface between Ca3Al2O6 (right side) and Ca9Al6O18 (left side) phases. The {101} planes of both the two phases are labeled by the black solid lines in the image. The increment of the lattice parameters of Ca9Al6O18 phase is clearly illustrated by these lines.
The brighter part in the BF image, while darker in HAADF image, is proved as mostly Ca3Al2O6 phase by EDP. Meanwhile, the other contrast is mostly Ca9Al6O18 phase. Normally these two phases are formed exactly in the same crystallographic orientation. So the EDPs can be assigned as both as Ca3Al2O6 and Ca9Al6O18 phases. However, the fewer cases occur that these two phases are almost, but not completely, in the same crystallographic direction. Figure 3 shows a detailed TEM analysis result for this case. Figure 3(a) is the HAADF image from one corner of the aggregated particles. The EDPs from area marked with numbers “1” and “2”, darker and brighter areas as discussed above, are shown in Fig. 3(b and c), respectively. One enlarged insert in Fig. 3(b) shows clearly two discrete spots from these two phases separately. It is obvious that Ca3Al2O6 and Ca9Al6O18 phases are not perfectly in the same crystallographic direction in area “1”. Accordingly, Ca3Al2O6 and Ca9Al6O18 phases in area “2” are exactly in the same direction as EDP shown in Fig. 3(c). The elementary maps of Ca, Al, O and Eu are shown in Fig. 3(d–g), respectively. From these elementary maps, it is obvious that the distribution of Ca, Al and O, even the rare Eu, are very homogenous. There seems to be no differences between Ca3Al2O6 and Ca9Al6O18 phases. So quantitative EDS analysis should be done carefully and profoundly.
Table 1 gives the chemical constitution (at. %) by the energy dispersive X-ray spectroscopy (EDS) analysis from those three different spots marked by numbers “1”, “2” and “3” in Fig. 3(a). It was confirmed that the relative content of Eu is higher in Ca9Al6O18 than in Ca3Al2O6 phase. The content of Eu is slightly higher in spot “2” than in “1” but with a lower Ca percentage. This verifies that the Eu3+ replaced Ca2+ in the particles. Due to the randomness of Eu3+ replacing Ca2+, it is necessary to have a large unit cell to contain these Eu3+ ions. Then there was formed as the Ca9Al6O18 phase, which the lattice parameter is doubled as that of Ca3Al2O6 phase. The replacement of Eu3+ for Ca2+ results in a different space group of Ca9Al6O18 phase, which can be proved by the occurrence of structural-extinction-spots, like (010) spot, in Figs 2(a) and 3(c). Based on this point, the structure of Ca9Al6O18 phase is preferred by the authors.
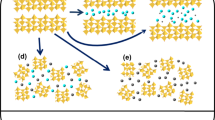
We know that the unit cell of Ca9Al6O18 contains 72 Ca, 48 A1 and 144 O atoms. The structure is built of six-fold rings centered on three-fold axes and composed of two types of distorted AlO4 tetrahedra. The holes in between the rings contain the Ca atoms. In the unit cell there are 80 such possible holes; 72 of them are filled up with Ca atoms leaving 8 vacant on threefold axes at position of (1/8, 1/8, 1/8) together with its symmetry-related positions. The extra atoms could fit into these 8 holes20. Since the sizes of Ca2+ and Eu3+ are comparable (0.099 nm and 0.095 nm, respectively), it is almost certain that there are two possible ways for Eu3+ ions to stay in the unit structure. First, Eu3+ ions could replace some of the Ca2+ ions and leaving some Ca2+ vacancies for balancing charges. Second, Eu3+ ions could go into these 8 holes of the structure. If Eu3+ ion occupies vacancies site such as (1/8, 1/8, 1/8), there should be some new vacancies of Ca2+ as near as possible in order to achieve a localized balancing of charges. The latter would probably not happen for the symmetry of the unit cell structure. So only small amount of Ca2+ ions were replaced by Eu3+ ions, which is already proved by FT-IR spectrum in Fig. 1(b). A schematic diagram is given in Fig. 4 showing the unit cell of Ca9Al6O18 phase and the replacement of parts of Ca by Eu. Figure 4(a) is the original unit cell of Ca9Al6O18 phase and Fig. 4(b) is the final result of the unit cell with parts of Ca replaced by Eu. Figure. 4(c,d) show only Ca atoms at 4a positions (Wyckoff position) for clarity as well as its partial replacement by Eu in the unit cell. This also matches the results of very little Eu doped into Ca9Al6O18 phase.
There are six types of Ca atoms in the structure of Ca9Al6O18. Two of them form distorted octahedra with O. The distortion in the octahedral arrangement of oxygen atoms and the presence of short Ca-O bonds indicates a certain amount of strain and hence there will be potential energy stored up in the structure. This potential energy might be available to assist the break-up of the structure by the action like water. So the “flash set” phenomenon of Ca3Al2O6 is attributed to its high reactivity with water20. While in our products, the Eu doped calcium aluminate (Ca3Al2O6 and Ca9Al6O18) is quite stable. The XRD pattern of the powder leaving in the room for months is almost the same as that from just-produced powder. This is also one advantage of our products. It should be noted that there are fragments on the aggregated particles, e.g. Area “3” in Fig. 3(a). The EDS result from this kind of fragment shows that it may be only calcium oxide (CaO) phase with very little Al solidified inside. There is no Eu detected by EDS in these fragments. This implies that it is not so easy for Eu3+ to substitute for Ca2+ in calcium oxide due to the strong bond between Ca2+ and O2−. While it becomes easier in more complicated structure of Ca9Al6O18 phase as discussed above.
The luminescent property of the as-prepared Eu-doped calcium aluminate sample is investigated. As the emission spectrum shown in Fig. 5 (λ ex = 277 nm), the sample emits characteristic red light. The emissions at (590 nm, 596 nm), (615 nm, 620 nm), (656 nm, 669 nm), and (689 nm, 701 nm) stem from 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3 and 5D0 → 7F4 transitions (4f → 4f), respectively. Generally, the magnetic dipole transition 5D0 → 7F1 is permitted. The electric dipole transition 5D0 → 7F2 is so sensitive to symmetry that it exceptionally allowed if Eu3+ ion occupies a non-inversion center site. Therefore, the strongest transition 5D0 → 7F2 at 620 nm indicates that Eu3+ ions locate non-inversion center sites in the calcium aluminate host. Moreover, Eu3+ ions are very sensitive to the crystal field. Owing to 4f electronic configuration interfused opposite 5d and crystal field asymmetry, f → f forbidden transition can be partly relaxed, which results in more intensive 5D0 → 7F2 transition than 5D0 → 7F1 21. In addition, Multi-state 7FJ of Eu3+ is split into multiple stark energy levels under the crystal field effect, so considerable splitting results appear in four groups of emission peaks of the 5D0 → 7FJ. The excitation spectrum of the sample by monitoring 5D0 → 7F2 transition is also carried out (Fig. 5). The peaks at 320–400 nm ascribe to the intra-configurational 4f → 4f transition of Eu3+ ions, in which the strong absorption at 387 nm belongs to 7F0 → 5L6 transition. Additionally, the strong absorption at 277 nm drives from the calcium aluminate host.
In order to investigate the photocatalytic activity of the as-prepared Eu-doped calcium aluminate sample, the UV-vis DRS of the sample is firstly performed to explore the light absorption property. As shown in Fig. 6(a), the as-prepared sample exhibits two absorption bands, which are in accord with the excitation spectrum in Fig. 5. Therefore, two absorption bands at 200–320 nm and 320–450 nm stem from transition from valence band (VB) to conduction band (CB) of the calcium aluminate host and 4f → 4f transition of Eu3+ ions, respectively. The methylene blue (MB) dye is used for target molecules to evaluate the degradation ability of the as-prepared sample. Figure 6(b) shows the dynamic curve of MB degradation. After running 15 min, the degradation rate of MB is more than 98%. Moreover, the kinetic curve of MB degradation can be approximated as a pseudo-first-order process22,23,24,25.
By plotting the ln(c 0/c) versus time and making linear fitting for kinetic curves (Fig. 6(c)), the removal rate constant k of MB is estimated to be 0.217 min−1. Furthermore, from the absorbance variations of MB solution in the photocatalytic reaction process (Fig. 6(d)), it has no shifting of the maximum absorption wavelength position of MB solution at 663 nm, and the absorption peak at 292 nm in ultraviolet region vanishes besides visible region. It implies that the benzene/heterocyclic rings of MB molecule may be completely decomposed, leading to the thorough mineralization of MB22,23,24,25. In order to assess the reusability of the sample which is crucial for its practical application, the circle runs experiments of MB solution photodegradation over Eu-doped calcium aluminate sample were performed. As shown in Fig. 7, the results indicated the photocatalytic ability of sample had not obviously loss after four recycles, indicating that the sample exhibits superior stability and durability.
The possible transfer behavior of charge carriers as well as luminescent and photocatalytic mechanism are shown in Fig. 8. Under the light excitation, the calcium aluminate host and Eu3+ ions are all excited at the same time. Electrons in the CB of calcium aluminate host and the ground state 7F0 of Eu3+ ions transfer into the corresponding CB and 5D0, 5D1, 5D2 and 5L6 states of them, respectively. At the luminescent process, electrons in the excitation state 5D1 and 5D2 return to 7F1, 7F2, 7F3, 7F4 states of Eu3+ ions to generate luminescence. It should be pointed out that part of the electron in the CB of the calcium aluminate host and 5L6 states can transfer into 5D0, 5D1, 5D2 states by means of multi-phonon assisted relaxation effect to enhance luminescent property. At the MB degradation process, part of the electrons in the CB of the calcium aluminate host migrate to the sample surface and are captured by O2 molecules in water to yield superoxide radicals (•O2 −). The superoxide radicals may further transform into hydroxyl radicals (•OH). Finally, the superoxide radicals, hydroxyl radicals and holes oxidize decompose MB dye.
Conclusion
A functional material of Eu-doped calcium aluminate was firstly obtained by the self-propagating combustion synthesis technique with low cost and low energy comsuption, which presents a significant decrease in the synthesis temperature compared with the solid-state calcination method using oxides (e.g. CaO and Al2O3) as starting material. The product is composed of two phases, i.e. Ca3Al2O6 and Ca9Al6O18, as well as exhibits the superior luminescent property and high-efficiency photocatalytic activity, which may have the potential application prospect for serving as phosphor in the display device and photocatalyst in the environment treatment, respectively.
Methods
According to the stoichiometric ratio of Ca2.98Al2O6: 0.02Eu3+, Eu2O3 was transferred to 250 mL beaker and dissolved via concentrated HNO3, then adding Al(NO3)3•9H2O, Ca(NO3)2•4H2O, CO(NH2)2 and appropriate distilled water. We keep on stirring, dissolving and heating until the solution was evaporated to be viscous. Subsequently, the beaker was put into a muffle furnace and kept at 500 °C. After a few minutes, the mixture started burning and last for 5–7 min. Finally, the obtained precursor was transferred into the corundum crucible and calcined at 900 °C for an additional 6 h to obtain the final white products.
X-ray diffraction (XRD) pattern was recorded by Rigaku D/max-2200 powder diffractometer. FT-IR spectrum was measured on FT-IR360 infrared spectrometer. The morphology was characterized utilizing scanning electron microscope (SEM, FEI Quanta 200FEG) and transmission electron microscope (TEM, FEI Tecnai G2 F30) with high angle annular dark field (HAADF) detector. Luminescent spectrum was measured via F4500 fluorescence spectrophotometer. The UV-visible diffuse reflectance spectrum (UV-vis DRS) was recorded on UV-vis spectrophotometer (PG, TU-1901).
The methylene blue (MB) solution (10 mg/L, 100 ml) containing 0.1 g sample was irradiated with a 300 W Xe arc lamp. Before the irradiation, it was stirred for 30 min in the dark environment to achieve the adsorption-desorption equilibrium between MB and sample. The absorbance of MB solution was monitored by UV-vis spectrophotometer (PG, TU-1901) every 5 min.
References
Palilla, F. C., Levine, A. K. & Tomkus, M. R. Fluorescent Properties of Alkaline Earth Aluminates of the Type MAl2O4 Activated by Divalent Europium. Journal of The Electrochemical Society 115, 642–644, doi:10.1149/1.2411379 (1968).
Han, S., Wang, Y., Zeng, W. & Chen, W. An outlook of rare-earth activated persistent luminescence mechanisms. Journal of Rare Earths 34, 245–250, doi:10.1016/S1002-0721(16)60021-2 (2016).
Gil, L. A., Campoy, J. C. P., Plaza, E. J. R. & de Souza, M. V. Conventional and anisotropic magnetic entropy change in HoAl2 ferromagnetic compound. Journal of Magnetism and Magnetic Materials 409, 45–49, doi:10.1016/j.jmmm.2016.02.085 (2016).
Estudillo-Wong, L. A., Luo, Y., Díaz-Real, J. A. & Alonso-Vante, N. Enhanced oxygen reduction reaction stability on platinum nanoparticles photo-deposited onto oxide-carbon composites. Applied Catalysis B: Environmental 187, 291–300, doi:10.1016/j.apcatb.2016.01.030 (2016).
Gomes, M. A., Lima, Á. S., Eguiluz, K. I. B. & Salazar-Banda, G. R. Wet chemical synthesis of rare earth-doped barium titanate nanoparticles. Journal of Materials Science 51, 4709–4727, doi:10.1007/s10853-016-9789-7 (2016).
Sprecher, B. et al. Life Cycle Inventory of the Production of Rare Earths and the Subsequent Production of NdFeB Rare Earth Permanent Magnets. Environmental Science & Technology 48, 3951–3958, doi:10.1021/es404596q (2014).
Shi, Y. et al. Factors Affecting the Growth of Multiseeded Superconducting Single Grains. Crystal Growth & Design 16, 5110–5117, doi:10.1021/acs.cgd.6b00685 (2016).
Weidenthaler, C. et al. Complex Rare-Earth Aluminum Hydrides: Mechanochemical Preparation, Crystal Structure and Potential for Hydrogen Storage. Journal of the American Chemical Society 131, 16735–16743, doi:10.1021/ja9042565 (2009).
Li, J., Li, J.-G., Li, X. & Sun, X. Tb3+/Eu3+ codoping of Lu3+-stabilized Gd3Al5O12 for tunable photoluminescence via efficient energy transfer. Journal of Alloys and Compounds 670, 161–169, doi:10.1016/j.jallcom.2016.02.013 (2016).
Liang, C.-J. & Siao, H.-Y. Calcining Temperatures of Sr1-3xEuxDy2xAl2O4 (x = 0−0.12) Phosphors Prepared Using the Potassium Carbonate Coprecipitation method. Journal of Alloys and Compounds 669, 38–45, doi:10.1016/j.jallcom.2016.01.227 (2016).
Sajan, S. J., Gopakumar, N., Anjana, P. S. & Madhukumar, K. Synthesis, characterization and mechanoluminescence of europium doped ZnxBa(1−x)Al2O4 (x = 0, 0.4, 0.5, 0.6, 0.8, 1.0) phosphor. Journal of Luminescence 174, 11–16, doi:10.1016/j.jlumin.2016.01.024 (2016).
Li, S., Yu, L., Sun, J. & Man, X. In-air self-reduction synthesis and photoluminescent properties of Eu2+–Eu3+ activated CaAl2SixO2x+4 phosphors. Ceramics International 42, 7968–7973, doi:10.1016/j.ceramint.2016.01.194 (2016).
Mendhe, M. S., Puppalwar, S. P. & Dhoble, S. J. Tunable luminescence properties and energy transfer in LaAl11O18:Eu,Tb phosphor. Luminescence 31, 881–887, doi:10.1002/bio.3046 (2016).
Sahu, I. P., Bisen, D. P., Brahme, N. & Tamrakar, R. K. Generation of White Light from Dysprosium-Doped Strontium Aluminate Phosphor by a Solid-State Reaction Method. Journal of Electronic Materials 45, 2222–2232, doi:10.1007/s11664-015-4284-5 (2016).
Lee, M.-H. & Jung, W.-S. Synthesis and luminescence spectra of two europium(II) aluminates, EuAl2O4 and EuAl12O19. Ceramics International 42, 3113–3120, doi:10.1016/j.ceramint.2015.10.100 (2016).
Seyed Mahdi, R., Aejung, K. & Mohammadreza, S. Enhanced Luminescence Properties of Combustion Synthesized Y2O3:Gd Nanostructure. Current Nanoscience 12, 244–249, doi:10.2174/1573413711666151026220101 (2016).
Rafiaei, S. M., Kim, A. & Shokouhimehr, M. Effect of Solvent on Nanostructure and Luminescence Properties of Combustion Synthesized Eu3+ Doped Yttria. Nanoscience and Nanotechnology Letters 6, 692–696, doi:10.1166/nnl.2014.1818 (2014).
An, L. J., Zhang, M. L., Liu, W. B. & Jiang, H. X. Sol-gel Synthesis of BaAl12O19: Mn Luminescent Material. Chemistry and Adhesion 2004, 270–272 (2004).
Tas, A. C. Chemical Preparation of the Binary Compounds in the Calcia-Alumina System by Self-Propagating Combustion Synthesis. Journal of the American Ceramic Society 81, 2853–2863, doi:10.1111/j.1151-2916.1998.tb02706.x (1998).
Mondal, P. & Jeffery, J. W. The crystal structure of tricalcium aluminate, Ca3Al2O6. Acta Crystallographica Section B 31, 689–697, doi:10.1107/S0567740875003639 (1975).
Sankara, R. & Subba Rao, G. V. Eu3+ Luminescence, Ce4+→Eu3+ Energy Transfer, and White‐Red Light Generation in Sr2CeO4. Journal of The Electrochemical Society 147, 2773–2779, doi:10.1149/1.1393605 (2000).
Dong, H. et al. Stability, durability and regeneration ability of a novel Ag-based photocatalyst, Ag2Nb4O11. Chemical Communications 50, 6596–6599, doi:10.1039/C4CC01183J (2014).
Dong, H. et al. Highly-effective photocatalytic properties and interfacial transfer efficiencies of charge carriers for the novel Ag2CO3/AgX heterojunctions achieved by surface modification. Dalton Transactions 43, 7282–7289, doi:10.1039/C4DT00058G (2014).
Dong, H. et al. A novel high-efficiency visible-light sensitive Ag2CO3 photocatalyst with universal photodegradation performances: Simple synthesis, reaction mechanism and first-principles study. Applied Catalysis B: Environmental 134–135, 46–54, doi:10.1016/j.apcatb.2012.12.041 (2013).
Dong, H. et al. An advanced Ag-based photocatalyst Ag2Ta4O11 with outstanding activity, durability and universality for removing organic dyes. Physical Chemistry Chemical Physics 16, 23915–23921, doi:10.1039/C4CP03494E (2014).
Acknowledgements
This work was supported by the National Natural Science Foundation of China with grant No. 51572054, 51621091. J.C. Rao thanks Dr. H.J. Dong and Dr. C.M. Li at Jiangsu University for revising the manuscript.
Author information
Authors and Affiliations
Contributions
J.C. Rao designed and leaded the research work; Y.C. Li, Y. Zhao, Z.L. Diao conducted the experiments; and J.C. Rao wrote the paper. Y.J. Wang, W. Wang, H. Ke, D.C. Jia and Y. Zhou joined the discussion of the experimental results and made some comments on the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rao, J., Wang, Y., Wang, W. et al. Mechanism of superior luminescent and high-efficiency photocatalytic properties of Eu-doped calcium aluminate by low-cost self-propagating combustion synthesis technique. Sci Rep 7, 2906 (2017). https://doi.org/10.1038/s41598-017-03099-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03099-9
This article is cited by
-
Nanocrystalline monocalcium aluminate “Krotite”: band gap engineering and near IR emission by selective excitation of native defects owing to annealing
Journal of Nanoparticle Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.