Abstract
House dust mite (HDM) allergy is a predominant cause for perennial allergic rhinitis (AR) in Europe. We recently reported that circulating erythrocyte numbers decrease after airway allergen challenge in a murine asthma model and in grass-pollen sensitized AR subjects. Consequently, we aimed to evaluate these findings in HDM sensitized AR subjects and the influence of preceding allergen immunotherapy. Seventy-seven (age 26.8 ± 7.3 years; 54.5% female) HDM-allergic rhinitis subjects previously enrolled in a randomized, monocentric sublingual immunotherapy (SLIT) trial at the Vienna Challenge Chamber (VCC) were included. Subjects had either received placebo (n = 22), low-dose HDM (n = 29) or high-dose HDM specific sublingual immunotherapy (n = 26) daily for 24 weeks. Blood sampling was performed before and after 6 hours of HDM allergen exposure. Overall, specific airway allergen challenge resulted in a significant decrease in circulating erythrocytes and hematocrit (p < 0.001), and elevation of leukocytes (p < 0.001), particularly segmented neutrophils (p < 0.001). Gender had no significant effect on the observed changes in circulating blood cells. Erythrocytes decreased and neutrophil counts increased significantly after airway allergen challenge regardless of preceding immunotherapy. These findings imply a rapid systemic mobilization of neutrophils occurring within immediate type hypersensitivity response upon a specific allergen challenge, which is possibly inversely linked with the erythrocyte numbers.
Similar content being viewed by others
Introduction
Allergic rhinitis (AR) is the most common cause for chronic rhinitis worldwide, affecting up to 30% of the human population1, 2. Most cases of perennial or “non-seasonal” AR in Europe are induced by house dust mite (HDM) allergens and result in a poorer health-related quality of life than seasonal rhinitis in affected subjects3. From a pathophysiological perspective, HDM-associated AR is an immunoglobulin E (IgE) mediated upper airway mucosal reaction to structural proteins and fecal proteases predominantly from the species Dermatophagoides pteronyssinus (Der p) and Dermatophagoides farinae (Der f) in Europe3, 4. Diagnosis of HDM allergen sensitization comprises skin-prick testing and specific IgE analysis on extracts and allergen molecules5, the latter overcoming non-specific reactions in SPT6. The current therapeutic standards for HDM associated AR include reduction of exposure and the use of immune modulating drugs. While the efficacy of specific allergen immunotherapy and, even more, sublingual immunotherapy (SLIT) were previously disputed7, a change of paradigms has taken place with the increasing number of controlled clinical trials conducted in HDM allergy patients using various formulations of HDM SLIT, such as tablets or drops. The clinical efficacy and long-term effects of HDM SLIT in AR is today undisputable in adults in European8, 9 and US10 studies, as well as in elderly patients11 and in children12,13,14,15.
In the absence of laboratory biomarkers correlating with the clinical success of allergen immunotherapy, clinical parameters, i.e. objective symptom-medication scores, subjective visual analogue scores, and percutaneous, conjunctival and respiratory allergen challenges are the current state of the art methods to determine the effects of allergen immunotherapy. Of those, allergen provocations in allergen challenge chambers represent the gold standard16,17,18. An environmental allergen challenge chamber was recently used to prove the clinical efficacy of a HDM sublingual tablet15. After 6-hour exposure the total nasal symptom score was a primary endpoint, the total ocular symptom scores and total symptom scores secondary end points in this study. Samples of this clinical trial were examined in the present study.
Erythrocytes, also known as red blood cells (RBC), represent the most abundant blood cells and are mainly responsible for oxygen-binding and transportation throughout the organism. An effect on, or involvement of, erythrocytes in the immune response after allergen challenge has not yet been investigated in allergic rhinitis, even though the crosstalk between erythrocytes and immune cells has gained interest in the pathophysiology of various immune-mediated diseases19, 20. For example, several damage-associated molecular patterns (DAMPs) have been identified in erythrocytes, such as heme, Hsp70 and IL-33, which can be released into the circulating blood upon intravascular hemolysis and potentiate inflammatory responses if not neutralized by specialized scavenger proteins20. We have previously reported that numbers of circulating erythrocytes significantly decreased after airway challenge with the specific allergen grass pollen21. Our findings deduced from a mouse model and verified in human AR subjects, were primarily attributed to a potential recruitment of erythrocytes to the respiratory mucosa as a site of hypoxia during specific allergen challenge and successive microepistaxis21. In accordance, increased free hemoglobin was previously found in nasal lavage samples after allergen challenge in AR subjects as a possible result of increased vascular permeability22. In this work we also observed in the human AR subjects that the 4 hour challenge with grass pollen allergens resulted in significant elevation of neutrophils in the peripheral blood, while eosinophil counts remained unchanged.
Consequently we hypothesized that successful allergen immunotherapy could neutralize the effects of an allergen challenge on erythrocytes and neutrophils, and possibly correlate with clinical improvement. The aim of the present study was therefore to i) confirm the previously observed changes in blood cell counts with a different allergen, HDM, in an environmental exposure chamber, and ii) to compare laboratory parameters for RBC and granulocytes before and after HDM challenge in AR subjects being treated with SLIT versus placebo15.
Results
Subject characteristics
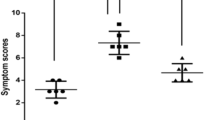
A total of seventy seven (n = 77) Caucasian subjects (54.5% female) with an average age of 26.8 ( ± 7.3) years were evaluated; all had confirmed AR to HDM allergens. According to the preceding drug therapy, subjects were divided in three groups15: group 1 had received placebo (n = 22), group 2 low dose (6 developmental units [DU] of MK-8237) (n = 29), and group 3 high dose (12 DU of MK-8237) (n = 26) HDM specific sublingual immunotherapy. Except for a higher proportion of females in the low dose subgroup, subject characteristics, preceding drug therapy and time since drug therapy were similar across groups (Table 1). To determine the lasting clinical benefit, allergen challenges were done in all three subject groups over 6 hours in the Vienna challenge chamber; blood cell counts before (baseline, “pre”) and immediately after the challenges (6 h time point, “post”) were compared. Compared to nasal symptom scores before participation in the sublingual immunotherapy trial, only subjects in group 3 (high dose SLIT) reported significantly less symptoms (−39%) during airway allergen challenge. (Supp. Fig. 3a). No significant differences in nasal symptom scores were found between subjects in group 1 (placebo) and group 2 (low dose SLIT) during airway challenge. (Supp. Fig. 3b+c).
Allergen challenge and erythrocytes
Absolute erythrocyte counts, hematocrit (p < 0.001, respectively) and hemoglobin (p = 0.014) values significantly decreased during allergen challenge in all AR subjects, independent of preceding treatment. MCH (mean corpuscular hemoglobin) and MCHC (mean corpuscular hemoglobin concentration) values (p = 0.002, p = 0.006, respectively) declined upon allergen challenge, whereas MCV (mean corpuscular volume) values remained almost constant. The decline in erythrocytes and hematocrit values after allergen challenge was observed similarly in female (p < 0.001 and p < 0.01) and in male (p < 0.01, respectively) subjects (Fig. 1).
In the subgroup analysis, erythrocytes, hematocrit and MCH (p < 0.05), but not hemoglobin (p = 0.081), decreased significantly after allergen challenge in the placebo-treated group.
These changes were likewise observed in the allergen immunotherapy groups: in those treated with low-dose SLIT, a significant decline in erythrocyte counts, hematocrit (p < 0.01, respectively) and hemoglobin (p < 0.05) was found similar to those treated with high-dose SLIT who had significantly lower erythrocyte counts and MCH levels (p < 0.05) after allergen challenge while the hematocrit did not reach statistical significance (p = 0.052).
Comparing the groups, significantly higher baseline levels of erythrocyte and hemoglobin levels were found in male subjects of the placebo group compared to male subjects treated with high dose SLIT (p < 0.05); a similar tendency in female subjects was found but did not reach statistical significance.
Allergen challenge and leukocytes
Overall, leukocytes and absolute numbers of segmented neutrophils (p < 0.001, respectively) were significantly increased after airway allergen challenge compared to baseline values before challenge. A significant increase of leukocyte and absolute segmented neutrophil numbers after challenge was observed in all groups (p < 0.01, respectively) regardless of preceding placebo or SLIT treatment. Absolute circulating eosinophil numbers slightly decreased (p < 0.05) compared to baseline values only in the placebo group, while in both SLIT treated groups eosinophil counts did not show statistically significant differences after 6 h of allergen challenge (Table 2).
Discussion
We previously reported a decrease in circulating erythrocytes after prolonged airway challenge with a specific allergen in grass pollen sensitized AR patients and, in analogy, in a murine model21. Our present study in HDM sensitized AR subjects confirms this phenomenon for an independent allergen. Moreover, the observed effect on red blood cells was not influenced by preceding allergen-specific immunotherapy compared to treatment with placebo. Therefore, the decline in red blood cells is not suitable as a biomarker for allergen immunotherapy, but is rather a surrogate for mucosal hyper-reactivity upon contact with respiratory allergens in sensitized subjects. Moreover, although total erythrocyte counts and hemoglobin levels in the peripheral blood were lower at the end of allergen challenge compared to baseline values before challenge, mean values remained in the normal reference ranges for the general population. To our knowledge, this is the first study in AR subjects sensitized to HDM reporting significant changes in circulating red blood parameters after HDM allergen challenge. These findings are consistent with the results of our previous study in grass-pollen sensitized AR subjects after allergen challenge, who had not undergone allergen specific immunotherap21. Both our studies together imply that the effect of allergen challenge on circulating erythrocyte levels is not limited to either HDM or grass pollen allergen, but rather represents involvement of RBC in a general pathophysiological mechanism.
A short-term decline of circulating erythrocytes after allergen exposure as found in our study can have several explanations. In acute allergic rhinitis mast cell induced inflammatory mediators, such as leukotrienes and prostaglandin, cause vascular hyper-permeability, vasodilation and chemotaxis of neutrophils and eosinophils to the local inflammation site following allergen exposure23. The increased microvascular leakage primarily leads to plasma extravasation making an acute systemic increase in hematocrit values more likely than a decrease, as found in our study. Thus, a dilution effect is unlikely. Further, an effect on erythropoiesis can be ruled out due to the relatively short duration between the two time points of blood sampling (6 hours of allergen challenge). In our setting, a removal of erythrocytes from the circulating blood stream seems the most likely explanation for the observed periphery decrease. Cysteinyl-leukotrienes (CysLTs), lipid inflammatory mediators which are released from immune cells in AR24, 25, have been shown to mediate erythrocyte clearance via erythrocyte apoptosis. Eryptosis has previously been described in inflammation and sepsis26, 27, but so far not in allergic disease. Foller et al. found that CysLTs can activate ion-channels on the erythrocyte surface resulting in Ca2+ influx which further leads to cell shrinkage and membrane scrambling28. This mechanism could explain an induced clearance of circulating erythrocytes by splenic macrophages and thus decrease in peripheral RBC counts. There were significantly less symptoms in the high dose HDM SLIT group and, following our hypothesis, less CysLTs should lead to less RBC degradation. Indeed, compared to baseline values there was a more prominent mean absolute RBC decline in the placebo (0.10 × 1012/l) than in the high dose SLIT group (0.04 × 1012/l) after allergen challenge. Furthermore, a subclinical bronchial reaction to airway allergen challenge, resulting in hypoxia, cannot be excluded. In this setting, a pulmonary redistribution of erythrocytes for forced oxygen loading is also a possible scenario.
Interestingly, we also observed changes in MCH and MCHC with inconsistent statistical significance in the subgroup analysis. Since MCH and MCHC are calculated laboratory values dependent on hemoglobin concentration and erythrocyte count (MCH) or hematocrit (MCHC), we attributed these findings to the prominent decline of erythrocyte counts and hematocrit as against more stable hemoglobin levels.
Allergen challenge further resulted in significant elevation of circulating leukocyte counts, specifically the absolute segmented neutrophil numbers in all groups including placebo. An increase in circulating neutrophils after allergen challenge in HDM-associated AR has been described before29, 30. As mentioned above, neutrophil chemotaxis to the site of infection or allergen-associated inflammation is an early innate immune response to potential pathogens, resulting in the induction of inflammatory cells and cytokines responsible for oxidative stress at the inflammation site29, 30. Further, the release of chromatin-based extracellular traps from neutrophils (NETs) as a defense mechanism against pathogens has recently gained interest in various diseases. In inflammatory respiratory diseases, NETs have been shown to form in the capillary plexuses of the lungs31 and can potentially entangle with red blood cells32. Dismantling NETs by DNase treatment improved lung function in murine asthma models and in some in vivo human studies31. A trapping of erythrocytes in neutrophil derived NETs at the site of inflammation, i.e. in the capillary plexuses of the nasal mucosa, could potentially explain a fraction of the decrease in their circulating counts. This trapping could further result in local hemolysis and release of DAMPs from erythrocytes20 potentially influencing the inflammatory reaction. A potential association between neutrophil recruitment and circulating erythrocyte decrease in specific allergen challenge would need to be evaluated in further studies.
Although subjects who had undergone high-dose immunotherapy (group 3) reported less rhinitis symptoms during allergen challenge, (Fig. S3) the decrease of circulating erythrocytes was statistically unaffected by preceding SLIT. Interestingly, however, there was a significant difference in erythrocyte and hemoglobin levels between the placebo and the high-dose SLIT group at baseline, with higher levels in placebo group AR. Probably due to higher subject numbers in both groups, this finding only reached statistical significance in male subjects. Elevation of red blood cells and oxygen-binding hemoglobin is a well-known mechanism for compensation of chronic low oxygen supply. Chronic hypoxic conditions resulting from living in high altitude areas, chronic obstructive pulmonary disease, but also cigarette smoking, have since long been associated with secondary erythrocytosis33. In allergic asthma, alterations in oxidant/antioxidant balance have been shown to shift towards increased oxidative stress, with increased superoxide dismutase activity and decreased glutathione peroxidase activity in erythrocytes34. Chronic allergic rhinitis partially limits the nasal airflow, but cannot result in relevant hypoxia if the respiratory airflow is unaffected. Accordingly, in our study, mean erythrocyte, hematocrit and hemoglobin levels remained in the normal reference ranges at both sampling times in all groups. However, the significantly higher baseline levels for all three parameters in untreated (placebo) compared to symptom reduced (SLIT) AR subjects, might indicate a subclinical difference in oxygen supply due to availability of additional nasal airflow. Another explanation for this observation might be a direct effect of the immunological therapy on the red blood cells in the SLIT groups, but no significant differences were found between red blood parameters in the high-dose and the low-dose SLIT groups, and surprisingly, the lowest mean values of erythrocyte counts, hemoglobin and hematocrit were found in the low dose SLIT group; altogether, the hypothesis of a dose-dependent drug effect is not support by the data.
In our study, there were no significant differences between female and male subjects apart from the known sex-dependent physiological divergence in erythrocyte counts, hemoglobin and hematocrit levels. While similar trends were found in all treatment subgroups, statistical significance was not always reached after further subdivision into male and female subjects.
Finally, only the eosinophil counts correlated with the treatment status of the subjects. Upon allergen challenge circulating eosinophil numbers remained unchanged in both HDM SLIT groups but slightly decreased in the placebo group. Eosinophil recruitment from the blood circulation and their arrest on activated endothelium and extravasation has been described in allergic airway diseases23, 35. There are various explanations for the mechanisms of allergen immunotherapy. Tregs 36 and Bregs 37 as sources of immunomodulatory cytokines IL-10 and TGFb counterregulate the Th2 cytokine dominance in allergy, including the Th2 cytokine IL-5. IL-5 is an important activator for eosinophils and acts in the nasal mucosa, especially in the late allergic reaction38. As was recently demonstrated in a mouse model39, SLIT could contribute to an overall lower Th2 bias.
Conclusion
To the best of our knowledge this is the first study addressing peripheral blood cell counts following allergen challenge in AR subjects after specific immunotherapy. Overall our study shows that (i) circulating erythrocyte numbers significantly decrease after specific allergen challenge in allergic rhinitis subjects, possibly due to leukotriene induced eryptosis; (ii) leukocyte counts, particularly segmented neutrophils, increase after allergen challenge, independent of preceding immunotherapy; (iii) placebo-treated subjects – possibly compensatory - have higher baseline levels of erythrocytes than subjects after effective SLIT treatment, (iv) only eosinophil dynamics differed between subjects after immunotherapy and subjects who had received placebo. These observations are in accordance with our previous findings on RBC dynamics after allergen challenge and imply an involvement of erythrocytes and neutrophils in the acute allergic response.
Methods
Subjects
In this monocentric study, otherwise healthy human subjects (n = 77) with a medical history of HDM allergy were recruited at the Vienna Challenge Chamber (VCC)15.
HDM allergen sensitization (Der p, Der f, or both) was assessed by specific IgE assessment and skin prick testing prior to study inclusion. All evaluated subjects had previously participated in a randomized, double-blind pharmacological trial on dose-related efficacy of HDM sublingual immunotherapy tablets15 and had received either placebo, low dose (6 DU) or high dose (12 DU) HDM sublingual immunotherapy (MK-8237 [Merck/ALK-Abelló]) for a total of 24 weeks. Data presented in the current study were assessed in volunteering subjects 3 to 16 months (⦰ 8.4 months) after MK-8237 trial completion (24 weeks SLIT and additional 2-weeks follow-up). (Table 1) The HDM allergen challenges were approved by the ethical committee “Österreichische Arbeitsgemeinschaft für Klinische Pharmakologie” (1811/2013) and data collection was conducted between 11/2013 and 10/2014 with volunteer subjects after gaining written informed consent of each participant. All studies in the VCC are strictly conducted in conformance with GCP (Good Clinical Practice).
Inclusion criteria
-
age 18–65 years
-
healthy individuals, except for allergic rhinitis and intermittent or mild asthma not requiring treatment and associated with normal baseline lung function (FEV1 ≥ 70% of predicted)
-
positive skin prick test (wheal ≥ 3 mm compared to standard control [saline solution]) and specific IgE ( ≥ 0.7 kU/l, radioallergosorbent test class [RAST] ≥ 2) results for Der p and/or Der f within the last 12 months preceding the study
Exclusion criteria
-
nasal abnormalities (septum perforation, polyps, malformations)
-
cold symptoms or acute infections of the upper respiratory tract within 3 weeks
-
active respiratory tract disease, other than mild asthma not requiring treatment
-
ongoing immunotherapy and/or immunomodulating medication
Interventions
Airway Allergen Challenge
A mixture of equal parts of Der p and Der f whole bodies (Allergon, Thermo Fisher Scientific, Ängelholm, Sweden) and a smaller part of dermatophagoides feces extract (Citeq Biologics, Groningen, The Netherlands) (ratio 10:10:1) was used for allergen provocation in the challenge chamber. Allergen products used for the mixture were provided by their respective manufacturers including certificates of analysis and dry stored at +2 to +10 °C (whole bodies) or frozen at −25 °C (feces) in their original containers. Airway allergen challenge was conducted in the VCC (a 54 m3 sealed provocation chamber) for 6 consecutive hours as previously described. (21) Airborne HDM allergen mixture concentration was monitored and kept constant. Every 15 minutes allergic symptoms, i.e. total nasal symptom score (TNSS - sneezing, obstructed, runny and itchy nose) were evaluated. Further, every 30 minutes nasal air flow volume (active anterior rhinomanometry) and every 60 min bronchial air flow volume (spirometry) values were assessed for safety reasons.
Blood Cell Collection
Blood was drawn immediately before and after 6 h of allergen challenge from periphery veins and sampled in ethylenediamine tetraacetic acid (EDTA) coated tubes. EDTA blood samples were shortly stored at +2 °C to +10 °C and sent to an ISO 9001-certified laboratory (Labors.at, Vienna, Austria) for differential blood cell count analysis, and determination of hemoglobin [Hb] and hematocrit [Hc].
Statistical Analysis
Data were analysed by ANOVA (sigma restricted parameterization) with three factors: group (placebo, low and high dose) and gender as between-subject factors and pre/post challenge as within-subject factor. Comparisons against placebo were performed applying linear contrasts. Normality of residuals was tested by Kolmogorov-Smirnov tests with Lilliefors correction. Homogeneity of variances was tested by Levene’s tests. For relative white blood-cell counts an arcsine transformation was applied. For all statistical tests p < 0.05 was considered significant. The software packages SPSS (version 20.0 for Windows, IBM Corp., USA) and STATISTICA (version 12.0; StatSoft Inc., USA) were used for statistical calculations.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Pawankar, R. Allergic diseases and asthma: a global public health concern and a call to action. The World Allergy Organization journal 7, 12, doi:10.1186/1939-4551-7-12 (2014).
Pawankar, R. C. G., Holgate, S. T., Lockey, R. F. World Health Organization. White Book on Allergy 2011–2012 Executive Summary. http://www.worldallergy.org/UserFiles/file/WAO-White-Book-on-Allergy_web.pdf (2011–2012).
Linneberg, A. et al. Burden of allergic respiratory disease: a systematic review. Clinical and molecular allergy: CMA 14, 12, doi:10.1186/s12948-016-0049-9 (2016).
Calderon, M. A. et al. Respiratory allergy caused by house dust mites: What do we really know? The Journal of allergy and clinical immunology 136, 38–48, doi:10.1016/j.jaci.2014.10.012 (2015).
Matricardi, P. M. et al. EAACI Molecular Allergology User’s Guide. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology 27 (Suppl 23), 1–250, doi:10.1111/pai.12563 (2016).
Foong, R. X., Roberts, G., Fox, A. T. & du Toit, G. Pilot study: assessing the clinical diagnosis of allergy in atopic children using a microarray assay in addition to skin prick testing and serum specific IgE. Clinical and molecular allergy: CMA 14, 8, doi:10.1186/s12948-016-0046-z (2016).
Nelson, H. S. Update on house dust mite immunotherapy: are more studies needed? Current opinion in allergy and clinical immunology 14, 542–548, doi:10.1097/ACI.0000000000000104 (2014).
Klimek, L. et al. SQ house dust mite (HDM) SLIT-tablet provides clinical improvement in HDM-induced allergic rhinitis. Expert review of clinical immunology 12, 369–377, doi:10.1586/1744666X.2016.1144473 (2016).
Demoly, P., Okamoto, Y., Yang, W. H., Devillier, P. & Bergmann, K. C. 300 IR HDM tablet: a sublingual immunotherapy tablet for the treatment of house dust mite-associated allergic rhinitis. Expert review of clinical immunology 12, 1141–1151, doi:10.1080/1744666X.2016.1237288 (2016).
Nolte, H. et al. Efficacy of house dust mite sublingual immunotherapy tablet in North American adolescents and adults in a randomized, placebo-controlled trial. The Journal of allergy and clinical immunology 138, 1631–1638, doi:10.1016/j.jaci.2016.06.044 (2016).
Bozek, A., Ignasiak, B., Filipowska, B. & Jarzab, J. House dust mite sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with allergic rhinitis. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 43, 242–248, doi:10.1111/cea.12039 (2013).
Anderson, H. M., Wood, R. A. & Busse, W. W. Dust Mite-Induced Perennial Allergic Rhinitis in Pediatric Patients and Sublingual Immunotherapy. The journal of allergy and clinical immunology. In practice 5, 46–51, doi:10.1016/j.jaip.2016.07.013 (2016).
Calderon, M. A. et al. House Dust Mite Respiratory Allergy: An Overview of Current Therapeutic Strategies. The journal of allergy and clinical immunology. In practice 3, 843–855, doi:10.1016/j.jaip.2015.06.019 (2015).
Pfaar, O. et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto- Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo journal international 23, 282–319, doi:10.1007/s40629-014-0032-2 (2014).
Nolte, H. et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. The Journal of allergy and clinical immunology 135, 1494–1501 e1496–1501.e6, doi:10.1016/j.jaci.2014.12.1911 (2015).
Horak, F. et al. Effect of continuous allergen challenge on clinical symptoms and mediator release in dust-mite-allergic patients. Allergy 53, 68–72, doi:10.1111/all.1998.53.issue-1 (1998).
Lueer, K. et al. Safety, efficacy and repeatability of a novel house dust mite allergen challenge technique in the Fraunhofer allergen challenge chamber. Allergy 71, 1693–1700, doi:10.1111/all.12947 (2016).
Ronborg, S. M., Mosbech, H. & Poulsen, L. K. Exposure chamber for allergen challenge. A placebo-controlled, double-blind trial in house-dust-mite asthma. Allergy 52, 821–828, doi:10.1111/all.1997.52.issue-8 (1997).
Buttari, B., Profumo, E. & Rigano, R. Crosstalk between red blood cells and the immune system and its impact on atherosclerosis. BioMed research international 2015, 616834–8, doi:10.1155/2015/616834 (2015).
Mendonca, R., Silveira, A. A. & Conran, N. Red cell DAMPs and inflammation. Inflammation research: official journal of the European Histamine Research Society… [et al.] 65, 665–678, doi:10.1007/s00011-016-0955-9 (2016).
Jordakieva, G. et al. Peripheral erythrocytes decrease upon specific respiratory challenge with grass pollen allergen in sensitized mice and in human subjects. PloS one 9, e86701, doi:10.1371/journal.pone.0086701 (2014).
Park, Y. J. et al. Nasal lavage concentrations of free hemoglobin as a marker of microepistaxis during nasal provocation testing. Allergy 57, 329–335, doi:10.1034/j.1398-9995.2002.1o3253.x (2002).
Cobanoglu, B., Toskala, E., Ural, A. & Cingi, C. Role of leukotriene antagonists and antihistamines in the treatment of allergic rhinitis. Current allergy and asthma reports 13, 203–208, doi:10.1007/s11882-013-0341-4 (2013).
Peters-Golden, M., Gleason, M. M. & Togias, A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 36, 689–703, doi:10.1111/j.1365-2222.2006.02498.x (2006).
Shirasaki, H. & Himi, T. Role of Cysteinyl Leukotrienes in Allergic Rhinitis. Adv Otorhinolaryngol 77, 40–45, doi:10.1159/000441871 (2016).
Pretorius, E., du Plooy, J. N. & Bester, J. A Comprehensive Review on Eryptosis. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 39, 1977–2000, doi:10.1159/000447895 (2016).
Lang, E. & Lang, F. Mechanisms and pathophysiological significance of eryptosis, the suicidal erythrocyte death. Seminars in cell & developmental biology 39, 35–42, doi:10.1016/j.semcdb.2015.01.009 (2015).
Foller, M. et al. Participation of leukotriene C(4) in the regulation of suicidal erythrocyte death. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society 60, 135–143 (2009).
Lavinskiene, S., Bajoriuniene, I., Malakauskas, K., Jeroch, J. & Sakalauskas, R. Sputum neutrophil count after bronchial allergen challenge is related to peripheral blood neutrophil chemotaxis in asthma patients. Inflammation research: official journal of the European Histamine Research Society… [et al.] 63, 951–959, doi:10.1007/s00011-014-0770-0 (2014).
Nakagome, K. & Nagata, M. Pathogenesis of airway inflammation in bronchial asthma. Auris, nasus, larynx 38, 555–563, doi:10.1016/j.anl.2011.01.011 (2011).
Malachowa, N., Kobayashi, S. D., Quinn, M. T. & DeLeo, F. R. NET Confusion. Frontiers in immunology 7, 259, doi:10.3389/fimmu.2016.00259 (2016).
Boneschansker, L., Inoue, Y., Oklu, R. & Irimia, D. Capillary plexuses are vulnerable to neutrophil extracellular traps. Integrative biology: quantitative biosciences from nano to macro 8, 149–155, doi:10.1039/c5ib00265f (2016).
Smith, J. R. & Landaw, S. A. Smokers’ polycythemia. The New England journal of medicine 298, 6–10, doi:10.1056/NEJM197801052980102 (1978).
Nadeem, A., Chhabra, S. K., Masood, A. & Raj, H. G. Increased oxidative stress and altered levels of antioxidants in asthma. The Journal of allergy and clinical immunology 111, 72–78, doi:10.1067/mai.2003.17 (2003).
Johansson, M. W. Activation states of blood eosinophils in asthma. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 44, 482–498, doi:10.1111/cea.12292 (2014).
Akdis, C. A. & Akdis, M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. The Journal of clinical investigation 124, 4678–4680, doi:10.1172/JCI78891 (2014).
van de Veen, W. et al. Role of regulatory B cells in immune tolerance to allergens and beyond. The Journal of allergy and clinical immunology 138, 654–665, doi:10.1016/j.jaci.2016.07.006 (2016).
Leaker, B. R. et al. The nasal mucosal late allergic reaction to grass pollen involves type 2 inflammation (IL-5 and IL-13), the inflammasome (IL-1beta), and complement. Mucosal immunology 10, 408–420, doi:10.1038/mi.2016.74 (2016).
Hagner, S. et al. House Dust Mite-Specific Sublingual Immunotherapy Prevents the Development of Allergic Inflammation in a Mouse Model of Experimental Asthma. International archives of allergy and immunology 170, 22–34, doi:10.1159/000446155 (2016).
Acknowledgements
We kindly thank Amelia Wein for language editing and proofreading. The study was supported by grant SFB F4606-B28 of the Austrian Science Fund FWF to EJJ.
Author information
Authors and Affiliations
Contributions
G.J. analyzed and interpreted the data, designed the figures and wrote the manuscript; M.K. performed statistical analysis, as well as data interpretation and manuscript revision. P.L. was involved in conception and design of the study, data interpretation and figure design. P.Z. and R.Z. both contributed to patient management, study design as well as to acquisition of data and critical revision of the manuscript. J.G.C. participated in drafting and critical revision of the manuscript. E.J.J. designed the study concept, contributed to interpretation of data and helped substantially in drafting of manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jordakieva, G., Kundi, M., Lemell, P. et al. Sublingual house dust mite immunotherapy has no impact on decrease of circulating erythrocytes upon airway allergen challenge in allergic rhinitis. Sci Rep 7, 2555 (2017). https://doi.org/10.1038/s41598-017-02321-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02321-y
This article is cited by
-
The impact of allergen exposure and specific immunotherapy on circulating blood cells in allergic rhinitis
World Allergy Organization Journal (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.