Abstract
Browning is the process of increasing the number of brite cells, which helps to increase energy expenditure and reduce obesity. Consumption of natural and non-toxic herbal extracts that possess the browning effect is an attractive anti-obesity strategy. In this study, we examined the browning effect of cinnamon extract. We found that cinnamon extract (CE) induced typical brown adipocyte multiocular phenotype in 3T3-L1 adipocytes. The treatment also increased brown adipocytes markers and reduced white adipocytes markers in the 3T3-L1 adipocytes. In ex vivo studies, we found that CE increased brown adipocytes markers in the subcutaneous adipocytes isolated from db/db mice and diet-induced obesity (DIO) mice. However, CE did not significantly affect UCP1 expression in the adipocytes isolated from perinephric adipose tissue and epididymal adipose tissue. β3-adernergic receptor (β3-AR) antagonist reduced the CE-enhanced UCP1 expression, suggesting an involvement of the β3-AR activity. Oral administration of CE significantly increased UCP1 expression in the subcutaneous adipose tissue in vivo and reduced the body weight of the DIO mice. Taken together, our data suggest that CE has a browning effect in subcutaneous adipocytes. Our study suggests a natural non-toxic herbal remedy to reduce obesity.
Similar content being viewed by others
Introduction
Obesity has become a public health crisis. Obese people are predisposed to a number of diseases, including cardiovascular disease, type 2 diabetes, hypertension, stroke and many types of cancers1. Strategies that can enhance energy expenditure and combat the epidemic of obesity are desperately needed.
There are two types of adipocytes, white adipocyte and brown adipocyte. White adipocytes are specialized to store chemical energy while brown adipocytes dissipate energy as heat. The thermogenic responses in brown adipocytes are dependent on the abundance of uncoupling protein 1 (UCP1) in the mitochondria2. UCP1 is an integral membrane protein unique to mitochondria in brown adipocytes and is responsible for the respiratory uncoupling during thermogenesis in the brown adipocytes. However, active brown fat is virtually absent or has low thermogenesis activity in obese people.
Browning is the induction of brown adipocyte markers including UCP1 in white adipocytes, these brown adipocyte-like cell are called brite cells or beige cells3, 4. These brite cells can be found in the white adipose tissues in rodents and human3, 4. Cold stress or in response to treatment with β3 selective adrenergic agonist increases the development of these brite cells in the white adipose tissue5, 6 although the origin of these brite cells is still controversial7. Abundance of brite or beige cells in white adipose tissue shows resistance to diet-induced obesity and improved glucose metabolism8,9,10,11.
Cinnamon is a spice produced from the bark of trees from the genus Cinnamomum (Cinnamomum zeylanicum and Cinnamon cassia) and belongs to the Lauraceae family12. Cinnamon is one of the most important spices used daily by people all over the world. Cinnamon exerts antioxidant, anti-inflammatory, antimicrobial and anticancer effects12, 13. Furthermore, cinnamon extract improves insulin sensitivity and has beneficial effects on the metabolism14, 15. A recent study suggests that cinnamon extract reduces lipid and glycogen accumulation in the livers of high fat diet-fed animal models and lowers glucose levels by increasing insulin secretion16,17,18. Other study showed that cinnamon extract regulated expression of multiple genes related to carbohydrate metabolism and lipogenesis in the adipose tissue of fructose-fed rats and had anti-hyperglycemic and anti-hyperlipidemic effects in diabetic animal models19. The anti-obesity effect of the extract is less studied. A study showed that the extract increased expression of glucose transporter in adipocytes20. However, the effects of the cinnamon extract on adipocyte differentiation are inconsistent and controversial21.
Our study aimed to find out the browning effect of the cinnamon extract (CE) with in vitro, ex vivo and in vivo studies. We showed that CE increased expressions of UCP1 and other brown adipocyte markers in subcutaneous adipocytes, β3-AR activity was involved in the CE-induced browning process.
Results
Cinnamon extract (CE) induces typical brown adipocyte multiocular phenotype in 3T3-L1 cells
Cinnamon, the bark of Cinnamomum cassia (Fig. 1a), was extracted by 70% ethanol in water and the dried cinnamon extract (CE) powder was reconstituted in DMSO for experiments. We first performed HPLC-ESI-MS to determine the chemical composition of CE for quality control. We have identified and quantified some of the bioactive compounds in the CE (Fig. 1b) with standards purchased from NICPBP National Institute for the Control of Pharmaceutical and Biological Products, China. The identified compounds in the CE are protocatechuic acid of 17.3033 mg/kg, catechin 302.0128 mg/kg, chlorogenic acid 25.1002 mg/kg, aesculetin 12.7499 mg/kg, quercetin 180.9573 mg/kg and icariin 51.1641 mg/kg.
We differentiated mouse embryo fibroblast 3T3-L1 to mature adipocytes for the study. Figure 2a showed the typical phenotype of white adipocytes, lipid droplets were stained by Oil Red O. We added CE (80 µg/ml) to the 3T3-L1 adipocytes on day 6 during the course of differentiation, typical brown adipocyte multiocular phenotype was observed after 24-hr incubation (Fig. 2b). The lipid content in the 3T3-L1 adipocytes is significantly reduced upon the CE treatment (Fig. 2c).
CE increases brown adipocyte marker gene expressions in 3T3-L1 adipocytes
We also found that CE significantly increased Ucp1 mRNA levels (Fig. 3a) and protein levels (Fig. 3b and Fig. S1) in 3T3-L1 adipocytes; the increase of UCP1 expression upon CE treatment is also demonstrated by immunofluorescence staining (Fig. 3c). Since CE increased Ucp1 mRNA level, we next examined if CE affected Ucp1 promoter activity. We transfected a Ucp1 −2979 bp reporter plasmid (Fig. 3d) to HEK293 cells and 3T3-L1 adipocytes, respectively. We found that the CE increased UCP1 promoter activities in these two cell models (Fig. 3e and f). Norepinephrine (NE)22 was used as a positive control to increase the UCP1 promoter activity in these cells (Fig. 3e and f). Since UCP1 is an integral membrane protein in the mitochondria, we used MitoTracker Green (Molecular Probes, Invitrogen USA) to stain mitochondria in the 3T3-L1 adipocytes to examine if CE enhanced mitochondrial biogenesis. As shown in Fig. 3g, CE increased mitochondrial protein biogenesis as indicated by a stronger staining in the CE-treated 3T3-L1 adipocytes when compared to the vehicle control adipocytes. Furthermore, CE also increased the expressions of other brown adipocyte marker genes including cell death-inducing DFFA-like effector A (Cidea), PR domain containing 16 (Prdm16), peroxisome proliferator-activated receptor gamma (PPARγ), PPARγ coactivator-1 (Pgc) and the fatty acid oxidation marker gene carnitine palmitoyltransferase 1 (Cpt1) (Fig. 4a). The enhanced PRDM16 is further suggested by its elevated protein level in the CE-treated 3T3-L1 adipocytes (Fig. 4b and Fig. S2). CE also reduced white adipocyte marker genes including dermatopontin (Dpt) and insulin-like growth factor (Igf) (Fig. 4c) in the 3T3-L1 adipocytes. All these data suggest that CE induces browning in 3T3-L1 adipocytes.
(a) Ucp1 mRNA expression, (b) UCP1 protein expression and (c) immunofluorescence staining of UCP1 in vehicle control and CE-treated 3T3-L1 cells. (d) Ucp1 promoter construct (−2979 to base +76) ligated into pGL3-basic luciferase reporter vector. Ucp1 promoter activity in (e) HEK293 cells and (f) 3T3-L1 cells. (g) MitoTracker Green (Molecular Probe) staining of the mitochondria in control and CE-treated 3T3-L1 cells. CE, cinnamon extract (80 µg/ml); NE, norepinephrine (10 µM). Ucp1, uncoupling protein 1; DAPI, (4′,6-diamidino-2-phenylindole staining). *p < 0.05, n = 3 individual experiments. Original magnification 20x.
Expressions of (a) brown adipocyte marker genes and (c) white adipocyte marker genes in control and CE-treated 3T3-L1 cells. (b) Protein expression of PRDM16 in control and CE-treated 3T3-L1 adipocytes. CE, cinnamon extract (80 µg/ml). Cidea, cell death-inducing DFFA-like effector A; Prdm16, PR domain containing 16; PPARγ, peroxisome proliferator-activated receptor gamma; Pgc, PPARγ coactivator-1; Cpt-1, carnitine palmitoyltransferase; Dpt, dermatopontin; Igf, insulin-like growth factor. *p < 0.05, n = 3 individual experiments.
CE increases brown adipocyte maker gene expressions in subcutaneous adipocytes isolated from C57BLKS db/db mice
To study the browning effect of CE ex vivo, we dissected subcutaneous adipose tissues from db/db mice. However, adipose tissue contains many cell types, including adipocytes, endothelial cells, preadipocytes and fibroblasts, it is difficult to determine whether the effect of CE is a direct action on adipocytes or an indirect action mediated by other cell types. Therefore, we freshly isolated mature adipocytes from subcutaneous adipose tissue dissected from db/db mice. We then treated these freshly isolated adipocytes with CE for 24 hr. We found that CE increased UCP1 protein (Fig. 5a and Fig. S3) and mRNA (Fig. 5b) expressions in these subcutaneous adipocytes, and other brown adipocyte markers including Cidea and Prdm16 (Fig. 5b). However, the CE treatment did not increase UCP1 expression in epididymal adipocytes isolated from these mice (Fig. 5c and Fig. S4). These results suggest that CE induces browning in subcutaneous adipocytes in db/db mice.
Expressions of (a) UCP1 protein and (b) brown adipocyte marker genes in subcutaneous adipocytes isolated from db/db mice. (c) Expression of UCP1 protein in epididymal adipocytes isolated from db/db mice. CE, cinnamon extract (80 µg/ml). Ucp1, uncoupling protein 1; cidea, cell death-inducing DFFA-like effector A; Prdm16, PR domain containing 16. *p < 0.05, n = 3 individual experiments.
CE increases brown adipocyte marker gene expressions in subcutaneous adipocytes isolated from DIO mice
Transgenic mice such as db/db mice might have developmental compensation and non-specific effects. In contrast, diet-induced obesity (DIO) mice might have similar physiology to human obesity and truly reflect the responsiveness to CE treatment. Therefore, we included DIO mice as another obesity model in the study. We fed 5-week-old C57BL/6 mice with high fat diet to induce DIO mice while control mice were fed matched control diet (Fig. 6a). Starting from the 14th week of the dietary intervention, the body weight of the DIO mice was significantly greater than that of the matched control-diet-fed mice (Fig. 6b). We then isolated the subcutaneous adipocytes from these mice and treated these adipocytes with CE. We found that CE increased UCP1 expression in the subcutaneous adipocytes isolated from DIO mice (Fig. 6c). The treatment also increased expressions of other brown adipocyte marker genes (Fig. 6d) and decreased expressions of white adipocyte marker genes (Fig. 6e). However, CE did not significantly increase the UCP1 expression in the subcutaneous adipocytes isolated from the matched control-diet-fed mice (Fig. 6c and Fig. S5). Since the propensity to express UCP1 upon stimulation differs between various white adipose tissue depots23; we examined if CE increased UCP1 expression in adipocytes isolated from other fat depots. Interestingly, we found that CE treatment neither increase UCP1 protein level (Fig. 6f and Fig. S6) nor the mRNA level (Fig. 6g) in adipocytes isolated from perinephric adipose tissue and epididymal adipose tissue in these DIO mice. These results suggest that CE has a browning effect in the subcutaneous adipocytes in DIO mice.
(a) High fat diet (HFD)-induced obesity mouse (DIO) and matched control diet-fed mouse. (b) Body weights of DIO mice and control diet-fed mice. (c) UCP1 protein expression in subcutaneous adipocytes isolated from the DIO mice and control diet-fed mice. (d) Expressions of brown adipocyte marker genes and (e) white adipocyte marker genes in subcutaneous adipocytes isolated from the DIO mice. (f) Protein expression and (g) mRNA expression of UCP1 in perinephric adipocytes (PAT) and epididymal adipocytes (EAT) isolated from the DIO mice. CE, cinnamon extract (80 µg/ml). Ucp1, uncoupling protein 1; Cidea, cell death-inducing DFFA-like effector A; Prdm16, PR domain containing 16. *p < 0.05, n = 3 individual experiments.
CE increases UCP1 expression by activating β3-adrenergic receptor
In adipocytes, UCP1 expression can be increased by β-adrenergic stimulation via the cyclic AMP (cAMP)-mediated pathways5, 24. Therefore, we examined if CE-increased UCP1 expression was mediated by the β-adrenergic signaling. We treated the 3T3-L1 adipocytes with CE and found that cAMP levels in these adipocytes were increased (Fig. 7a), implying an involvement of the β-adrenergic receptor (β-AR) activity. Next, we preincubated the 3T3-L1 adipocytes with either β2-AR antagonist ICI1185551 (Sigma-Aldrich) or β3-AR antagonist SR59230A (Sigma-Aldrich), before we treated the adipocytes with CE. ICI 118,551 possesses a high degree of selectivity and specificity for the beta 2-adrenoceptor25. We found that inhibition of β2-AR did not have significant effect on the Ucp1 expression (Fig. 7b). However, inhibition of β3-AR significantly reduced the CE-enhanced Ucp1 and Prdm16 expression in the 3T3-L1 adipocytes (Fig. 7b). SR 59230 A is a selective antagonist of the β3-AR, but is subsequently shown to also act at α1-AR at high doses. To ensure the involvement of β3 but not α1 in the CE-enhanced UCP1 expression, we used doxazosin mesylate (Tocris) which is a selective α1-AR. We found that inhibition of α1-AR did not have significant effect on Ucp1 expression (Fig. 7b). Taken together, our data suggest that β3-adrenergic receptor plays a role in the CE-enhanced Ucp1 expression in 3T3-L1 adipocytes.
(a) cyclic AMP (cAMP) levels in control and CE-treated 3T3-L1 cells. Expressions of brown adipocyte marker genes in (b) 3T3-L1 cells, (c) subcutaneous adipocytes isolated from db/db mice and (d) subcutaneous adipocytes isolated from diet induced obesity (DIO) mice. CE, cinnamon extract (80 µg/ml); SR, SR59230A (1 µM); ICI, ICI118551 (1 mM); doxazosin mesylate, DM (1 µM). Ucp1, uncoupling protein 1; Cidea, cell death-inducing DFFA-like effector A; Prdm16, PR domain containing 16. *p < 0.05, **p < 0.01, n = 3 individual experiments.
To further suggest the involvement of β3-AR in the CE-enhanced Ucp1 expression in white adipocytes, we isolated the adipocytes from the subcutaneous adipose tissue dissected from db/db mice and from DIO mice, respectively. Then, we pre-treated these adipocytes with either β2-AR antagonist ICI118551 or β3-AR antagonist SR59230A, before we treated them with CE. We found that inhibition of β3-AR significantly reduced the CE-enhanced Ucp1 expression in the subcutaneous adipocytes dissected from db/db mice (Fig. 7c) and from DIO mice (Fig. 7d). These results clearly demonstrated that β3-AR was involved in the CE-enhanced Ucp1 expression in the subcutaneous adipocytes.
Oral administration of CE reduces body weight and increases UCP1 expression in the subcutaneous adipocytes in DIO mice
Next, we tried to examine if CE increased Ucp1 expression in subcutaneous adipocytes and reduced body weight in vivo. We fed the DIO mice with either vehicle control or CE (500 mg/kg body weight)26, 27 for 15 consecutive days. The CE treatment significantly reduced the body weight of the DIO mice (Fig. 8a). The reduction of body weight was unlikely due to changes in organ weight or food intake because the treatment did not have significant effect on organ weight and food intake (data not shown). Although the CE treatment slightly but significantly reduced the mass of perinephric adipose tissue (Fig. 8b) and increased the mass of SAT (Fig. 8B), the total white adipose tissue mass of these mice was not affected (Fig. 8c). The reduction of body weight was unlikely due to detrimental damage of the CE treatment to the mice because the treatment did not affect aspartate aminotransferase (AST) activity (Fig. 8d) or induce apoptosis in the subcutaneous adipose tissue (Fig. 8e and Fig. S7). Interestingly, the CE treatment increased UCP1 expression in the subcutaneous adipose tissue as clearly indicated by the immunostaining (Fig. 9). Therefore, our in vivo study suggests that CE treatment increases UCP1 expression in subcutaneous adipose tissue and reduces body weight of the DIO mice.
(a) Body weight of vehicle control and CE-treated mice. (b) Weights of different fat depots, presented in the percentage of body weights. (c) Total white adipose tissue mass of the mice. (d) Aspartate aminotransferase (AST) levels in the mice. (e) Expression of caspase 3 and cleaved caspase 3 in the subcutaneous adipose of the vehicle control and CE-treated mice. CE, cinnamon extract (500 mg/kg body weight). SAT, subcutaneous adipose tissue; PAT, perinephric adipose tissue; EAT, epididymal adipose tissue; BAT, brown adipose tissue. *p < 0.05, n = 3 individual experiments.
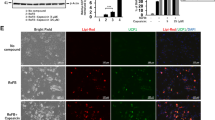
Immunostaining of UCP1 (left panel) and DAPI (4′,6-diamidino-2-phenylindole staining) (middle panel) in the subcutaneous tissues isolated from vehicle control and CE-treated DIO mice. Merged images are shown in the right panel. CE, cinnamon extract (500 mg/kg body weight). UCP1, uncoupling protein 1. Original magnification 20x.
Discussion
Upregulation of metabolism and increase heat generation reduce body weight. It is well known that UCP1 is the critical protein for thermogenesis. Our data clearly demonstrated that CE significantly increased the expressions of UCP1 and other brown adipocyte markers in subcutaneous adipocytes and 3T3-L1 adipocytes. The CE treatment also significantly increased mitochondrial protein biogenesis. In the animal study, we found that CE increased UCP1 expression in subcutaneous adipocytes in vivo and reduced the body weight of the DIO mice. It is reasonable to postulate that CE increases UCP1 expression and hence the heat production, which leads to a reduction in body weight. Interestingly, the treatment did not significantly affect UCP1 expression in adipocytes isolated from perinephric adipose tissue and epididymal adipose tissue in these mice. Our data also suggest that β3-AR antagonist reduced the CE-enhanced UCP1 expression, suggesting the CE-enhanced UCP1 expression is mediated by β3-AR activity. Our study suggests a natural non-toxic common herbal remedy to reduce obesity.
It is interesting to investigate how CE activates β3-AR. In this study, we used the extract of cinnamon which contains many bioactive compounds. For example, cinnamon contains a lot of derivatives such as cinnamaldehyde, cinnamic acid, cinnamate and numerous polyphenols12. Identifying which of these bioactive compounds induce browning by activating β3-AR will be an interesting study. The extract of cinnamon has been shown to have many functions. In animal studies, on top of the antioxidant, anti-inflammatory, antimicrobial and anticancer effects13, cinnamon is also found to have beneficial effects in Alzheimer’s disease12. However, further investigation is needed to provide more clinical evidence. In other studies, cinnamon lowers glucose and lipid levels in type 2 diabetes patients, it also reduces fasting serum glucose, triglyceride, low density lipoprotein cholesterol and total cholesterol in these patients28. However, other human clinical trials with cinnamon resulted in different findings21. Nevertheless, cinnamon supplements are sold as preventative or therapeutic supplement for various diseases including type 2 diabetes, metabolic syndrome, insulin resistance, asthma, arthritis, cancers and elevated cholesterol21. A study also showed that Cinnulin PF®, a commercial cinnamon extract (Integrity Nutraceutical, USA) improved fasting blood sugar levels, reduced systolic blood pressure and changed body fat composition in human29.
Herbal remedies that can induce browning is less studied. Recently, it is reported that berberine induced the development of brown-like adipocytes in inguinal, but not in epididymal adipose depots30. Ginsenoside Rb1 promotes browning in 3T3-L1 adipocytes31. Here, we reported that cinnamon induced browning in the subcutaneous adipocytes of the obesity mouse models. In agreement with other studies, adipocytes in the other white adipose depots such as perinephric adipose tissue and epididymal adipose tissue cannot be induced to develop brown adipocyte phenotypes. The reason underlying these observations is not known. However, genes in the visceral and subcutaneous adipose tissue of obese subjects are differentially expressed32, which may explain their different responses to the same treatment. Indeed, a large accumulation of brite cells during cold exposure can be found in the subcutaneous inguinal adipose tissue, but is rather seldom observed in epididymal or perigonadal adipose tissues23.
Interestingly, the origin of the brite cells is controversial. Nowadays, many consider that there are three types of adipocytes—brown, brite and white; while the other consider brite adipocytes are not an independent cell type but they belong to the brown adipocytes. Up to present, there is no evidence suggesting that the functions of brown and brite adipocytes are different. Furthermore, classical brown and brite adipocytes in vivo are remarkably alike regarding to both the molecular and morphological markers7. Further investigation is needed to find out the origin of the brite cells in our models.
UCP1 is an integral membrane protein unique to brown adipocyte mitochondria, it acts as proton channel to uncouple oxidative phosphorylation by dissipating the proton gradient across the inner mitochondrial membrane. Physiologically, UCP1 is the protein capable of mediating adaptive nonshivering thermogenesis in the cold and it is essential for the recruitment of adaptive adrenergic nonshivering thermogenesis. Therefore, UCP1 enables separation of lipid oxidation from ATP production and allows a higher metabolic rate and conversion of nutritional energy to heat energy. Thermogenic responses in brown fat cells are fully UCP1-dependent. Prdm16 is able to program fibroblast to become brown adipocytes, and subcutaneous implantation of Prdm16 resulted in brown adipocyte-containing fat pads33. Prdm16 enhances nuclear receptor-dependent transcription of the brown fat-specific Ucp1 gene34. Our study also showed that cinnamon increased Prdm16 expression in 3T3-L1 adipocytes and the subcutaneous adipocytes isolated from db/db mice and DIO mice. Therefore, there is a possibility that CE increases UCP1 expression by increasing Prdm16 expression.
Our study clearly demonstrated that CE treatment induced browning in the subcutaneous adipocytes isolated from obesity mouse models. Since active brown fat is virtually absent or has low thermogenesis activity in obese people, consumption of non-toxic herbal remedies that can induce browning in obese subject should be an attractive strategy to reduce obesity.
Methods
Preparation of cinnamon extracts
Cinnamon was purchased from the School of Chinese Medicine, Hong Kong Baptist University. Cinnamon extraction (100 g) was reflux-extracted twice with 70% ethanol (1:10, w/v) for 3 hr each. The extracts were filtered and evaporated under vacuum. The cinnamon extract was analyzed with reversed phase chromatography and Agilent 6540 UHD Accurate Mass Q-TOF LC/MS.
Animal handling
All animal experimentation was approved and conducted in accordance with the guidelines from Hong Kong Baptist University and was endorsed by the University Human and Animal Subject Committee and the Department of Health, the Government of Hong Kong Special Administration Region. Male mice C57BL/6 (C57) of 5 weeks old and male C57BLKS db/db mice of 5 weeks’ old were purchased form the Chinese University of Hong Kong. C57BL/6 (C57) mice were randomly selected to have either control diet (D12450J Research Diets), or high fat diet (D12762 Research Diets) which was used to induce obesity. Both diet and water were supplied ad libitum. Body weight of each mouse was recorded every week. After 3 months of dietary intervention, the diet-induced obesity (DIO) mouse models were used for the experiments.
Isolation of adipocytes
Isolation of adipocytes from subcutaneous adipose tissues (SAT), perinephric adipose tissue (PAT) and epididymal adipose tissue (EAT) were performed as described elsewhere35, 36. Briefly, SAT was dissected from the bilateral superficial subcutaneous white adipose deposits between the skin and muscle fascia just anterior to the lower segment of the hind limbs35, 36. PAT is dissected from the adipose capsule of kidney in the mice. EAT is dissected from the fat pad over the epididymis. These fat pads were digested for 1 hr at 37 °C with collagenase in Krebs-Ringer Buffer (12 mM HEPES, 121 mM NaCl, 4.9 mM KCl, 1.2 mM MgSO4, and 0.33 mM CaCl2) supplemented with 3 mM glucose and 1% fatty acid-free BSA, filtered through nylon mesh. Adipocytes were collected from the upper phase after centrifugation. The isolated adipocytes were counted using a hemocytometer.
Oil Red-O staining
Lipids are stained by Oil Red O staining. To quantify staining, Oil Red-O was extracted from the cells with isopropanol containing 4% Nonidet P-40, and optical density was then measured at a wavelength of 520 nm.
3T3-L1 preadipocyte differentiation
3T3-L1 preadipocytes were induced to differentiate to mature white adipocytes with differentiation inducing medium containing 1 mM dexamethasone, 0.5 mM isobutylmethylxanthine and 1.67 mM insulin in DMEM (Dulbecco’s Modified Eagle’s medium) with 10% FBS for 4 days before switching to DMEM with only 10% FBS and 10 µg/ml insulin for an additional 3 days37.
Western blot
Proteins were prepared as described36. Briefly, after boiling the protein for 5 min, the supernatant was separated onto 8% SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad). The membranes were then blocked with milk and were incubated with corresponding antibodies (Santa Cruz) overnight at 4 °C. Then, the membranes were washed with Tris-buffered saline (TBS) and TBS-Tween20 (TBST) followed by incubation with corresponding secondary antibodies. The signals were detected by ECL detection system (Amersham Biosciences).
Real time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen). Reverse transcription was performed with oligo-dT using MMLV reverse transcriptase (Promega, USA) according to the manufacturer’s protocol. Quantitative real time PCR was carried out by monitoring the increase in fluorescence of SYBR green with the ViiA 7 Real Time PCR System (Applied Biosystems, USA). The primer sets were synthesized by Invitrogen. Ucp1, forward: ACTGCCACACCTCCAGTCATT, reverse: CTTTGCCTCACTCAGGATTGG. Cidea, forward: TGCTCTTCTGTATCGCCCAGT, reverse: GCCGTGTTAAGGAATCTGCTG. Prdm16, forward: CAGCACGGTGAAGCCATTC, reverse: GCGTGCATCCGCTTGTG. Cpt1 forward: GCTGGAGGTGGCTTTGGT, reverse: GCTTGGCGGATGTGGTTC. Pparγ forward: GCCCTTTGGTGACTTTATGGA, reverse: GCAGCAGGTTGTCTTGGATG. Pgc1 forward: GCAACATGCTCAAGCCAAAC, reverse: TGCAGTTCCAGAGAGTTCCA. Igf forward: GGACCAGAGACCCTTTGCGGG, reverse: GGCTGCTTTTGTAGGCTTCAGTGG. Dpt forward: GGTGGCTACGGGTACCCATA, reverse: GTCAGAGCCTTCCTTCTTGC. Each sample was amplified in triplicate for quantification. Data were analyzed by relative quantitation using the ∆∆Ct method and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Cryosectioning
Fresh adipose tissues dissected from the mice were cut into slices at 8 µM and fixed with a 10% formaldehyde solution. The formaldehyde was then rinsed before immunostaining35, 38. The nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) (ThermoFisher Scientific). Images were viewed under a fluorescence microscope.
Transient reporter assay
A ucp1 −6300 bp reporter plasmid was kindly provided by Prof Anne-Marie Cassard-Doulcier (Univ Paris-Sud, Faculté de médecine Paris-Sud, France)39. The ucp1 promoter construct from base −2979 to base +76 was ligated into pGL3-basic luciferase reporter vector (Promega). HEK293 cells or 3T3-L1 cells grown in 24-well plates were co-transfected with 0.5 µg of ucp1-luc and 0.02 µg of pRLSV40 encoding Renilla luciferase (rLuc) (Promega). Control cells were co-transfected with 0.5 ug empty pGL3-basic luciferase reporter vector and 0.02 µg pRL-SV40 encoding rLuc. 48 hr-post transfection, cells were treated with cinnamon extract for 24 hr and Dual-Luciferase assay (Promega) was performed. The luciferase readings for each samples were normalized against the rLuc levels40.
cAMP direct immunoassay
Cells were grown in 24-well plates. After treatment, cAMP levels were quantified by cAMP direct immunoassay kit (BioVision) following the manufacturer’s protocol.
MitoTracker Green staining
Cells were grown in 96-well plates. After treatment, mitochondrial proteins were labeled with MitoTracker® (Molecular Probes) following the manufacturer’s protocol.
Aspartate aminotransferase activity measurement
The aspartate aminotransferase (AST) activity was measured by the aspartate aminotransferase assay kit (Abcam) following the manufacturer’s protocol.
References
Kwan, H. Y. et al. The anticancer and antiobesity effects of Mediterranean diet. Crit Rev Food Sci Nutr 57, 82–94, doi:10.1080/10408398.2013.852510 (2017).
Hondares, E. et al. Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-coactivator (PGC)-1alpha gene transcription: an autoregulatory loop controls PGC-alpha expression in adipocytes via peroxisome proliferator-activated receptor-gamma coactivation. Endocrinology 147, 2829–2838, doi:10.1210/en.2006-0070 (2006).
Enerback, S. The origins of brown adipose tissue. N Engl J Med 360, 2021–2023, doi:10.1056/NEJMcibr0809610 (2009).
Petrovic, N. et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285, 7153–7164, doi:10.1074/jbc.M109.053942 (2010).
Himms-Hagen, J. et al. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279, C670–681 (2000).
Xue, B. et al. Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Mol Cell Biol 25, 8311–8322, doi:10.1128/MCB.25.18.8311-8322.2005 (2005).
Rosenwald, M. & Wolfrum, C. The origin and definition of brite versus white and classical brown adipocytes. Adipoyctes 3, 4–9, doi:10.4161/adip.26232 (2014).
Tsukiyama-Kohar, K. et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med 7, 1128–1132, doi:10.1038/nm1001-1128 (2001).
Hansen, J. B. et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl Acad Sci USA 101, 4112–4117, doi:10.1073/pnas.0301964101 (2004).
Wang, H. et al. Liver X receptor alpha is a transcriptional repressor of the uncoupling protein 1 gene and the brown fat phenotype. Mol Cell Biol 28, 5187–2200, doi:10.1128/MCB.01479-07 (2008).
Romanatto, T. et al. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem 284, 36213–36222, doi:10.1074/jbc.M109.030874 (2009).
Hariri, M. & Ghiasvand, R. Cinnamon and Chronic Diseases. Adv Exp Med Biol. 929, 1–24, doi:10.1007/978-3-319-41342-6_1 (2016).
Cao, H., Urban, J. F. Jr. & Anderson, R. A. Cinnamon polyphenol extract affects immune responses by regulating anti- and proinflammatory and glucose transporter gene expression in mouse macrophages. J Nutr 138, 833–840 (2008).
Couturier, K. et al. Cinnamon improves insulin sensitivity and alters the body composition in an animal model of the metabolic syndrome. Archives Biochem Biophys 501, 158–161, doi:10.1016/j.abb.2010.05.032 (2010).
Sheng, X., Zhang, Y., Gong, Z., Huang, C. & Zang, Y. Q. Improved Insulin Resistance and Lipid Metabolism by Cinnamon Extract through Activation of Peroxisome Proliferator-Activated Receptors. PPAR Res. 2008, 581348–9, doi:10.1155/2008/581348 (2008).
Kim, S. H., Hyun, S. H. & Choung, S. Y. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacology 104, 119–23, doi:10.1016/j.jep.2005.08.059 (2006).
Soliman, M. M., Attia, H. F., El-Shazly, S. A. & Saleh, O. M. Biomedical Effects of Cinnamon Extract on Obesity and Diabetes Relevance in Wistar Rats. Am J Biochem Mol Biol 2, 133–145, doi:10.3923/ajbmb.2012.133.145 (2012).
Sartorius, T. et al. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS 9, e92358, doi:10.1371/journal.pone.0092358 (2014).
Kim, S. H. & Choung, S. Y. Antihyperglycemic and antihyperlipidemic action of Cinnamomi Cassiae (Cinnamon bark) extract in C57BL/Ks db/db mice. Arch Pharm Res 33, 325–333, doi:10.1007/s12272-010-0219-0 (2010).
Cao, H., Graves, D. J. & Anderson, R. A. Cinnamon extract regulates glucose transporter and insulin-signaling gene expression in mouse adipocytes. Phytomedicine 17, 1027–1032, doi:10.1016/j.phymed.2010.03.023 (2010).
Rafehi, H., Ververis, K. & Karagiannis, T. C. Controversies surrounding the clinical potential of cinnamon for the management of diabetes. Diabetes, Obesity and Metabolism 14, 493–499, doi:10.1111/j.1463-1326.2011.01538.x (2012).
Collins, S. B-adrenergic signaling networks in adipocytes for recruiting stored fat and energy expenditure. Frontiers in Endocrinology 2, 102, doi:10.3389/fendo.2011.00102 (2012).
Seale, P. et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121, 96–105, doi:10.1172/JCI44271 (2011).
Xue, Y. et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab 9, 99–109, doi:10.1016/j.cmet.2008.11.009 (2009).
Bilski, A. J., Halliday, S. E., Fitzgerald, J. D. & Wale, J. L. The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551). J Cardiovasc Pharmacol. 5, 430–437, doi:10.1097/00005344-198305000-00013 (1983).
Hong, J. W. et al. Anti-inflammatory activity of cinnamon water extract in vivo and in vitro LPS-induced models. BMC Complementary and Alternative Medicine 12, 237, doi:10.1186/1472-6882-12-237 (2012).
Qin, B. et al. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract 62, 139–148, doi:10.1016/S0168-8227(03)00173-6 (2003).
Khan, A., Safdar, M., Ali Khan, M. M., Khattak, K. N. & Anderson, R. A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 26, 3215–3218, doi:10.2337/diacare.26.12.3215 (2003).
Ziegenfuss, T. N., Hofheins, J. E., Mendel, R. W., Landis, J. & Anderson, R. A. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J Int Soc Sports Nutr. 28(3), 45–53, doi:10.1186/1550-2783-3-2-45 (2006).
Zhang, Z. et al. Berberine activates thermogenesis in white and brown adipose tissue. Nature Commun 5, 5493, doi:10.1038/ncomms6493 (2014).
Mu, Q. et al. Ginsenoside Rb1 promotes browning through regulation of PPARγ in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 466, 530–535, doi:10.1016/j.bbrc.2015.09.064 (2015).
Linder, K., Arner, P., Flores-Morales, A., Tollet-Egnell, P. & Norstedt, G. Differentially expressed genes in visceral or subcutaneous adipose tissue of obese men and women. J Lipid Res 45, 148–154, doi:10.1194/jlr.M300256-JLR200 (2004).
Kajimura, S. et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 460, 1154–1158, doi:10.1038/nature08262 (2009).
Iida, S., Chen, W., Nakadai, T., Ohkuma, Y. & Roeder, R. G. PRDM16 enhances nuclear receptor-dependent transcription of the brown fat-specific Ucp1 gene through interactions with Mediator subunit MED1. Genes Dev 29, 308–321, doi:10.1101/gad.252809.114 (2015).
Ahmadian, M. et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13, 739–748, doi:10.1016/j.cmet.2011.05.002 (2011).
Kwan, H. Y. et al. Subcutaneous adipocytes promote melanoma cell growth by activating the Akt signaling pathway: role of palmitic acid. J Biol Chem 289, 30525–30537, doi:10.1074/jbc.M114.593210 (2014).
Wang, Y. & Sul, H. S. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab 9, 287–302, doi:10.1016/j.cmet.2009.01.013 (2009).
Kwan, H. Y. et al. Lipidomic-based investigation into the regulatory effect of Schisandrin B on palmitic acid level in non-alcoholic steatotic livers. Sci Rep 5, 9114, doi:10.1038/srep09114 (2015).
del Mar Gonzalez-Barroso, M. et al. Transcriptional activation of the human ucp1 gene in a rodent cell line. Synergism of retinoids, isoproterenol, and thiazolidinedione is mediated by a multipartite response element. J Biol Chem 275, 31722–31732, doi:10.1074/jbc.M001678200 (2000).
Kwan, H. Y. et al. The anticancer effect of oridonin is mediated by fatty acid synthase suppression in human colorectal cancer cells. J Gastroenterol. 48, 182–192, doi:10.1007/s00535-012-0612-1 (2013).
Acknowledgements
This work was partially supported by Health and Medical Research Fund (HMRF/14-15/03), the Hong Kong Baptist University grants FRG2/14-15/017 and FRG2/16-17/010, Innovation and Technology Commission of Hong Kong (UIM/290) and Consun Pharmaceutical Group Limited.
Author information
Authors and Affiliations
Contributions
H.Y.K., W.F.F. and Z.L.Y. generated the idea. H.Y.K., J.W., T.S., X.J.C., B.L., X.F., C.L.C., R.H.Y.L. participated in and executed the experiments in the study. H.Y.K. wrote the manuscript A.K.W.T., Q.B.H., W.F.F. and Z.L.Y. provided critical comments.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwan, H.Y., Wu, J., Su, T. et al. Cinnamon induces browning in subcutaneous adipocytes. Sci Rep 7, 2447 (2017). https://doi.org/10.1038/s41598-017-02263-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02263-5
This article is cited by
-
An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications
Reviews in Endocrine and Metabolic Disorders (2024)
-
Cinnamaldehyde supplementation acts as an insulin mimetic compound improving glucose metabolism during adolescence, but not during adulthood, in healthy male rats
Hormones (2023)
-
Signal transducer and activator of transcription-3 drives the high-fat diet-associated prostate cancer growth
Cell Death & Disease (2019)
-
High dose of linagliptin induces thermogenic beige adipocytes in the subcutaneous white adipose tissue in diet-induced obese C57BL/6 mice
Endocrine (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.