Abstract
Aim of this study was to study the tolerability of opioid analgesia by performing a network meta-analysis (NMA) of randomized-controlled trials (RCTs) which investigated effectiveness of opioids for the management of chronic pain. Research articles reporting outcomes of RCT/s comparing 2 or more opioid analgesics for the management of chronic pain were obtained by database search. Bayesian NMAs were performed to combine direct comparisons between treatments with that of indirect simulated evidence. Study endpoints were: incidence of adverse events, incidence of constipation, trial withdrawal rate, and patient satisfaction with treatment. Outcomes were also compared with conventional meta-analyses. Thirty-two studies investigating 10 opioid drugs fulfilled the eligibility criteria. Tapentadol treatment was top-ranking owing to lower incidence of overall adverse events, constipation, and least trial withdrawal rate. Tapentadol was followed by oxycodone-naloxone combination in providing better tolerability and less trial withdrawal rate. Patient satisfaction was found to be higher with oxycodone-naloxone followed by fentanyl and tapentadol. These results were in agreement with those achieved with conventional meta-analyses. Tapentadol and oxycodone-naloxone are found to exhibit better tolerability characteristics in comparison with other opioid drugs for the management of chronic pain and are associated with low trial withdrawal rate and better patient satisfaction.
Similar content being viewed by others
Introduction
Chronic pain is defined as the pain lasting for more than 3 months and is characterized by a continuum with an inherently uncertain prognosis1, 2. Pain in its severe form significantly affects physical and mental health3, 4. Up to 70% of cancer patients suffer from etiological or iatrogenic pain5. In US alone, approximately 100 million individuals suffer from chronic pain6. At least 20% of adult population in western countries experience chronic pain at any stage of life7,8,9 and approximately 40% exhibit their dissatisfaction over adequate pain management3.
Opioid receptor agonists are widely used to treat chronic pain and are considered as the standard of care10, 11. Short-term use of opioids for chronic pain is associated with reduction in pain intensity, improved functional outcomes but with significant side effects and discontinuation rates12,13,14,15. Recently issued Center for Disease Control and Prevention (CDC) guidelines for prescribing opioids for chronic pain stress for risk assessment as a continuous process during opioid therapy16, 17.
Whereas, use of opioids receptor agonists (hereinafter opioids) for chronic cancer and non-cancer pain is reviewed18, 19, a head-to-head comparison of the risks in terms of tolerability and adherence associated with different types of opioid analgesics is not available in literature. We have carried out a systematic review of literature in order to identify RCTs which addressed this issue and performed network meta-analyses to evaluate the tolerability characteristics, trial withdrawal rate and patient satisfaction with opioid treatment for chronic pain and have compared the outcomes with conventional meta-analyses.
Method
This systematic review and meta-analysis was performed by following the Cochrane Handbook for Systematic Reviews guidelines20 and is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRSIMA) statement21.
Literature Search
Embase, Ovid SP, and PubMed/Medline electronic databases were searched for the relevant RCT reports published during the inception date of respective database and June 2016. Primary MeSH/keywords used were: Opioid-chronic pain, opioid agonist-chronic pain, opioid receptor agonist-chronic pain, buprenorphine-chronic pain, fentanyl-chronic pain, hydromorphone-chronic pain, methadone-chronic pain, morphine-chronic pain, naloxone-chronic pain, oxycodone-chronic pain, oxymorphone-chronic pain, tapentadol-chronic pain, tramadol-chronic pain. Secondary MeSH/keywords used with any of primary terms were: osteoarthritis, low back pain, cancer pain, musculoskeletal pain, neuropathic pain, and nociceptive. Tertiary MeSH/keywords used with any of primary and/or secondary combination/s were: efficacy, safety, tolerability, randomized trial, trial withdrawal and patient outcomes. Reference lists of the selected articles were also seen for additional study reports. Eligibility of the trials was assessed by two researchers independently who then unified the list of selected studies mutually. Inter-rater reliability was good (kappa = 0.94).
Eligibility Criteria
To be included in the meta-analysis a study, (a) had to be a RCT recruiting patients with chronic pain (cancer or non-cancer) to treat with an opioid drug either alone or in combination with N-methyl D-aspartic acid (NMDA) receptor antagonist for the management of chronic pain; (b) evaluated the outcomes against a comparator opioid either alone or in combination with NMDA receptor antagonist treated group; and (c) reported at least one of the following endpoints: (1) incidence of adverse events; (2) incidence of constipation; (3) trial withdrawal rate; and (4) patient satisfaction (satisfaction, convenience, improvement, or relief; patient’s excellent or good response to an appraisal regarding the effectiveness of opioid therapy using a Likert scale or a dichotomous tool). A study/arm was excluded if tested an opioid or combination of opioids against a placebo group; used an opioid in combination with a non-opioid analgesic drug other than NMDA) receptor antagonist; intervened for cancer breakthrough pain or non-cancer acute pain; or had single-arm design.
Data and Analyses
Data were extracted by two authors independently who then unified the output by attaining consensus on dubious situations. Incidence of adverse events was prespecified as the primary outcome measure, whereas the incidence of constipation, trial withdrawal rate and patient satisfaction with treatment were used as secondary endpoints. For the assessment of the quality of included studies, Cochrane Collaboration’s Tool for the Assessment of Quality of RCTs was used.
Network meta-analyses utilized Bayesian random effects Poisson regression based probabilistic modelling with Markov-Chain Monte-Carlo method. This model preserves randomized treatment comparisons within the trials. Model used total number of events and accumulated patients/patient-weeks to estimate odds ratios. Prior distribution for treatment effects was none-informative. Model involved calculations on 50,000 iterations with 20,000 iterations as burn-in. Statistical heterogeneity between the trials was estimated from between-trial variance (τ2) of the posterior distribution. Ranking probabilities were examined either with rankograms or by obtaining surface under the cumulative ranking (SUCRA; a simple numerical summary of the probabilities ranging between 100% (the best) and 0% (the worst). Statistical procedures were performed with WinBUGS software (version 1.4) by using NetMetaXL interface22.
Convergence of the iterative simulations was assessed by using the Brooks-Gelman-Rubin method by comparing within-chain and between-chain variances to calculate the potential scale reduction factor (PSRF; good convergence was considered when PSRF was close to 1) as well as by noting whether the Monte Carlo error is less than 5% of the standard deviation of the effect estimates and between-study variance. Assessment of inconsistency of direct and indirect evidence of the treatment network was performed by comparing the deviance residuals and deviance information criterion (DIC) statistics in fitted consistency and inconsistency models23. For the visual examination, the posterior mean deviance of the individual data points in the inconsistency model were plotted against their posterior mean deviance in the consistency model to identify possible loops in the network representing inconsistency.
Conventional meta-analyses were carried out with Stata software (Stata Corporation, Texas, USA) by using the numeric values of the study endpoints to achieve individual and overall odds ratios between the comparator groups. A particular opioid drug was compared with all possible comparator opioids. Between study heterogeneity was assessed by the chi-squared (chi2) and I-squared (I2) indices. A p < 0.1 and I2 > 50% were considered to indicate significant statistical heterogeneity.
Results
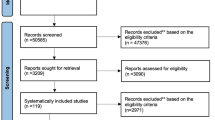
During the literature search over 3500 titles and abstracts screened of which 137 were selected for full-text research article retrieval. Observation of the eligibility criteria led to the final selection of 32 research articles24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55 reporting the outcomes of the RCTs (Fig. 1).
Important characteristics of the included trials are presented in Appendix S2. In general, trials were of moderate to high methodological quality. Appropriate methods of allocation concealment were described for 18 trials (56%). Blinding of outcome assessment was also reported for 18 trial (56%). An assessment summary is presented in Table S1.
Overall network consisted of 10 opioid treatment regimens. These were: buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone, oxycodone-naloxone, oxymorphone, tapentadol, and tramadol. Visual illustrations showing the network of treatment regimens for study endpoints are presented as Fig. (S1a–c). In all analyses, convergence of the iterative simulations was reached.
No significant inconsistency between direct and indirect evidence was observed for any of the study endpoints. Figure S2a–d show the plotting of posterior mean deviance of the individual data points in the inconsistency model against their posterior mean deviance in the consistency model for all four endpoints.
Incidence of Adverse Events
Conventional meta-analysis revealed that tapentadol treatment was associated with significantly (p < 0.00001) less incidence of adverse events (AEs) in comparison with other opioids. There was no significant difference in the incidence of AEs between oxycodone-naloxone, morphine, fentanyl, oxycodone, or tramadol and their comparator opioids (Table 1 and Figure S3a).
In NMA of the incidence of AEs, total number of interventions were 9 which were carried out in 25 studies involving 280,292 patient-weeks. Network, intervention, and direct comparison characteristics of this NMA are given in Tables S2–4. Oxycodone was the most investigated opioid (17 studies; compared with 9 different opioids) followed by morphine and hydromorphone (7 studies each). This NMA revealed that least incidence of AEs was associated with tapentadol treatment followed by oxycodone-naloxone, tramadol and oxycodone (Table 2). Probability for ranking top among the opioids was 0.698 for tapentadol and the probability for ranking second was 0.26 for oxycodone-naloxone combination (Figure S4a). The SUCRA probabilities for various opioids were: tapentadol (0.945), oxycodone-naloxone (0.652), tramadol (0.651), oxycodone (0.507), fentanyl (0.467), morphine (0.437), hydromorphone (0.420), buprenorphine (0.368), and oxymorphone (0.054) (Figure S5a).
Incidence of constipation
Conventional meta-analysis revealed that tapentadol (p < 0.00001), morphine (p = 0.007), fentanyl (p = 0.015), and oxycodone-naloxone (p = 0.042) treatment was associated with significantly less incidence of constipation in comparison with their comparator opioids. There was no significant difference in the incidence between oxycodone or tramadol and their comparator opioids (Table 1 and Figure S3b).
In the NMA, total number of interventions were 9 which were carried out in 25 studies in which 2831 events occurred in 286,864 patient-weeks. Network, intervention, and direct comparison characteristics of this NMA are given in Tables S2–4. Oxycodone was the most investigated opioid (17 studies; compared with 7 different opioids) followed by oxycodone-naloxone (7 studies) and tapentadol (6 studies). This NMA revealed that least incidence of constipation was associated with tapentadol treatment followed by oxycodone-naloxone, fentanyl and tramadol (Table 3). Probability for ranking top among the opioids was 0.516 for tapentadol whereas the probability for ranking second best was 0.4464 for oxycodone-naloxone combination (Figure S4b). SUCRA probabilities for various opioids were: tapentadol (0.913), oxycodone-naloxone (0.829), fentanyl (0.641), tramadol (0.598), morphine (0.389), oxycodone (0.373), buprenorphine (0.364), oxymorphone (0.268) and hydromorphone (0.126) (Figure S5b).
Trail withdrawal rate
Conventional meta-analysis revealed that tapentadol (p = 0.006) and oxycodone-naloxone (p = 0.057) treatment had significantly less odds of trial withdrawal rate in comparison with other opioids. There was no significant difference in the odds ratios between oxycodone, fentanyl, hydromorphone, buprenorphine and their comparator opioids (Table 1 and Figure S3c).
In NMA, total number of interventions were 10 which were carried out in 27 studies in 12,207 patients of which 4822 withdrew. Network, intervention, and direct comparison characteristics of this NMA are given in Tables S2–4. Oxycodone was the most investigated opioid (18 studies; compared with 7 different opioids) followed by morphine (8 studies), oxycodone-naloxone and tapentadol (7 studies each). This NMA revealed that least trial withdrawal was associated with tapentadol treatment followed by oxycodone-naloxone, fentanyl and hydromorphone (Table 4). Probability for ranking top among the opioids was 0.76 for tapentadol whereas the probability for ranking second best was 0.224 for oxycodone-naloxone combination (Figure S4c). SUCRA probabilities for various opioids were: tapentadol (0.961), oxycodone-naloxone (0.669), fentanyl (0.661), hydromorphone (0.543), oxycodone (0.536), buprenorphine (0.406), methadone (0.399), tramadol (0.338), morphine (0.314), and oxymorphone (0.173) (Figure S5c).
Patient satisfaction
Conventional meta-analysis revealed that oxycodone-naloxone treatment had significantly better patient satisfaction rate in comparison with other opioids (p < 0.0001). There was no significant difference in the patient satisfaction rate between either of tapentadol, oxycodone, fentanyl, hydromorphone, or buprenorphine and their comparator opioids (Table 1 and Figure S3d).
In NMA, total number of interventions were 8 which were carried out in 15 studies in 6,560 patients of which 3918 were satisfied with the treatment. Network, intervention, and direct comparison characteristics of this NMA are given in Tables S2–4. Oxycodone was the most investigated opioid (10 studies; compared with 6 different opioids) followed by morphine (5 studies) and tapentadol (4 studies each). This NMA revealed that highest patient satisfaction was associated with oxycodone-naloxone treatment, followed by fentanyl and tapentadol (Table 5). Probability for ranking top among the opioids was 0.89 for oxycodone-naloxone whereas the probability for ranking second highest was 0.743 for fentanyl (Figure S4d). SUCRA probabilities for various opioids were: oxycodone-naloxone (0.984), fentanyl (0.821), tapentadol (0.631), oxycodone (0.396), buprenorphine (0.371), morphine (0.309), hydromorphone (0.294), and tramadol (0.194) (Figure S5d).
Forest graphs of all NMAs are given in supporting information as Figure S5a–d.
Discussion
Tapentadol treatment to patients with chronic pain conditions has been found to be associated with least incidence of overall adverse events as well as constipation and trial withdrawal rate in this mixed-treatment meta-analysis. Oxycodone-naloxone combination also exhibited good tolerability with lesser trial withdrawal rate. Patient satisfaction was found to be higher with oxycodone-naloxone followed by fentanyl and tapentadol. These results were in agreement with those achieved by the conventional meta-analyses.
Recently issued CDC guidelines for prescribing opioids for chronic pain recommend that opioids should be prescribed after risk assessment, and if risks outweigh benefits, a discontinuation strategy should be planned in advance. It is also recommended that lowest effective dosage should be prescribed earlier and then a re-assessment be made when considering increasing dosage equivalent to 50 mg/day morphine while avoiding concurrent opioids and benzodiazepines. A re-assessment of benefits and harms of continued opioid therapy should be made in less than every third month16, 17.
However, optimal and judicious use of opioids is still far away from the desired situation. A number of potentially serious adverse effects and aberrant behaviors such as opioid abuse, addiction, and diversion make it difficult for physicians to prescribe opioids liberally56. Therefore, not only the under-treatment of pain is recognized as a major problem for patients which has societal implications6 but also the opioids are underutilized drugs for chronic pain especially in elderly57 due to physician’s concerns about the uncertain efficacy, untoward side effects and addiction58, 59.
Benefits of opioids are generally limited by their tolerability which is reported to cause up to 31% discontinuation rate14, 15. Constipation, nausea, vomiting, dizziness, somnolence, pruritus, fatigue and anorexia are commonly observed in opioid treated patients. A review of 40 studies found that 25% of the opioid treated patients discontinued treatment due to an adverse event and in a subset of studies there was no significant difference in the incidence of opioid-related AEs in individuals of over or under 65 years of age without significant comorbidities60.
Tapentadol is a centrally acting synthetic opioid which not only act as opioid receptor agonist but also as a norepinephrine reuptake inhibitor61, 62. The controlled release formulation of tapentadol can provide analgesia for 12 hours. For chronic pain conditions, it is suitable because its µ-receptor activation is a secondary mechanism and therefore a relatively low degree of µ-receptor activation, in comparison with potent opioids, causes a slower onset of tolerance62. Review of head-to-head trials of tapentadol with other opioids such as oxycodone controlled release and oxycodone-naloxone has also shown that tapentadol is at least comparable in providing pain relief in moderate/severe chronic nociceptive as well as neuropathic pain conditions besides improving health status and quality of life63, 64. On the other hand, incidences of common opioid-related adverse events such as nausea and/or vomiting, and constipation as well as discontinuation rate are found to be significantly lower in the tapentadol treated groups in comparison with other opioid treated groups64.
Oxycodone-naloxone combination was developed basically to reduce the incidence of opioid-induced constipation. In a typical tablet, naloxone is added as an opioid receptor antagonist to prevent oxycodone activity in the gut while its prolonged release and extensive metabolism in the liver leads to low bioavailability (2%) which remains insufficient to antagonize oxycodone action centrally48, 65. In the present study, oxycodone-naloxone combination has been found to attain second rank after tapentadol with regards to its benefits in low incidence of adverse events, constipation and trial withdrawal. However, in patient satisfaction, it attained first rank. Beyond the present study too, it has been reported that oxycodone-naloxone combination is safe and well tolerable and is superior in effectiveness to oxycodone66,67,68.
Opioids therapy for chronic pain is challenging due to clinical, adverse, and end-organ effects as well as due to their potential interactions with other medications. Although, tolerability is a major issue with opioid therapy, nevertheless, opioid analgesia provides good relief with manageable adverse effects to selected patients suffering from chronic pain. Centrally acting analgesics with a dual mechanism of action offer therapies with improved tolerability. Tapentadol and oxycodone-naloxone appear to advance opioid therapy in this direction. However, a better patient-centered care in opioid therapy for chronic pain is more important keeping in view the high inter-individual variability in responsiveness to opioids and an earlier start of medication can reduce the progression of pain.
There can be several factors to affect the outcomes of this mixed-treatment meta-analysis including route and mode of drug administration, ethnicity and geographical distribution of patients, treatment duration, and history of opioid use. Modes of drug administration in the present study varied across the included studies and for some modes as well as routes of administration, less data (studies) were available to classify each mode as a separate regimen, therefore, impact of mode of administration will need to be further studied in larger datasets. Although, recent trend is towards more concentrated dosage and controlled-release preparations with oral and transdermal opioid formulations but other factors may also play a role in determining the suitability of a drug delivery system e.g. the patient’s preferences, delivery efficiency, potential complications of a system, and financial cost of formulation or device69.
Ethnic differences in response to pain management are reported by many researchers. Besides, inconsistencies in pain medication prescription, different ethnic groups were reported to use pain medications in different quantities even when equal access e.g. with patient-controlled analgesia was ensured70. History of opioid use may be associated with inadequacy of opioid treatment and or efficacy and may have potential for consequences such as illicit drug use, misuse of prescription drugs, psychiatric distress, etc.71. To evaluate the effects of these factors on overall outcomes, a relatively larger dataset is required that can be used to identify the prognostic factors to help decision-making in the clinical use of opioids and in selecting subgroup of patients for a particular opioid therapy.
Some other limitations are also needed to be account for while interpreting the outcomes observed herein. Number of studies investigating some types of opioids were much less, especially for methadone and oxymorphone. Many of the included studies also involved open-label designs which might had introduced a risk of bias because patients or investigators might had taken/prescribed concomitant treatments to enhance efficacy or to improve tolerability based on their knowledge and beliefs of treatment allocation66. However, it has been opined that potential benefits of an open-label design may outweigh those of a blinded design in this population43 and not blinding the study could be sometimes intentionally directed by the need to mimic a daily clinical trite where therapeutic flexibility is needed11. This may also be attributed to the difficulties in performing controlled trials in this population. Additionally, trial duration was relatively smaller in some studies which necessitates the assessment of trial duration on the overall effectiveness.
Conclusion
Tapentadol treatment in chronic pain conditions is associated with least incidence of overall adverse events and constipation, as well as with least trial withdrawal rate. Oxycodone-naloxone combination also share superiority with tapentadol in providing better tolerability and less trial withdrawal rate. Patient satisfaction was found to be higher with oxycodone-naloxone followed by fentanyl and tapentadol. Conventional meta-analyses also endorsed these findings.
References
Merskey, H. & Bogduk, N. Classification of chronic pain: Descriptions of chronic pain syndromes and definitions of pain terms (2nd edition) 1–16 (IASP Press, 1994).
Pergolizzi, J. et al. The chronic pain conundrum: should we CHANGE from relying on past history to assessing prognostic factors? Curr. Med. Res. Opin. 28, 249–56, doi:10.1185/03007995.2011.651525 (2012).
Breivik, H., Collett, B., Ventafridda, V., Cohen, R. & Gallacher, D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain. 10, 287–333, doi:10.1016/j.ejpain.2005.06.009 (2006).
Kroenke, K. et al. Association between anxiety, health related quality of life and functional impairment in primary care patients with chronic pain. Gen. Hosp. Psychiatry. 35, 359–65, doi:10.1016/j.genhosppsych.2013.03.020 (2013).
Brennan, F., Carr, D. B. & Cousins, M. Pain management: a fundamental human right. Anesth. Analg. 105, 205–221, doi:10.1213/01.ane.0000268145.52345.55 (2007).
The American Academy of Pain Medicine. AAPM Facts and Figures on Pain. http://www.painmed.org/patientcenter/facts_on_pain.aspx (last accessed March 3, 2017).
Elliott, A. M., Smith, B. H., Penny, K. I., Smith, W. C. M. & Chambers, W. A. The epidemiology of chronic pain in the community. Lancet. 354, 1248–52, doi:10.1016/S0140-6736(99)03057-3 (1999).
Gureje, O., Simon, G. E. & Von Korff, M. A cross-national study of the course of persistent pain in primary care. Pain. 95, 195–200, doi:10.1016/S0304-3959(00)00483-8 (2001).
Sjoegren, P., Ekholm, O., Peuckmann, V. & Groenbaek, M. Epidemiology of chronic pain in Denmark: an update. Eur. J. Pain. 13, 287–92, doi:10.1016/j.ejpain.2008.04.007 (2009).
Boudreau, D. et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol. Drug Saf. 18, 1166–75, doi:10.1002/pds.v18:12 (2009).
Nuckols, T. K. et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann. Intern. Med. 160, 38–47, doi:10.7326/0003-4819-160-1-201401070-00732 (2014).
Kalso, E., Edwards, J. E., Moore, A. & McQuay, H. J. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 112, 372–380, doi:10.1016/j.pain.2004.09.019 (2004).
Eisenberg, E., McNichol, E. D. & Carr, D. M. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin. JAMA. 293, 3043–3052, doi:10.1001/jama.293.24.3043 (2005).
Furlan, A. D., Sandoval, J. A., Mailis-Gagnon, A. & Tunks, E. Review: Opioids are more effective than placebo but not other analgesics for chronic noncancer pain. CMAJ. 174, 1589–1594, doi:10.1503/cmaj.051528 (2006).
Avouac, J., Gossec, L. & Dougados, M. Efficacy and safety of opioids for osteoarthritis: A meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 15, 957–965, doi:10.1016/j.joca.2007.02.006 (2007).
Dowell, D., Haegerich, T. M. & Chou, R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 65, 1–49, doi:10.15585/mmwr.rr6501e1 (2016).
Dowell, D., Haegerich, T. M. & Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016. JAMA. 315, 1624–45, doi:10.1001/jama.2016.1464 (2016).
Noble, M. et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst. Rev. 1, CD006605, doi:10.1002/14651858.CD006605.pub2 (2010).
Chou, R. et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 162, 276–86, doi:10.7326/M14-2559 (2015).
Higgins, J. P. T., Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.3. http://handbook.cochrane.org/.
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G., PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–41, doi:10.1016/j.ijsu.2010.02.007 (2010).
Brown, S. et al. A Microsoft-Excel-based tool for running and critically appraising network meta-analyses—an overview and application of NetMetaXL. Systematic Rev. 3, 110, doi:10.1186/2046-4053-3-110 (2014).
Dias, S. et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med. Decis. Making. 33, 641–656, doi:10.1177/0272989X12455847 (2013).
Ahmedzai, S. H. et al. A randomized, double-blind, active-controlled, double-dummy, parallel-group study to determine the safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate/severe, chronic cancer pain. Palliat. Med. 26, 50–60, doi:10.1177/0269216311418869 (2012).
Allan, L. et al. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ. 322, 1154–58, doi:10.1136/bmj.322.7295.1154 (2001).
Allan, L., Richarz, U., Simpson, K. & Slappendel, R. Transdermal fentanyl versus sustained release oral morphine in strong-opioid naïve patients with chronic low back pain. Spine (Phila Pa 1976). 30, 2484–90, doi:10.1097/01.brs.0000186860.23078.a8 (2005).
Baron, R. et al. Tolerability, safety, and quality of life with tapentadol prolonged release (PR) compared with oxycodone/naloxone PR in patients with severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, phase 3b/4 trial. Pain Pract. 16, 600–9, doi:10.1111/papr.2016.16.issue-5 (2016).
Binsfeld, H., Szczepanski, L., Waechter, S., Richarz, U. & Sabatowski, R. A randomized study to demonstrate noninferiority of once-daily OROS(®) hydromorphone with twice-daily sustained-release oxycodone for moderate to severe chronic noncancer pain. Pain Pract. 10, 404–15, doi:10.1111/j.1533-2500.2009.00342.x (2010).
Etropolski, M., Kelly, K., Okamoto, A. & Rauschkolb, C. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Adv. Ther. 28, 401–17, doi:10.1007/s12325-011-0018-0 (2011).
Etropolski, M. et al. Safety and tolerability of tapentadol extended release in moderate to severe chronic osteoarthritis or low back pain management: pooled analysis of randomized controlled trials. Adv. Ther. 31, 604–20, doi:10.1007/s12325-014-0128-6 (2014).
Hale, M., Tudor, I. C., Khanna, S. & Thipphawong, J. Efficacy and tolerability of once-daily OROS hydromorphone and twice-daily extended-release oxycodone in patients with chronic, moderate to severe osteoarthritis pain: results of a 6-week, randomized, open-label, noninferiority analysis. Clin. Ther. 29, 874–88, doi:10.1016/j.clinthera.2007.05.016 (2007).
Hale, M., Upmalis, D., Okamoto, A., Lange, C. & Rauschkolb, C. Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, double-blind study. Curr. Med. Res. Opin. 25, 1095–1104, doi:10.1185/03007990902816970 (2009).
Hanna, M., Thipphawong, J. & 118 Study Group. A randomized, double-blind comparison of OROS(R) hydromorphone and controlled-release morphine for the control of chronic cancer pain. BMC Palliat. Care. 7, 17, doi:10.1186/1472-684X-7-17 (2008).
Hanna, M., Tuca, A. & Thipphawong, J. An open-label, 1-year extension study of the long-term safety and efficacy of once-daily OROS(R) hydromorphone in patients with chronic cancer pain. BMC Palliat. Care. 8, 14, doi:10.1186/1472-684X-8-14 (2009).
Hartrick, C., Van Hove, I., Stegmann, J. U., Oh, C. & Upmalis, D. Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end-stage joint disease: a 10-day, phase III, randomized, double-blind, active- and placebo-controlled study. Clin. Ther. 31, 260–71, doi:10.1016/j.clinthera.2009.02.009 (2009).
Imanaka, K. et al. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Curr. Med. Res. Opin. 29, 1399–409, doi:10.1185/03007995.2013.831816 (2013).
Karlsson, M. & Berggren, A. C. Efficacy and safety of low-dose transdermal buprenorphine patches (5, 10, and 20 microg/h) versus prolonged-release tramadol tablets (75, 100, 150, and 200 mg) in patients with chronic osteoarthritis pain: a 12-week, randomized, open-label, controlled, parallel-group noninferiority study. Clin. Ther. 31, 503–13, doi:10.1016/j.clinthera.2009.03.001 (2009).
Kavanagh, S. et al. Pain relief and tolerability balance of immediate release tapentadol or oxycodone treatment for patients with moderate to severe osteoarthritis or low back pain. Pain Med. 13, 1110–20, doi:10.1111/j.1526-4637.2012.01454.x (2012).
Lange, B. et al. Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain. Adv. Ther. 27, 381–99, doi:10.1007/s12325-010-0036-3 (2010).
Leng, X. et al. Effectiveness and safety of transdermal buprenorphine versus sustained-release tramadol in patients with moderate to severe musculoskeletal pain: an 8-week, randomized, double-blind, double-dummy, multicenter, active-controlled, noninferiority study. Clin. J. Pain. 31, 612–20, doi:10.1097/AJP.0000000000000144 (2015).
Löwenstein, O. et al. Efficacy and safety of combined prolonged-release oxycodone and naloxone in the management of moderate/severe chronic non-malignant pain: results of a prospectively designed pooled analysis of two randomised, double-blind clinical trials. BMC Clin. Pharmacol. 10, 12, doi:10.1186/1472-6904-10-12 (2010).
Matsumoto, A. K., Babul, N. & Ahdieh, H. Oxymorphone extended-release tablets relieve moderate to severe pain and improve physical function in osteoarthritis: results of a randomized, double-blind, placebo- and active-controlled phase III trial. Pain Med. 6, 357–66, doi:10.1111/j.1526-4637.2005.00057.x (2005).
Mercadante, S. et al. Sustained-release oral morphine versus transdermal fentanyl and oral methadone in cancer pain management. Eur. J. Pain. 12, 1040–6, doi:10.1016/j.ejpain.2008.01.013 (2008).
Neumann, A. M. et al. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexistent opioid addiction. J. Addict. Dis. 32, 68–78, doi:10.1080/10550887.2012.759872 (2013).
Nicholson, B., Ross, E., Sasaki, J. & Weil, A. Randomized trial comparing polymer-coated extended-release morphine sulfate to controlled-release oxycodone HCl in moderate to severe nonmalignant pain. Curr. Med. Res. Opin. 22, 1503–14, doi:10.1185/030079906X115603 (2006).
Richarz, U., Waechter, S., Sabatowski, R., Szczepanski, L. & Binsfeld, H. Sustained safety and efficacy of once-daily hydromorphone extended-release (OROS® hydromorphone ER) compared with twice-daily oxycodone controlled-release over 52 weeks in patients with moderate to severe chronic noncancer pain. Pain Pract. 13, 30–40, doi:10.1111/papr.2012.13.issue-1 (2013).
Steiner, D., Munera, C., Hale, M., Ripa, S. & Landau, C. Efficacy and safety of buprenorphine transdermal system (BTDS) for chronic moderate to severe low back pain: a randomized, double-blind study. J. Pain. 12, 1163–73, doi:10.1016/j.jpain.2011.06.003 (2011).
Ueberall, M. A. & Mueller-Schwefe, G. H. Safety and efficacy of oxycodone/naloxone vs. oxycodone vs. morphine for the treatment of chronic low back pain: results of a 12 week prospective, randomized, open-label blinded endpoint streamlined study with prolonged-release preparations. Curr. Med. Res. Opin. 31, 1413–29, doi:10.1185/03007995.2015.1047747 (2015).
Ueberall, M. A., Eberhardt, A. & Mueller-Schwefe, G. H. Quality of life under oxycodone/naloxone, oxycodone, or morphine treatment for chronic low back pain in routine clinical practice. Int. J. Gen. Med. 9, 39–51, doi:10.2147/IJGM.S94685 (2016).
van Seventer, R., Smit, J. M., Schipper, R. M., Wicks, M. A. & Zuurmond, W. W. Comparison of TTS-fentanyl with sustained-release oral morphine in the treatment of patients not using opioids for mild-to-moderate pain. Curr. Med. Res. Opin. 19, 457–69, doi:10.1185/030079903125002045 (2003).
Vondrackova, D. et al. Analgesic efficacy and safety of oxycodone in combination with naloxone as prolonged release tablets in patients with moderate to severe chronic pain. J. Pain. 9, 1144–54, doi:10.1016/j.jpain.2008.06.014 (2008).
Wallace, M. S. & Thipphawong, J. Clinical trial results with OROS® hydromorphone. J. Pain Sympt. Manage. 33, S25–32, doi:10.1016/j.jpainsymman.2006.09.008 (2007).
Webster, L. R. et al. Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J. Pain. 7, 937–46, doi:10.1016/j.jpain.2006.05.005 (2006).
Webster, L. R., Slevin, K. A., Narayana, A., Earl, C. Q. & Yang, R. Fentanyl buccal tablet compared with immediate-release oxycodone for the management of breakthrough pain in opioid-tolerant patients with chronic cancer and noncancer pain: a randomized, double-blind, crossover study followed by a 12-week open-label phase to evaluate patient outcomes. Pain Med. 14, 1332–45, doi:10.1111/pme.12184 (2013).
Yu, S. et al. Safety and efficacy of once-daily hydromorphone extended-release versus twice-daily oxycodone hydrochloride controlled-release in Chinese patients with cancer pain: a phase 3, randomized, double-blind, multicenter study. J Pain. 15, 835–44, doi:10.1016/j.jpain.2014.04.008 (2014).
Volkow, N. D. & McLellan, A. T. Opioid abuse in chronic pain – Misconceptions and mitigation strategies. N. Engl. J. Med. 374, 1253–63, doi:10.1056/NEJMra1507771 (2016).
Auret, K. & Schug, S. A. Underutilization of opioids in elderly patients with chronic pain: Approaches to correcting the problem. Drugs Aging. 22, 641–654, doi:10.2165/00002512-200522080-00002 (2005).
Upshur, C. C., Luckmann, R. S. & Savageau, J. A. Primary care provider concerns about management of chronic pain in community clinic populations. J. Gen. Intern. Med. 21, 652–5, doi:10.1111/j.1525-1497.2006.00412.x (2006).
Lin, J. J., Alfandre, D. & Moore, C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin. J. Pain. 23, 799–803, doi:10.1097/AJP.0b013e3181565cf1 (2007).
Papaleontiou, M. et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 58, 1353–69, doi:10.1111/j.1532-5415.2010.02920.x (2010).
Tzschentke, T. M. et al. Tapentadol hydrochloride: a next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today (Barc). 45, 483–96 (2009).
Vadivelu, N., Timchenko, A., Huang, Y. & Sinatra, R. Tapentadol extended-release for treatment of chronic pain: a review. J. Pain Res. 4, 211–8, doi:10.2147/JPR.S14842 (2011).
Afilalo, M. & Morlion, B. Efficacy of tapentadol ER for managing moderate to severe chronic pain. Pain Physician. 16, 27–40 (2013).
Baron. R. et al. Tapentadol prolonged release for chronic pain: a review of clinical trials and 5 years of routine clinical practice data. Pain Pract., doi:10.1111/papr.12515 (2016).
Mercadante, S. & Giarratano, A. Combined oral prolonged-release oxycodone and naloxone in chronic pain management. Expert Opin. Investig. Drugs. 22, 161–6, doi:10.1517/13543784.2013.752460 (2013).
Sandner-Kiesling, A. et al. Long-term efficacy and safety of combined prolonged-release oxycodone and naloxone in the management of non-cancer chronic pain. Int. J. Clin. Pract. 64, 1–12, doi:10.1111/ijcp.2010.64.issue-6 (2010).
Schutter, U., Grunert, S., Meyer, C., Schmidt, T. & Nolte, T. Innovative pain therapy with a fixed combination of prolonged-release oxycodone/naloxone: a large observational study under conditions of daily practice. Cur. Med. Res. Opin. 26, 1377–87, doi:10.1185/03007991003787318 (2010).
Blagden, M., Hafer, J., Duerr, H., Hopp, M. & Bosse, B. Long-term evaluation of combined prolonged-release oxycodone and naloxone in patients with moderate-to-severe chronic pain: pooled analysis of extension phases of two phase III trials. Neurogastroenterol. Motil. 26, 1792–801, doi:10.1111/nmo.2014.26.issue-12 (2014).
Martin, C. et al. Controlled-release of opioids for improved pain management. Materials Today. 19, 491–502, doi:10.1016/j.mattod.2016.01.016 (2016).
Campbel, C. M. & Edwards, R. R. Ethnic differences in pain and pain management. Pain Manage. 2, 219–230, doi:10.2217/pmt.12.7 (2012).
Rosenblum, A., Marsch, L. A., Joseph, H. & Portenoy, R. K. Opioids and the Treatment of Chronic Pain: Controversies, Current Status, and Future Directions. Exp. Clin. Psychopharmacol. 16, 405–416, doi:10.1037/a0013628 (2008).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 31300137). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: R.Z.H. Performed literature search and extracted data: Z.D.M., S.R.W. and L.H.Y. Analyzed the data: C.L., R.Z.H. and J.Y. Drafted the manuscript: Z.D.M., M.A.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meng, Z., Yu, J., Acuff, M. et al. Tolerability of Opioid Analgesia for Chronic Pain: A Network Meta-Analysis. Sci Rep 7, 1995 (2017). https://doi.org/10.1038/s41598-017-02209-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02209-x
This article is cited by
-
A systematic review and network meta-analysis of pharmaceutical interventions used to manage chronic pain
Scientific Reports (2024)
-
Tapentadol versus klassische WHO-III-Opioide bei chronischen Rückenschmerzen
MMW - Fortschritte der Medizin (2022)
-
Oxycodone/Naloxone Prolonged Release: A Review in Severe Chronic Pain
Clinical Drug Investigation (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.