Abstract
Prognostic biomarkers for the pancreatic neuroendocrine tumors are needed. Proteomic study on insulinoma has been rarely reported. We identified the differential expression of proteins between insulinoma and their paired tissues by proteomic analysis, and evaluated the prognostic significance of specific proteins in pancreatic neuroendocrine tumors including insulinoma. The differential expression of select proteins was validated in more than 300 tumors using immunohistochemical staining and western blot. Methylation of UCH-L1 promoter in tumors was examined by methylation specific PCR and validated by sequencing. The concurrent expression of UCH-L1 and α-internexin was correlated with the prognosis in 2 independent collectives of patients with tumors. Sixty-two and 219 proteins were significantly down-regulated and up-regulated in insulinomas, respectively. Demethylation of UCH-L1 promoter was associated with UCH-L1 expression in tumors (p = 0.002). The concurrent expression of UCH-L1 and α-internexin in pancreatic neuroendocrine tumors was significantly associated with better overall survival and disease-free survival in the combination of both cohorts (log rank p = 3.90 × 10−4 and p = 3.75 × 10−5, respectively) and in each of cohorts. The prognostic value of both proteins was also validated in patients with stage II and III tumors (p = 0.017 and p = 0.006, respectively). The proteins UCH-L1 and α-internexin could be independent prognostic biomarkers of pancreatic neuroendocrine tumors.
Similar content being viewed by others
Introduction
Pancreatic neuroendocrine tumors (PNETs) are uncommon with an incidence of 1–10 /million/year1,2,3,4,5,6,7. The incidence and prevalence of PNETs have increased over the past 30 years, not only in western countries but also in Asia1, 3, 8,9,10,11,12,13,14,15. Insulinoma is a main type of PNETs and causes hyperinsulinemic hypoglycemia3, 16, 17. The molecular alterations underlying insulinoma tumorigenesis are not well addressed5, 17. Islet β-cell specific ablation of the MEN1 gene causes insulinoma in mice18; while frequent loss of heterogeneity (LOH) of MEN1 gene is found in human insulinomas19, MEN1 gene mutations are rarely found20, 21. Insights into the molecular alterations in sporadic insulinoma are crucial not only for deciphering tumorigenesis in insulinoma and other PNETs but also for discovering biomarkers of prognosis. The clinicopathological criteria for PNETs prognosis are improved considerably by the ENETS and WHO staging and grading systems. PNETs tend to relapse after resection, even if the tumors originally had lower stage and lower grade. Thus, molecular biomarkers are required for predicting relapse and prognosis of PNETs.
Recently, a number of molecular profiling studies on PNETs have been reported21,22,23,24,25,26,27,28; these studies revealed somatic mutation of some genes and abnormal expression of miRNA and message RNA in PNETs. These molecular alterations might play roles in the tumorigenesis of PNETs, and could be correlated with the prognosis of PNETs. However, proteomic study on sporadic insulinoma has been rarely reported.
We previously demonstrated that α-internexin was extensively expressed in PNETs and could be a novel prognostic biomarker for overall survival29. However, α-internexin could not be used as a marker for disease-free survival29. As tumor recurrence is the predominant cause of death in PNET, if molecular biomarkers could be identified to predict the relapse or the aggressive behaviours of PNET in an individual patient before the recurrence happens, the patient would benefit from more stringent surveillance and more aggressive antitumor therapy.
Therefore, the aims of the present study were to investigate the differential expression of proteins between sporadic insulinoma and paired pancreas by proteomic analysis and to examine if some proteins could be molecular prognostic biomarkers for insulinomas and other PNETs.
Results
Clinicopathological Characteristics of All Patients and Tumors
All PNETs studied were well-differentiated. The clinicopathological features of each tumor/patient were listed in detail in Supplementary Table S1, and summarized in Table 1. Of 306 patients, 103 (33.6%) underwent enucleations, 65 (21.2%) had either head, body or tail resection, 59 (19.3%) had tail resection and splenectomy and 56 (18.3%) underwent Whipple procedure; the surgical procedures were not well documented in 23 patients (7.5%). Two hundred and forty-seven patients were followed up (80.7%) and median time of follow-up was 68 months.
Differential Expression of Proteins in Insulinomas and Bioinformatic Analysis
Using quantitative proteomics approach, we assessed the global changes of the proteome by comparing the mean of relative abundance of proteins identified in 4 insulinomas with that of 4 paired pancreatic tissue samples. In this study, 5279 proteins were identified across all 8 samples, 3476 proteins were identified with more than two unique peptides (Supplementary Table S2). Quantitative analysis of the changes of the 3476 proteins between tumors and paired tissues revealed that 2021 proteins including housekeeping ones such as ribosomal proteins, GAPDH, tubulin were similarly expressed in both tumoral and paired tissues, while 1455 proteins were differentially expressed in tumor tissue and paired pancreatic tissue (Fig. 1). We identified that 219 of 1455 proteins were significantly up-regulated or expressed only in tumor tissues and 62 proteins were significantly down-regulated in tumor tissue or expressed only in paired pancreatic tissue. Among the 219 proteins which were up-regulated in tumor tissues, UCH-L1 was one of the most highly expressed proteins, the tumor/para-tumor ratio being 55.4, P = 0.016 (Fig. 1, the orange dot).
Volcano plot showing fold change of identified proteins between tumors and paired pancreatic specimens. The x-axis represents fold-changes of PNET versus para-tumor tissue (log2 of fold change), and the y-axis represents the statistical significance p-value (−log10 of p-value, n = 4). The orange dots represent UCH-L1.
Bioinformatic analyses revealed that 24%, 15% and 11.6% of proteins were from cytoplasm, membrane and nucleus, respectively (Supplementary Fig. S1). These proteins were associated with a number of signal pathways, such as cytoskeleton signaling, protein ubiquitination pathway, VEGF signaling, mTOR and PI3K/AKT pathway (Supplementary Table S3).
Validation of the Expressions of UCH-L1 and Other proteins in Subgroups of PNETs
Differential expression of UCH-L1, MAP1B, MAP2, VCAN, CDK4 and PDX-1 was verified in more than 40 PNETs, including insulinomas and non-insulinomas by IHC but the expression of CaSR was only validated in 29 insulinomas (Supplementary Table S4 and Fig. 2). Furthermore, expression of protein UCH-L1, CDK4 and CaSR was confirmed by Western blot in 10 samples (Fig. 2b, Supplementary Table S4). By IHC, these proteins were extensively expressed in PNETs but were either not expressed or expressed at reduced levels in peritumoral tissues (Fig. 2a, Supplementary Table S4). PDX-1 was only expressed in 36 of 41 insulinomas. CDK4 was expressed in more than 98% of PNETs and 83% of peritumoral specimens (Fig. 2b, Supplementary Table S4). The biological and functional features of proteins UCH-L1, MAP1B, MAP2, VCAN, PDX-1, CDK4 and α-internexin were summarized in Table 2. Three of these proteins (MAP1B, MAP2 and α-internexin) are associated with cytoskeleton organization and PDX-1 is crucial for insulin expression30. In western blot analysis, intensity of protein band in each sample was quantified and listed in Table 3. For example, the signal of UCH-L1 in tumor # 5 (UCH-L1/β-actin = 0.342) was 8.3-fold stronger than that in its para-tumor tissue #5 N (UCH-L1/β-actin = 0.041) (Fig. 2b). A statistic analysis on ratio of UCH-L1/β-actin was performed. The ratio of UCH-L1/β-actin in tumor specimens was significantly higher than that in paired tissue or normal pancreatic specimens (p = 0.0095, Mann-Whitney U test, Table 3). The results from western blot were comparable to those from IHC. As UCH-L1 was one of the most highly expressed proteins in insulinomas and a number of studies showed that UCH-L1 was associated with biological behaviors in many types of tumors, we focused on UCH-L1 in the present study.
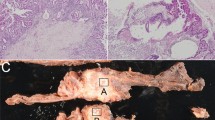
Representative examples of validated protein expression in PNETs and paired pancreatic specimens. Part a: IHC results. Left panel: HE staining of tumors and their paired pancreatic specimens; middle panel 1: expression of UCH-L1, MAP1B and VCAN in tumor #121, #52 (upper) and #292, respectively; middle panel 2: HE staining of tumors and their paired pancreatic specimens; right panel: negative expression of UCH-L1 in tumor #31 with a few positive cells, positive expression of MAP2 and CaSR in tumor #257 and #52, respectively; lower panel: expression of PDX-1 in insulinoma #36 and the negative expression of PDX-1 in tumor #63. Scale bar: 100 µm. Part b: Western blot results. Upper panel: expression of UCHL-1 in 4 PNETs, reduced expression of UCH-L1 in para-tumour tissue (#5 N) and no expression of UCH-L1 in 3 normal pancreatic specimens; upper middle panel 1: expression of CDK4 in 4 PNETs, 1 para-tumor tissue (#5 N) and 2 normal pancreatic specimens, no expression of CDK4 in 1 normal pancreatic specimen (Nor #3); lower middle panel 2: expression of CaSR in 2 PNETs and no expression of CaSR in 2 PNETs, para-tumor tissue (#5 N) and 3 normal pancreatic specimens; lower panel: β-actin was used as internal control. The identification of tumor was shown in Supplementary Table S1.
Methylation of UCH-L1 promoter in tumors
It is reported that expression of the UCH-L1 gene is mainly regulated by promoter methylation status in several non-endocrine tumors31, 32. To study the mechanisms underlying the differential expression of UCH-L1 in PNETs, we checked UCH-L1 promoter methylation in PNETs. We examined the promoter methylation status of UCH-L1 in 21 fresh frozen PNET specimens, 9 paired peritumoral tissue samples and 3 normal pancreatic tissues using MSP (Fig. 3a–c), and the results were confirmed by bisulfite sequencing (Fig. 3d). The methylation of UCH-L1 promoter was found in 20 of 20 samples without UCH-L1 expression and in 3 of 13 samples with expression, respectively. Conversely, demethylation of UCH-L1 promoter was found in 13 of 13 samples with UCH-L1 expression and in 9 of 20 samples without expression, respectively, p = 0.002 (Fisher exact test). As negative and positive controls of methylation status of the UCH-L1, cell lines SH-SY5Y and SW480 showed complete demethylation and methylation of UCH-L1 gene promoter, respectively (Fig. 3c). TE buffer was used as blank control (Fig. 3c). The data suggested that hypo- or demethylation of the UCH-L1 gene promoter was significantly associated with UCH-L1 protein expression in PNETs.
Promoter methylation of UCH-L1 in cancer cell lines and PNETs. The methylation of UCH-L1 promoter was more common in tumors without expression of UCH-L1 protein and para-tumor tissues (#289 N, #88 N). Demethylation was frequently seen in tumours with UCHL1 expression. U: unmethylated; M: methylated. Cell lines SW480 and SH-SY5Y were used as methylation and unmethylation controls, respectively, and TE buffer as blank control. Sequencing PCR products confirmed the MSP results.
Correlation of Clinicopathological Features/Prognosis with UCH-L1 Expression
When we evaluated the prognosis in PNET patients, 10 patients who died of unknown reasons were excluded. UCH-L1 expression was associated with disease-free survival in 104 patients with insulinomas (p = 0.047, Supplementary Table S5). Thus, we naturally extended our analysis to other PNETs, and we found that UCH-L1 expression was also associated with disease-free survival in 235 patients with PNETs (p = 0.013, Supplementary Table S5). UCH-L1 expression was also associated with less recurrence (p = 0.071, Supplementary Table S5). Moreover, Kaplan-Meier analysis showed that UCH-L1 expression in patients of collective I was associated with better overall survival (p = 0.034, Supplementary Fig. S2A) and disease-free survival (p = 0.019, Supplementary Fig. S2B). Multivariate analysis (Cox model), however, revealed that the UCH-L1 expression in PNETs was not associated with overall survival but still correlated with disease-free survival (Supplementary Table S6-a, model A, p = 0.463 and 6-b, model A, p = 0.018, respectively). These data suggested that UCH-L1 could be an independent prognostic marker of disease-free survival but not for overall survival.
Correlation of Clinicopathological Features/Prognosis with UCH-L1 and α-internexin Expression
In our recent study on PNETs29, we found that the expression of α-internexin was significantly associated with overall survival but not with disease-free survival. We hypothesized that combination of both UCH-L1 and α-internexin might be useful in evaluating both overall survival and disease-free survival. The concurrent expression of UCH-L1 and α-internexin significantly correlated with better overall survival and disease-free survival in collective I (Fig. 4A, p = 0.024 and Fig. 4B, p = 0.004, respectively). Similarly, in collective II, the patients with concurrent expression of both proteins had a favorable overall survival (Fig. 4C, p = 0.014) and a better disease-free survival (Fig. 4D, p = 0.009). In the combination of both collectives, the concurrent expression of UCH-L1 and α-internexin proteins significantly correlated with a better overall survival (Fig. 4E, p = 3.90 × 10–4; Cox analysis: HR 0.141, 95% CI 0.018 to 1.088, p = 0.060, see Supplementary Table S6-a, model B) and a better disease-free survival (Fig. 4F, p = 3.75 × 10−5, Cox: HR 0.215, 95% CI 0.064 to 0.716, p = 0.012, see Supplementary Table S6-b, model B). The data suggested that concurrent expression of UCH-L1 and α-internexin could be an independent prognostic biomarker of PNETs.
Correlation of Simultaneous Expression of UCH-L1 and α-internexin with Prognosis in 2 Collectives of Patients. Left panel: overall survival; right panel: disease free survival. The simultaneous expression of UCH-L1 and α-internexin in tumors was correlated with better overall survival and disease free survival in collective I (A and B, respectively) and a better overall survival with a statistical trend in collective II (C) and favorable disease free survival in collective II (D) as well as in the combination of collective I + collective II (E and F). The expression of both proteins in tumors was significantly associated with favourable overall survival (G) and disease free survival (H) in patients with stage II and III PNETs.
Interestingly, we found that the concurrent expression of UCH-L1 and α-internexin in patients with stage II and III was significantly associated with better overall survival (Fig. 4G, p = 0.017) and disease-free survival (Fig. 4H, p = 0.006, Cox analysis: HR 0.167, 95% CI 0.038 to 0.731, p = 0.017, Supplementary Table S7).
The cross-sectional analysis was consistent with the findings revealed by Kaplan-Meier analysis and Cox’s proportional hazard model. In the combination of both collectives, the concurrent expression of both proteins was significantly associated with lower stages (p = 9.26 × 10−5), less recurrence (p = 4.53 × 10−6) and smaller tumor size (p = 1.79 × 10−5) (Table 4). Notably, only one of 60 patients (1.7%) with the expression of both proteins was dead while 37 of 170 patients (21.8%) whose tumors were without the expression of both proteins died of disease (p = 7.79 × 10−5, Table 4). In addition, 57 of 60 patients (95%) with the concurrent expression of both proteins had disease-free survival whereas 106 of 168 patients (63%) without concurrent expression of both proteins had disease-free survival at the last follow-up (p = 6.10 × 10−7, Table 4).
We also analyzed the prognostic value of the two proteins in subgroups of PNETs. Interestingly, the simultaneous expression of UCH-L1 and α-internexin in insulinomas was correlated with better overall survival of patients (Log rank, p = 0.042, Fig. 5A) but insignificantly with better disease-free survival (Log rank, p = 0.073, Fig. 5B). The simultaneous expression of UCH-L1 and α-internexin in patients with non-insulinomas was associateded with better overall survival and disease-free survival (Log rank, p = 0.046 and p = 0.027, Fig. 5C and D, respectively). Similarly, concurrent expression of both proteins was significantly associated with favourable overall survival and disease-free survival in patients with functional PNETs (Log rank, p = 0.016 and p = 0.004, Fig. 5E and F, respectively) or with NF (Log rank, p = 0.041 and p = 0.023, Fig. 5G and H, respectively).
Correlation of Concurrent Expression of UCH-L1 and α-internexin with Prognosis in Subgroups of PNETs. Left panel: overall survival; right panel: disease-free survival. The simultaneous expression of UCH-L1 and α-internexin was correlated with better overall survival and disease-free survival in either insulinomas (Fig. 5A and B, respectively) or non-insulinomas (Fig. 5C and D, respectively). Similarly, the concurrent expression of both proteins was significantly associated with favourable overall survival and disease-free survival in either functional PNETs (Fig. 5E and F, respectively) or NF (Fig. 5G and H, respectively).
Using multivariate analysis (Cox’s proportional hazard model), the concurrent expression of both protein is not significantly associated with better prognosis in each of subgroup of PNETs, although a statistical trend could be seen (p value between 0.150 to 0.08, see Supplementary Table S8), likely due to the smaller sample sizes when we divided all PNETs into 4 groups.
Discussion
Tumor biomarkers have been used for diagnosis, management and prognosis by predicting tumor behavior or monitoring response to treatment. The biomarkers can be DNA, RNA and proteins; for example, high Akt expression could predict a nonresponse to chemotherapy in gastro-entero -pancreatic neuroendocrine tumors (GEP-NETs)33, over-expression of FGF13 mRNA is an independent predictor of a shorter progression-free survival23 and expression of KIT protein is an independent prognostic marker of mortality of PNETs34 and high expression of CD68 protein correlates with nonfunctional PNETs recurrence35.
A previous gene expression profiling study on human insulinoma found a number of genes that might play an important role in the pathogenesis of insulinoma36. Whole exome sequencing of insulinoma revealed a hotspot mutation of YY1 gene that could play a role in the tumorigenesis of insulinoma21. Both studies, however, did not identify applicable molecular markers for evaluating prognosis of insulinomas. Recently, a study showed that CUX1 mediates progression and angiogenesis in murine neuroendocrine tumors and is associated with malignant behaviors in human insulinomas37. Another interesting study revealed that TPD52 protein was associated with survival of patients with insulinomas using proteomic approach27. It has not been addressed whether these proteins could be used as a prognostic marker in other subtypes of PNETs. The expression profiling study on PNETs found that down-regulation of PTEN or TSC2 correlated with tumor aggressiveness but as addressed by the authors, PTEN and TSC2 might not be independent prognostic biomarkers at multivariate analysis23. Thus, more reliable independent prognostic biomarkers for PNETs are needed to predict unfavorable outcome in a patient with PNET so that the patient would benefit by receiving individualized treatment and surveillance based on prognostic markers.
Mass spectrometry brings higher accuracy and detection capability, and allows simultaneous detecting of thousands of proteins in a single study, thereby leading to discovering novel and more-reliable biomarkers for tumors27, 38 or potential therapeutic targets33. Here, we found more than 200 proteins differentially expressed in insulinoma by mass-spectrometry-based proteomic analysis and validated the expressions of several proteins not only in insulinomas but also in PNETs as a whole. In the present study, we used para-tumor tissue as the control, which contains both islets and exocrine tissue, based on previous studies demonstrating that non-islet pancreas was an important origin of PNETs. For example, a NIH study using a combination of genetic and morphological analysis strongly suggested that PNETs are derived from the ductal/acinar system39. Perren et al. also revealed that MEN1-associated PNETs arise from at least two different cell compartments of the pancreas, i.e. islets and exocrine ducts40. It is not surprising that PNETs might originate from either endocrine or exocrine cellular compartment because β cells could differentiate from nonendocrine epithelial cells (duct and acinar cells) in the human pancreas41. Para-tumor tissue has been used as control for PNETs by others with meaningful results22. We thus believe that para-tumor tissue is an appropriate control in our proteomic analysis of PNETs.
The present proteomic study identified the differential expression of proteins between tumor tissue and paired pancreatic tissue while Alkatout et al. compared the protein expression between benign insulinoma and malignant insulinoma27. This is the major difference between the 2 proteomic studies on insulinomas. Alkatout et al. revealed a number of protein differentially expressed between benign and malignant tumors, for example, expression of GSN and TPD52 proteins was upregulated in benign insulinomas, both by 3.9 fold27. Interestingly, we also found expression of GSN and TPD52 was upregulated in benign insulinomas, by 4.3 and 3.7 fold, respectively.
Our previous study demonstrated that α-internexin, a component of cytoskeleton, is extensively expressed in PNETs and could be a novel prognostic biomarker for overall survival29. In our proteomic analysis, UCH-L1 was one of the proteins that were most strongly expressed in insulinomas. Our data showed that UCH-L1 might be an independent prognostic marker of disease-free survival in PNETs but not for overall survival. Thus, it was conceivable to combine UCH-L1 with α-internexin to assess their prognostic value in PNETs. As we demonstrated, the concurrent expression of both proteins proves valuable for predicting either disease-free survival or overall survival in each collective as well as in the combination of both cohorts. Moreover, the patients whose tumor expressed both proteins have lower probability of recurrence in each cohort and in the combination of both cohorts.
Another advantage of present study was that we assessed the prognostic value of these 2 proteins in a subgroup of patients with tumor stage II and stage III. Among 1072 patients with PNETs from 8 European cancer centers, only 1 of 248 patients with tumor stage I (ENETS staging) died of disease, in contrast, almost half of patients with tumor stage IV died of diseases42. In our most recent study on 977 PNETs patients43, we found that all of the patients with tumor stage I were alive without disease but more than 90% of patients with tumor stage IV died of disease or alive with tumor at the last follow-up. In fact, most patients with stage IV PNETs died of the disease eventually. Thus, it is more needed to predict the prognosis in patients with stage II and III than that in patients with stage I (very favorable outcome) or stage IV (the worst prognosis). However, few studies previously focused on PNET patients with tumor stage II and stage III. Our present data showed that concurrent expression of UCH-L1 and α-internexin could be prognostic biomarker for PNETs of stage II and stage III.
It is also interesting to note that the concurrent expression of both proteins might be of prognostic value in subtypes of PNETs, e.g. NF/functional PNETs or insulinoma/non-insulinoma although the correlation of concurrent expression of both proteins with survival does not reach the significance by using multivariate analysis. The multivariate analysis (Cox’s model) showed only a statistical trend between the concurrent expression of UCH-L1 and α-internexin and prognosis in each of the subgroups of PNETs (p value between 0.15 to 0.08), it is likely due to the sample sizes correspondingly reduced after we divided all PNETs into 4 groups.
UCH-L1 belongs to a deubiquitinases family which plays important roles in various cancer and may be potential therapeutic targets44. A number of studies showed that UCH-L1 was associated with many types of cancers including colorectal carcinoma45 and prostate cancer46. However, whether UCH-L1 inhibits tumor growth and tumor progression is controversial. The expression of UCH-L1 in tumor cells enhances their invasive potential in vitro and in vivo by regulating cell adhesion through Akt-mediated pathway, suggesting that the protein is an upstream regulator of Akt47. Some studies also showed that expression of UCH-L1 contributed to cell malignant transformation, tumor growth, metastasis and worse prognosis46, 48, 49, suggesting UCH-L1 is an oncogene product50. In contrast, other researchers find that expression of UCH-L1 induces apoptosis in breast cancer51 and, UCH-L1 knockdown in ovarian cancer cell lines caused increased proliferation44, suggesting that UCH-L1 is a tumor suppressor gene in these endocrine tumors. Two other studies also suggested UCH-L1 was a tumor suppressor in nasopharyngeal carcinoma and liver cancer31, 52. Hence, it seems that function of UCH-L1 gene (protein) and its clinical implication maybe tumor-type dependent.
Recently, an interesting study showed UCH-L1 expression in a group of well-differentiated GEP-NETs and the protein level was significantly higher among localized GEP-NETs compared with metastatic tumors53. The study suggested that loss of UCH-L1 expression was an independent risk factor associated with metastatic NETs at the surgery, which was consistent with our present finding that UCH-L1 expression was significantly associated with localized PNETs. That study, however, included only 11 PNETs and was unable to analyze the prognosis due to the small number of cases53. Moreover, UCH-L1 expression was only positive in 40% of cervical neuroendocrine carcinomas, a highly aggressive NETs54. In the present study, we found that combination of UCH-L1 with α-internexin could be better and more reliable for evaluating prognosis in PNETs than using single protein biomarker because expression of UCH-L1 alone was associated with disease-free survival only, whereas α-internexin was only correlated with overall survival.
Our study has some limits. At least half of patients were followed up retrospectively; the limitations of retrospective study could exist in the study, such as the variability in different hospitals, tissue availability might lead to the potential bias. Although minimal significant difference was found between patients with available specimens and those without, sample selection based on specimen availability could still introduce subtle systemic biases when sample size was small. The median duration of follow-up (68 months) was not very long for PNETs patients as many of them could survive for 5 years or more, even with recurrence. Although some bias may exist, the two independent cohorts of patients in present study and enough number of patients (more than 300) could diminish the bias as possible as we could.
In conclusion, we analyzed the proteomic profile of sporadic insulinoma and validated the expression of several proteins in a large number of PNETs. Our findings suggested that simultaneous expression of UCH-L1 and α-internexin is an independent prognostic marker for PNETs in 2 different cohorts of the patients and in the combination of both cohorts. More interestingly, the concurrent expression of both proteins is a valuable prognostic marker in a subgroup of patients with stage II and stage III tumors.
Materials and Methods
Clinicopathological characteristics
One hundred and seventy tumors from 164 patients with PNETs evaluated between 1989 and 2014 at Peking Union Medical College Hospital (collective I) were enrolled in the present study. Clinical follow-up data of 117 patients were obtained from 1989 until 2016. In addition, 144 tumors from 142 PNETs patients outside Peking Union Medical College Hospital (collective II) which were evaluated between 1992 and 2014 at the First Affiliated Hospital of Sun Yat-Sen University, Memorial Sloan-Kettering Cancer Center (New York), and Cedars-Sinai Medical Center (Los Angeles) were enrolled in the study, and 130 patients were followed up from 1992 until 2016 (see Supplementary Table S1). The selection of patients from each hospital was based on tumor tissue availability. All patients of the 2 cohorts underwent curative resection of primary tumors and metastasis. The duration of follow-up was calculated from the date of surgery to the date of recurrence, death, or last follow-up. Overall, 314 tumors from 306 patients and 127 paired pancreatic tissue specimens were studied. The research was approved by the Scientific Ethics Committee of Peking Union Medical College Hospital, the First Affiliated Hospital of Sun Yat-Sen University, Memorial Sloan-Kettering Cancer Center and Cedars-Sinai Medical Center, the written informed consent was obtained from all subjects, all methods were performed in accordance with the relevant guidelines and regulations. The diagnostic criteria for PNETs were identical to those used in our previous studies29, 55, 56. For example, the clinical and laboratory diagnostic criteria of insulinoma included symptoms of hypoglycemia, hypoglycemia (serum levels of glucose <50 mg/dl), hyperinsulinemia (elevated serum levels of insulin or high serum levels of proinsulin at time of hypoglycemia), please see our previously published paper55 and most recently published paper57. The pathological diagnosis of all PNETs was made by 2 experienced pathologists. We analyzed tumor grade and stage in 252 and 305 patients who had relevant data detail, respectively, according to ENETS guideline42, 58.
Protein extraction, separation and in-gel digestion
Workflow for proteomic analysis was shown in Supplementary Fig. S3.
We deliberately selected 4 representative patients (2 female and 2 male, age 25, 40, 51, and 71) with typical insulinomas (2 G1 and 2 G2, size 1.5, 1.8, 2, and 2 cm, 2 at body/tail and 2 at head/neck of pancreas, all without lymph node or remote metastasis). Total protein was extracted from each of 4 insulinomas and their paired pancreatic tissues, and separated by electrophoresis. The protein in-gel was digested by trypsin before nanospray LC/MS/MS analysis. Please see Supplementary Method.
Nanospray LC/MS/MS analysis and database search
The resulting peptides from 16 fractions of each sample were sequentially analyzed by nanoLC-MS/MS using an UltiMate 3000 RSLCnano System (Thermo Scientific/Dionex) coupled to Q-Exactive hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Scientific, Bremen). The experimental procedures of mass spectrometer in detail were described in Supplementary Method.
Raw data files were searched against the NCBI/UniprotKB human protein sequence databases using the Proteome Discoverer 1.4 software (Thermo, San Jose, CA) based on the SEQUEST algorithm. The false positive discovery rates (FDR) is set on 1%.
Spectral count based label-free quantization and statistical analysis
Protein quantification used the normalized spectral abundance factors (NSAFs) method59, 60 to calculate the protein relative abundance for each identified protein. In order to quantitatively describe the relative abundance, the ppm (part per million) was chosen as the unit and, the 1,000,000 ppm value was assigned to each proteome profile. A ppm value at the range of 0 to 1,000,000 ppm for each identified protein in each proteome profile was calculated based on its normalized NSAF. NSAFs were calculated as follow: NSAFN = (SN/LN)/(Σn i=1Si/Li), N is protein index, SN is the number of peptide spectra matched to the protein, LN is the length of protein N, N is the total number of proteins in the input database; NSAF values should range from 0 to 1, with values closer to 1 indicating higher protein levels. NSAFs were used to rank proteins within a particular analysis and to compare the relatively concentration of each identified protein across all 8 analyzed samples.
Validating the proteins expression by immunohistochemical staining (IHC) and Western blot
Proteomic analysis showed that proteins UCH-L1, MAP1B, MAP2, VCAN, PDX-1 and CDK4 were highly expressed in all of insulinomas but not (or weakly expressed) in the paired controls. Thus, the expression of these proteins was validated by immunohistochemistry in more than 40 randomly selected PNETs. Randomization was achieved by picking every 3rd patient from a patient list. The expression of CaSR was only validated in 29 insulinomas due to limitation of tissue amount. The sections of paraffin-embedded tumor tissue and paired para-tumoral tissue were antigen-retrieved by heating and stained with appropriate antibodies (see Supplementary Method for detail). The results were interpreted by 2 persons blinded to clinical data and patients’ outcome. We defined <20% tumor cells with staining of protein as negative expression, i.e. (±) and (-), similar to our previous report29, 55. Expression of UCH-L1, CDK4 and CaSR was further confirmed in 10 fresh frozen tissues by Western blot similar to the method previously described29, with anti-UCH-L1, anti-CDK4 and anti-CaSR at 1:1000, 1:800 and 1:500 dilution, respectively. Selection of the 10 fresh frozen samples (6 tumors and 4 paired tissue) was mostly based on tissue availability. The median size of the 6 tumors were 2 cm (range 1.5–4). β-actin was used as an internal control. Protein quantification was performed by reading the density of bands and, a statistic analysis (Mann-Whitney U test) on ratio of UCH-L1/β-actin was performed.
Bioinformatic Analysis
The subcellular location of the proteins which were identified in tumors and the associated signal pathways were bioinformatically analyzed. The biological and functional features of proteins UCH-L1, MAP1B, MAP2, VCAN, PDX-1, CDK4 and α-internexin were also analyzed.
Detecting the methylation of UCH-L1 promoter by methylation-specific PCR (MSP) and bisulfite sequencing
Human cancer cell lines SW480 and SH-SY5Y were cultured in DMEM medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (HyClone, Logan, UT). Genomic DNA was isolated from the 2 cell lines, 21 fresh frozen tumoral, 9 paired tissue samples and 3 normal pancreatic tissues by ZR Genomic DNA II Kit (Zymo Research), then bisulfite-modified as we previously reported29. The selection of the 33 fresh frozen tumor samples was mainly based on UCH-L1 expression status as well as tissue availability. The methylation or demethylation of UCH-L1 promoter was detected by MSP. DNA from UCH-L1 gene unmethylated cell line SH-SY5Y and methylated cell line SW480 was used as negative and positive control, respectively. TE buffer was used as blank control of PCR. PCR conditions and primer sets were summarized in Supplementary Method. MSP results were confirmed by sequencing PCR products29, 55. We correlated promoter methylation status with protein expression, using statistic analysis.
Correlating the concurrent expression of UCH-L1 and α-internexin with clinicopathological characteristics
At first, we correlated the expression of UCH-L1 protein with the clinicopathological features of 154 insulinomas and 314 PNETs, respectively. After excluding 10 patients who died of unknown reasons, the prognostic value of UCH-L1 protein was assessed in collective I by Kaplan-Meier plots. In our previous study, we found that expression of α-internexin was significantly associated with overall survival in patients with PNETs29. Thus, in present study, the concurrent expression of both UCH-L1 and α-internexin were correlated with clinicopathological features including prognosis in each independent collective and in the combination of 2 cohorts (n = 230). The prognostic value of UCH-L1 and α-internexin was further evaluated in 131 patients with stage II and stage III tumors. Moreover, the prognostic value of concurrent expression of UCH-L1 and α-internexin was assessed in subgroup of PNETs, i.e. insulinoma vs. non-insulinoma and NF vs functional PNETs, respectively.
Workflow of each step of experiments
A diagram showed the number of cases used in each step of experiments, see Supplementary Table S9.
Statistical analysis
SPSS statistics software version 20.0 was used for statistical analysis. Significance was calculated using Fisher’s exact test, χ-test for categorical variables and Mann-Whitney U test for continuous variables. Survival of patients was analyzed by Kaplan-Meier analysis and log-rank test. Cox’s proportional hazard model was used for multivariate analysis. Two-tailed test was used in all of statistical analysis. P ≤ 0.05 was considered significant.
References
Halfdanarson, T. R., Rubin, J., Farnell, M. B., Grant, C. S. & Petersen, G. M. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer 15, 409–427, doi:10.1677/ERC-07-0221 (2008).
Metz, D. C. & Jensen, R. T. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 135, 1469–1492, doi:10.1053/j.gastro.2008.05.047 (2008).
Modlin, I. M. et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9, 61–72, doi:10.1016/S1470-2045(07)70410-2 (2008).
de Herder, W. W. Gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Best Pract Res Clin Gastroenterol 26, 689–690, doi:10.1016/j.bpg.2013.01.005 (2012).
de Wilde, R. F., Edil, B. H., Hruban, R. H. & Maitra, A. Well-differentiated pancreatic neuroendocrine tumors: from genetics to therapy. Nat Rev Gastroenterol Hepatol 9, 199–208, doi:10.1038/nrgastro.2012.9 (2012).
Ito, T., Igarashi, H. & Jensen, R. T. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Pract Res Clin Gastroenterol 26, 737–753, doi:10.1016/j.bpg.2012.12.003 (2012).
Asa, S. L. Pancreatic endocrine tumors. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 24(Suppl 2), S66–77, doi:10.1038/modpathol.2010.127 (2011).
Hauso, O. et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer 113, 2655–2664, doi:10.1002/cncr.v113:10 (2008).
Yao, J. C. et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 26, 3063–3072, doi:10.1200/JCO.2007.15.4377 (2008).
Fraenkel, M., Kim, M. K., Faggiano, A. & Valk, G. D. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol 26, 691–703, doi:10.1016/j.bpg.2013.01.006 (2012).
Rindi, G. & Wiedenmann, B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol 8, 54–64, doi:10.1038/nrendo.2011.120 (2012).
Falconi, M. et al. Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology 84, 196–211, doi:10.1159/000098012 (2006).
Fraenkel, M., Kim, M., Faggiano, A., de Herder, W. W. & Valk, G. D. Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer 21, R153–163, doi:10.1530/erc-13-0125 (2014).
Li, A. F. et al. A 35-year retrospective study of carcinoid tumors in Taiwan: differences in distribution with a high probability of associated second primary malignancies. Cancer 112, 274–283, doi:10.1002/cncr.23159 (2008).
Ito, T. et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol 45, 234–243, doi:10.1007/s00535-009-0194-8 (2010).
Oberg, K. & Eriksson, B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol 19, 753–781, doi:10.1016/j.bpg.2005.06.002 (2005).
Jonkers, Y. M., Ramaekers, F. C. & Speel, E. J. Molecular alterations during insulinoma tumorigenesis. Biochim Biophys Acta 1775, 313–332, doi:10.1016/j.bbcan.2007.05.004 (2007).
Bertolino, P. et al. Pancreatic beta-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res 63, 4836–4841 (2003).
Jiang, W. J. et al. Frequent loss of heterozygosity at MEN-1 gene and chromosome 22q in insulinomas and its significance. Zhonghua Yi Xue Za Zhi 84, 1705–1709 (2004).
Cupisti, K. et al. Lack of MEN1 gene mutations in 27 sporadic insulinomas. Eur J Clin Invest 30, 325–329, doi:10.1046/j.1365-2362.2000.00620.x (2000).
Cao, Y. et al. Whole exome sequencing of insulinoma reveals recurrent T372R mutations in YY1. Nat Commun 4, 2810, doi:10.1038/ncomms3810 (2013).
Roldo, C. et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 24, 4677–4684, doi:10.1200/JCO.2005.05.5194 (2006).
Missiaglia, E. et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 28, 245–255, doi:10.1200/JCO.2008.21.5988 (2010).
Speisky, D. et al. Molecular profiling of pancreatic neuroendocrine tumors in sporadic and Von Hippel-Lindau patients. Clin Cancer Res 18, 2838–2849, doi:10.1158/1078-0432.CCR-11-2759 (2012).
Jiang, X. et al. miR-144/451 Promote Cell Proliferation via Targeting PTEN/AKT Pathway in Insulinomas. Endocrinology 156, 2429–2439, doi:10.1210/en.2014-1966 (2015).
Jiao, Y. et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331, 1199–1203, doi:10.1126/science.1200609 (2011).
Alkatout, I. et al. Novel prognostic markers revealed by a proteomic approach separating benign from malignant insulinomas. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc 28, 69–79, doi:10.1038/modpathol.2014.82 (2015).
Capurso, G. et al. Gene expression profiles of progressive pancreatic endocrine tumours and their liver metastases reveal potential novel markers and therapeutic targets. Endocr Relat Cancer 13, 541–558, doi:10.1677/erc.1.01153 (2006).
Liu, B. et al. alpha-Internexin: a novel biomarker for pancreatic neuroendocrine tumor aggressiveness. J Clin Endocrinol Metab 99, E786–795, doi:10.1210/jc.2013-2874 (2014).
Ferber, S. et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 6, 568–572, doi:10.1038/75050 (2000).
Yu, J. et al. Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology 48, 508–518, doi:10.1002/hep.v48:2 (2008).
Heitzer, E. et al. Differential survival trends of stage II colorectal cancer patients relate to promoter methylation status of PCDH10, SPARC, and UCHL1. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc 27, 906–915, doi:10.1038/modpathol.2013.204 (2014).
O’Toole, D. et al. Molecular markers associated with response to chemotherapy in gastro-entero-pancreatic neuroendocrine tumors. Endocr Relat Cancer 17, 847–856, doi:10.1677/ERC-09-0204 (2010).
Zhang, L. et al. KIT is an independent prognostic marker for pancreatic endocrine tumors: a finding derived from analysis of islet cell differentiation markers. The American journal of surgical pathology 33, 1562–1569, doi:10.1097/PAS.0b013e3181ac675b (2009).
Wei, I. H. et al. Tumor-associated macrophages are a useful biomarker to predict recurrence after surgical resection of nonfunctional pancreatic neuroendocrine tumors. Annals of surgery 260, 1088–1094, doi:10.1097/SLA.0000000000000262 (2014).
Wang, X. C. et al. Gene expression profiling in human insulinoma tissue: genes involved in the insulin secretion pathway and cloning of novel full-length cDNAs. Endocr Relat Cancer 11, 295–303, doi:10.1677/erc.0.0110295 (2004).
Krug, S. et al. CUX1: a modulator of tumour aggressiveness in pancreatic neuroendocrine neoplasms. Endocr Relat Cancer 21, 879–890, doi:10.1530/erc-14-0152 (2014).
Kulasingam, V. & Diamandis, E. P. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat Clin Pract Oncol 5, 588–599, doi:10.1038/ncponc1187 (2008).
Vortmeyer, A. O., Huang, S., Lubensky, I. & Zhuang, Z. Non-islet origin of pancreatic islet cell tumors. J Clin Endocrinol Metab 89, 1934–1938, doi:10.1210/jc.2003-031575 (2004).
Perren, A. et al. Multiple endocrine neoplasia type 1 (MEN1): loss of one MEN1 allele in tumors and monohormonal endocrine cell clusters but not in islet hyperplasia of the pancreas. J Clin Endocrinol Metab 92, 1118–1128, doi:10.1210/jc.2006-1944 (2007).
Hao, E. et al. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med 12, 310–316, doi:10.1038/nm1367 (2006).
Rindi, G. et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst 104, 764–777, doi:10.1093/jnci/djs208 (2012).
Zhu, L. M. et al. Differences and Similarities in the Clinicopathological Features of Pancreatic Neuroendocrine Tumors in China and the United States: A Multicenter Study. Medicine 95, e2836, doi:10.1097/MD.0000000000002836 (2016).
Pfoh, R., Lacdao, I. K. & Saridakis, V. Deubiquitinases and the new therapeutic opportunities offered to cancer. Endocr Relat Cancer 22, T35–54, doi:10.1530/erc-14-0516 (2015).
Yamazaki, T. et al. PGP9.5 as a marker for invasive colorectal cancer. Clin Cancer Res 8, 192–195 (2002).
Jang, M. J., Baek, S. H. & Kim, J. H. UCH-L1 promotes cancer metastasis in prostate cancer cells through EMT induction. Cancer Lett 302, 128–135, doi:10.1016/j.canlet.2011.01.006 (2011).
Kim, H. J. et al. Ubiquitin C-terminal hydrolase-L1 is a key regulator of tumor cell invasion and metastasis. Oncogene 28, 117–127, doi:10.1038/onc.2008.364 (2009).
Mandelker, D. L. et al. PGP9.5 promoter methylation is an independent prognostic factor for esophageal squamous cell carcinoma. Cancer Res 65, 4963–4968, doi:10.1158/0008-5472.CAN-04-3923 (2005).
Seliger, B. et al. Ubiquitin COOH-terminal hydrolase 1: a biomarker of renal cell carcinoma associated with enhanced tumor cell proliferation and migration. Clin Cancer Res 13, 27–37, doi:10.1158/1078-0432.CCR-06-0824 (2007).
Bheda, A., Shackelford, J. & Pagano, J. S. Expression and functional studies of ubiquitin C-terminal hydrolase L1 regulated genes. PLoS One 4, e6764, doi:10.1371/journal.pone.0006764 (2009).
Wang, W. J. et al. Over-expression of ubiquitin carboxy terminal hydrolase-L1 induces apoptosis in breast cancer cells. Int J Oncol 33, 1037–1045 (2008).
Li, L. et al. The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma. Clin Cancer Res 16, 2949–2958, doi:10.1158/1078-0432.CCR-09-3178 (2010).
Kleiman, D. A. et al. Silencing of UCHL1 by CpG promoter hyper-methylation is associated with metastatic gastroenteropancreatic well-differentiated neuroendocrine (carcinoid) tumors. Ann Surg Oncol 21(Suppl 4), S672–679, doi:10.1245/s10434-014-3787-2 (2014).
McCluggage, W. G., Kennedy, K. & Busam, K. J. An immunohistochemical study of cervical neuroendocrine carcinomas: Neoplasms that are commonly TTF1 positive and which may express CK20 and P63. The American journal of surgical pathology 34, 525–532, doi:10.1097/PAS.0b013e3181d1d457 (2010).
Mei, M. et al. Clinical implications of microsatellite instability and MLH1 gene inactivation in sporadic insulinomas. J Clin Endocrinol Metab 94, 3448–3457, doi:10.1210/jc.2009-0173 (2009).
Wang, Y. H. et al. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: A single-institution analysis (1995-2012) in South China. BMC Endocr Disord 12, 30, doi:10.1186/1472-6823-12-30 (2012).
Run Yu, N. N. N. et al. A Clinicopathological Study of Malignant Insulinoma in a Contemporary Series. Pancreas. doi:10.1097/MPA.0000000000000718 (2017).
Rindi, G. et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 449, 395–401, doi:10.1007/s00428-006-0250-1 (2006).
Florens, L. et al. Analyzing chromatin remodeling complexes using shotgun proteomics and normalized spectral abundance factors. Methods 40, 303–311, doi:10.1016/j.ymeth.2006.07.028 (2006).
Paoletti, A. C. et al. Quantitative proteomic analysis of distinct mammalian Mediator complexes using normalized spectral abundance factors. Proc Natl Acad Sci USA 103, 18928–18933, doi:10.1073/pnas.0606379103 (2006).
Acknowledgements
We appreciate Dr Tao Xu (Department of Epidemiology and Statistics, Institute of Basic Medical Sciences, Peking Union Medical College, Chinese Academy of Medical Sciences & School of Basic Medicine Sciences) for his great help of statistic supports. This research was supported by the National Natural Sciences Foundation of China (grants No. 81250021 and 81450015 to Yuan-Jia Chen).
Author information
Authors and Affiliations
Contributions
Y.-J.C., designed and supervised the project; Y.-L.S., R.Y., X.-W.Q., T.-T.S., R.Y., J.C., L.S., B.L., L.T., prepared the samples; J.-J.H. and X.-W.Q., performed proteomic analysis; Y.-L.S., X.-W.Q., Y.-H.W. and H.-L.G., detected the gene methylation and expression; C.-M.B., C.-M.L., T.-P.Z., Y.-P.Z., J.C., D.-R.Z., Y.X., B.L., R.Y., L.S., and L.T., clinically and pathologically diagnosed the patients; T.-P.Z. and Y.-P.Z., performed the surgery; Y.-J.C., Y.-L.S., R.Y., Y.Z., L.T. and C.-M.L., analyzed the data; Y.-L.S., X.-W.Q., J.C., B.L., L.S., L.T and T.-T.S., followed up patients; Y.-J.C., R.Y. and Y.-L.S., wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, YL., Yu, R., Qiao, XW. et al. Prognostic relevance of UCH-L1 and α-internexin in pancreatic neuroendocrine tumors. Sci Rep 7, 2205 (2017). https://doi.org/10.1038/s41598-017-02051-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02051-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.