Abstract
Monocrotophos (MCP) is an organophosphorus pesticide that is median-toxic to fish. MCP pesticide resulted in an increase of 17 beta estradiol following a decrease in testosterone in male goldfish (Carassius auratus). To fully understand the mechanism of MCP pesticide that causes the imbalance between male and female hormones, we determined the levels of plasma cholesterol, spermatic steroidogenic acute regulatory protein mRNA, steroidogenesis enzyme mRNA, plasma sex hormone synthesis intermediates, and effectual hormones in male goldfish exposed to MCP pesticide at nominal concentrations of 0.01, 0.10, and 1.00 mg/L for 21 days in a semi-static exposure system. The results indicated that MCP pesticide (a) led to decreased steroidogenic acute regulatory protein mRNA levels; (b) decreased mRNA levels of cholesterol side chain cleavage enzyme and cytochrome P450 17 alpha hydroxylase, which are steroidogenesis enzymes involved in androgen synthesis; and (c) increased cytochrome P450 aromatase mRNA levels, a steroidogenesis enzyme involved in the synthesis of effectual estrogen. The present study provides evidence that MCP pesticide affects synthesis and conversion of sex steroids through multiple targets in male goldfish.
Similar content being viewed by others
Introduction
Monocrotophos (MCP, CAS number 6923-22-4) is an organophosphorus pesticide that is high-toxic to birds, median-toxic to fish, and listed as a UNEP Prior Informed Consent chemical. Production, management, and use of MCP pesticides have been comprehensively banned in China since January 1, 2007, but it is still extensively detected in China and some other developing countries. MCP was detected in snow pea samples from western China in 20101. MCP residues in brinjal, okra, cucurbits, crucifers, and green chilies were 0.023–1.140 mg/kg in the Andaman Islands, India2, and its concentration in the industrial wastewater near Lucknow City, India, was 8.32 ± 3.9 μg/L3.
In our previous studies, it was demonstrated that MCP pesticide was a potential environmental estrogen4, and it was furthermore determined that MCP pesticide induced mRNA expression of aromatizing enzyme in the gonads of male goldfish (Carassius auratus) and resulted in an increase of 17 beta estradiol (E2) following a decrease in testosterone (T)5. The changes in the E2 and T levels were in good agreement with the increase in aromatase expression; however, this might not be the only mechanism by which MCP pesticide disrupts the balance of sex hormones in male fish because a series of enzymatic reactions are involved in sex hormone synthesis in teleosts.
In theory, the process of sex hormone synthesis provides numerous potential targets for MCP pesticide. First, cholesterol serves as precursor to all steroid hormones, and some xenobiotics disturbed the synthesis of sex hormones by affecting cholesterol levels6,7,8. Second, the synthesis of sex hormones starts only when cholesterol is transported to the inner mitochondrial membrane. Steroidogenic acute regulatory protein (StAR) plays an important role in this delivery process, and thus, low StAR levels will lead to a sharp decline or even interruption of sex hormone synthesis. For example, beta sitosterol exposure changed gonadal transcript levels of StAR in goldfish, leading to lower concentrations of T9. Third, after being transported to the inner mitochondrial membrane, cholesterol is translated into pregnenolone under the catalysis of the cholesterol side chain cleavage enzyme (CYP11A1/P450scc). The conversion from pregnenolone to effectual hormones requires the participation of 3 beta hydroxysteroid dehydrogenase (3 beta HSD), cytochrome P450 17 alpha hydroxylase (CYP17/P450c17/P45017alpha), 17 beta hydroxysteroid dehydrogenase (17 beta HSD), cytochrome P450 aromatase (CYP19/P450arom), 20 beta hydroxysteroid dehydrogenase (20 beta HSD), cytochrome P45011beta (CYP11beta/P45011beta), and 11 beta hydroxysteroid dehydrogenase (11 beta HSD), all of which are potential target sites of xenobiotics. Govoroun et al. demonstrated that E2 inhibited spermatic P450c17, 3 beta HSD, and P45011beta gene expression in rainbow trout (Oncorhynchus mykiss)10. MCP pesticide might affect synthesis and conversion of sex hormones via a number of pathways, such as changing the contents of synthesis substrates or influencing gene expression and activities of steroidogenesis enzymes.
This study was conducted to fully understand the mechanism by which MCP pesticide causes an imbalance between male and female hormones in male goldfish. First, effects on plasma total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were examined to determine whether a lack of substrates for sex hormone synthesis is responsible for inhibited T levels. Second, spermatic StAR mRNA levels were quantified, which could indicate the influence of MCP on the transport of cholesterol from the outer to the inner mitochondrial membrane. Third, mRNA levels of eight kinds of steroidogenesis enzymes and plasma levels of six kinds of sex hormone synthesis intermediates and four kinds of effectual hormones were determined, to elucidate the effects of MCP pesticide on steroidogenesis in male goldfish.
Results
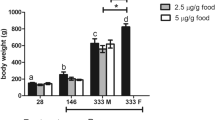
Effects of MCP pesticide on gonadosomatic index (GSI)
The fishes were sampled in summer with degraded gonads. GSI of the control male goldfish was 0.14 ± 0.06%, which was not different from that in any of the MCP pesticide treatments studied (Fig. 1).
Effects of MCP pesticide on cholesterol levels
In the control group, the content of plasma HDL-C was higher than that of LDL-C. TC, HDL-C, and LDL-C levels were not influenced by MCP pesticide exposure (Fig. 2).
Effects of MCP pesticide on gene expression of StAR and steroidogenesis enzymes
Fragments encoding P450scc, 3-beta-HSD, 20-beta-HSD, 17-beta-HSD1, P45011beta, and 11-beta-HSD2 genes of goldfish were amplified and cloned. The deduced amino acid sequences showed high homology to those of other fish species, containing typical P450 conserved features as indicated in Table 1 11,12,13,14.
Gene expression levels of eight steroidogenesis enzymes, as well as StAR were detected. A significant decrease in StAR gene transcriptions was caused by MCP pesticide exposure (P < 0.01, η2 > 0.15, Fig. 3C). Transcription levels of P450scc, which is related to synthesis of pregnenolone, were significantly down-regulated in all three exposure groups (P < 0.01, η2 > 0.15, Fig. 3A). Significant down-regulation of P450c17, which is a key enzyme involved in androstenedione synthesis, was also observed (P < 0.01, η2 > 0.15, Fig. 3B), whereas 3-beta-HSD mRNA levels exhibited no significant changes (Fig. 3C). Gene expression of steroidogenesis enzymes involved in synthesis of effectual androgen and estrogen was decreased and increased, respectively, by MCP pesticide exposure. Specifically, expression of P45011beta (P < 0.01, η2 > 0.15, Fig. 3C) and 11-beta-HSD2 (P < 0.01, η2 > 0.15, Fig. 3A) was down-regulated after MCP pesticide treatment, and P450arom A mRNA levels were significantly up-regulated in the 0.10 mg/L exposure group (η2 > 0.15, Fig. 3C), although 17-beta-HSD1 mRNA levels were not significantly changed in any exposure group. The conversion of 17 alpha hydroxyprogesterone to 17 alpha, 20 beta dihydroxy-4-pregnen-3-one is catalyzed by 20-beta-HSD, and only the 0.01 mg/L exposure group showed up-regulation of its expression (P < 0.01, Fig. 3A).
Effects of MCP pesticide on plasma sex hormone levels
Effects of MCP pesticide on plasma levels of six kinds of intermediates, as well as four kinds of effectual hormones were determined. The highest pesticide concentration (1.00 mg/L) significantly decreased plasma pregnenolone levels (F 3,36 = 4.413, P < 0.05, η2 > 0.15, Fig. 4A). Androstenedione is an important precursor of male and female sex hormones, and decreases in the levels of plasma androstenedione (P < 0.01, η2 > 0.15, Fig. 4E) and one of its substrates, 17 alpha hydroxyprogesterone (P < 0.01, η2 > 0.15, Fig. 4D), were detected; however, dehydroepiandrosterone, which is another substrate for androstenedione, exhibited no significant changes in any of the three exposure groups (Fig. 4C). Plasma 17 alpha hydroxypregnenolone (F 3,36 = 5.826, P < 0.05, η2 > 0.15, Fig. 4B) and progesterone (F 3,36 = 3.020, P < 0.05, η2 > 0.15, Fig. 5A) levels, which are also involved in synthesis and transformation of androstenedione, were increased by pesticide exposure, with significant changes detected only in the highest dose group for progesterone. There were no significant changes in 17 alpha, 20 beta double hydroxyprogesterone (Fig. 5B), 11 beta hydroxytestosterone (Fig. 5C), 11-keto testosterone (11-KT) (Fig. 5D), or estrone (Fig. 4F) levels in any of the groups tested.
Discussion
In this study, mechanisms by which MCP pesticide affects sex hormone synthesis and conversion of male goldfish were discussed from four aspects: (a) cholesterol levels, (b) gene expression of StAR and steroidogenesis enzymes; (c) intermediate products of the process of sex hormone synthesis; and (d) levels of effectual hormones (Fig. 6).
In bony fish, the substrates of steroid synthesis are mainly provided by exogenous cholesterol11. For example, climbing perch (Anabas testudineus) used plasma cholesterol as the raw material of sex hormone synthesis15. Low-density lipoproteins (LDL) play a leading role in mammals, but most cholesterol was transported by high-density lipoproteins (HDL) in fish7, 16, 17. In this study, plasma levels of LDL-C and HDL-C were both maintained following MCP pesticide exposure, suggesting that the content of raw material for sex hormone synthesis was not affected.
StAR can transfer cholesterol to the inner mitochondrial membrane, providing a reaction substrate for synthesis of pregnenolone, which is catalyzed by P450scc. In rainbow trout, StAR transcriptional levels were closely related to changes in plasma steroid hormones18. P450scc catalyzes the first step of steroidogenesis, making it a key enzyme during this process. MCP pesticide inhibited gene expression levels of StAR in male goldfish testes, leading to the reduction of cholesterol that was transported to the inner mitochondrial membrane, and the decrease in plasma levels of pregnenolone caused by MCP pesticide exposure is consistent with the inhibition of P450scc gene expression.
Pregnenolone, 17α-hydroxypregnenolone, and dehydroepiandrosterone can be converted to progesterone, 17α-hydroxyprogesterone, and androstenedione, respectively. These reactions were all catalyzed by 3-beta-HSD. P450c17 is one of the key enzymes of the steroidogenesis pathway in teleostean gonads. There are two kinds of P450c17 in bony fish: P450c17-I and P450c17-II. P450c17-I shows both 17α-hydroxylase and 17,20-lyase activity, whereas P450c17-II has only 17α-hydroxylase activity. Pregnenolone and progesterone can be converted to 17α-hydroxypregnenolone and 17α-hydroxyprogesterone, respectively, by 17α-hydroxylase activity; 17,20-lyase activity can break the chemical bonds between C17 and C20 and transform 17α-hydroxypregnenolone into dehydroepiandrosterone or 17α-hydroxyprogesterone into androstenedione. We found a significant reduction of plasma T in male goldfish after MCP pesticide exposure in our previous study5, and we observed that MCP pesticide exposure resulted in decreased plasma androstenedione in this study, which is the substrate of T. The down-regulation of androgens by MCP pesticide corresponded to decreases in P450c17 mRNA levels.
Estradiol is formed stepwise, starting with aromatization of androstenedione to estrone, followed by conversion of estrone to E2 via 17-beta-HSD activity, or conversion of androstenedione to T via 17-beta-HSD, followed by aromatization of T to E2. The intermediates androstenedione and estrone have low bioactivity, whereas T and E2 are effectual hormones with high bioactivity. MCP pesticide increased P450arom A gene expression in male goldfish gonads, and this is consistent with our previous study5.
P45011beta catalyzes T into 11-beta-hydroxytestosterone19, and 11-beta-HSD2 further converts 11-beta-hydroxytestosterone into 11-KT20. The reduction of P45011beta gene expression led to inhibition of 11-KT synthesis10, 21, whereas Kusakabe et al. suggested that plasma 11-KT levels had less relevance to 11-beta-HSD2 gene expression in the testes22. In this study, we observed significant declines of 11-beta-HSD2 and P45011beta mRNA levels, but there was no significant change in 11-KT.
Androgen is responsible for development of the testes. Based on the appearance and histologic features of the testes, the development process in goldfish can be classified into six periods23. Individuals were sampled in August in this study, when testes had degraded to period III after spermiation, and GSI of the control male goldfish was as low as 0.14 ± 0.06%. Although an abnormally low plasma concentration of T was observed concomitant with an increase in MCP pesticide concentration in our previous study5, GSI was not affected in this study. This may be explained by the unchanged 11-KT levels, because in general 11-KT, rather than T, is the dominant androgen in most fish species, including goldfish.
Studies on endocrine disruption of exogenous compounds, particularly regarding steroidogenesis, have focused mainly on gene expression and activity of steroidogenesis enzymes24, 25, especially aromatase26, 27, although multiple factors could be involved in steroidogenesis. In this research, we demonstrated that MCP pesticide acted on many target sites, affecting the transport of cholesterol and gene expression of steroidogenesis enzymes, leading to abnormal levels of sex hormone synthesis intermediates and effectual hormones, and thus resulting in a proportional imbalance of sex hormones in male goldfish.
Materials and Methods
Fish exposure and sample protocol
Goldfish (8.4 ± 0.6 cm standard length; 17.6 ± 3.6 g wet mass) were obtained from a local dealer in Qingdao, China. Following acclimation in the laboratory at 24–26 °C and under a 16 h light/8 h dark cycle for two weeks, fish were exposed to 0.01, 0.10, and 1.00 mg/L MCP pesticide (40% water-soluble preparation), the concentrations of which were 1/10000, 1/1000, and 1/100 of the 96-h LC50 (about 100 mg/L, our unpublished results), respectively5. Experiments were conducted in 70 L aquaria containing 50 L dechlorinated tap water using a semi-static toxicity test. Twenty liters of water was renewed daily to keep the MCP concentrations constant. Fish were fed a non-estrogenic pelletized diet daily. Additionally, the fish were handled according to the National Institute of Health Guidelines for the handling and care of experimental animals. The animal utilization protocol was approved by the Institutional Animal Care and Use Committee of the Ocean University of China.
After 21 days of exposure28,29,30,31,32,33, goldfish were anesthetized in 75 mg/L MS-222 (Sigma-Aldrich, St. Louis, MO, USA). Blood was taken from the caudal vein using chilled heparinized syringes. After centrifugation (700 g, 15 min, 4 °C or 2810 g, 10 min, 4 °C), plasma was frozen at −80 °C for cholesterol or hormone quantification. Individuals were identified as male or female during dissection. The testes were weighed and related to body weight to determine GSI (GSI = gonad weight/body weight × 100%), and then gonad samples were frozen in liquid nitrogen and stored at −80 °C for steroidogenesis enzyme gene quantification. In addition, ovaries and testes collected from unexposed fish were frozen in liquid nitrogen and stored at −80 °C for steroidogenesis enzyme gene cloning.
Cholesterol quantification
After twice dilution with 0.7% saline, plasma was analyzed using a fully automatic biochemistry analyzer to determine the content of TC, HDL-C, and LDL-C, with commercial kits obtained from Shanghai Fenghui Medical Science and Technology Co., LTD, Shanghai Rongsheng Biological Pharmaceutical Co., LTD, Beijing Beihua Kangtai Clinical Reagent Co., LTD, and Shenzhen-Mindray Biological Medical Electronics Co., LTD, China, respectively. The assay detection ranges were 0–20 mmol/L for TC, 0.05–6.0 mmol/L for HDL-C, and 0–9 mmol/L for LDL-C, respectively. The inter- and intra-assay coefficients of variation for TC and LDL-C were ≤5%. The inter- and intra-assay coefficients of variation for HDL-C were ≤2.5% and ≤4.0%, respectively.
cDNA fragment cloning and mRNA quantification of steroidogenesis enzyme genes
Isolation of total RNA from gonad tissue was accomplished using phenolic reagent TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Extracted RNA was measured by spectrometry at OD260/280 prior to being treated with DNase I (Promega, Madison, WI, USA). M-MLV first strand reverse transcription reaction I (Invitrogen, Carlsbad, CA, USA) was built with 1.0 μL Oligo(dT)12–18 (500 μg/mL), 5.5 μL total RNA, 1.0 μL 10 mM each dNTP, and 4.5 μL diethypyrocarbonate (DEPC) water, incubated at 65 °C for 5 min, and then rapidly frozen. Reverse transcription reaction II was built with 4.0 μL 5× first strand synthesis buffer, 2.0 μL 0.1 mol/L DTT, 1.0 μL cloned ribonuclease inhibitor (40 U/μL, Takara Bio Inc., Shiga, Japan), and 12.0 μL reverse transcription reaction I, hatched at 37 °C for 2 min, and then hatched at 37 °C for 50 min after 1 μL M-MLV reverse transcriptase (200 U/μL) was added. The reaction was terminated by heating the reaction mixture at 70 °C for 15 min.
To clone cDNA fragments of steroidogenesis enzyme genes, including P450scc, 3-beta-HSD, 20-beta-HSD, 17-beta-HSD1, P45011beta, and 11-beta-HSD2, from goldfish gonads, primers were designed for conserved amino acid sequence regions based on alignments of related gene sequences in other fish species that are closely related to goldfish (Table 2). Primers designed by Primer Premier 5.0 were compounded by Sangon Biological Engineering Technology & Services Co., Ltd., Shanghai, China. The polymerase chain reaction (PCR) was conducted with an initial denaturation step at 94 °C for 5 min, followed by 40 cycles at 94 °C for 30s, annealing temperature (Table 2) for 30s, 72 °C for 1 min, and a final extension step at 72 °C for 5 min. Amplified PCR products were cloned in pGM-T easy vector (Takara Bio Inc., Shiga, Japan) and sequenced (Sangon Biological Engineering Technology & Services Co., Ltd., Shanghai, China). MegAlign and GeneDoc programs in DNAStar were used to analyze the homology of the deduced amino acid sequences of goldfish steroidogenesis enzymes with those of other vertebrates.
Quantitative real-time PCR assays were developed to examine the expression patterns of steroidogenesis enzyme genes and StAR in response to MCP pesticide based on the cloned goldfish steroidogenesis enzyme cDNA fragments and StAR, P450c17, P450arom A, beta actin (internal control 1), and 18S rRNA (internal control 2) sequences for goldfish published in GenBank. Primers designed by Primer Premier 5.0 were compounded by Sangon Biological Engineering Technology & Services Co., Ltd., Shanghai, China (Table 3). Real-time PCR was performed in 20 μL reaction mixtures consisting of SYBR® Premix Ex Taq TM II (Takara Bio Inc., Dalian, China), 0.4 μL ROX Reference Dye II (Takara Bio Inc., Shiga, Japan), 0.8 μL sense primer (10 μM), 0.8 μL anti-sense primer (10 μM), 4.0 μL cDNA, and 4.0 μL ultrapure water (sterile). The real-time PCR reactions were incubated at 95 °C for 30s, followed by 40 cycles of 95 °C for 5s, and 60 °C for 30s. Melting curve analyses were conducted to differentiate between the desired PCR products and primer-dimers or DNA contaminants. In addition, 2% agarose gel electrophoresis of the PCR products was performed to confirm the presence of single amplicons of the correct predicted size (data not shown). Gene expression was measured in duplicate. Neither beta actin nor 18S rRNA levels were affected by any of the experimental conditions in the study. The relative target gene mRNA levels were normalized using the geometric mean of the beta actin and 18S rRNA gene expression levels following the formula 2−ΔΔCt and plotted on a logarithmic scale34.
Hormone quantification
Fish hormone ELISA Kits (R&D Systems, Inc., Minneapolis, MN, USA) were used to quantify plasma pregnenolone, progesterone, 17 alpha hydroxypregnenolone, 17 alpha hydroxyprogesterone, 17 alpha, 20 beta double hydroxyprogesterone, dehydroepiandrosterone, androstenedione, estrone, 11 beta hydroxytestosterone, and 11-KT levels according to the manufacturer’s instructions. The concentration of each hormone was calculated from the linear part of the standard curve, and plasma was diluted when the hormone concentration exceeded the linear range.
Statistics
All data were tested for normality and homoscedasticity. Kruskal-Wallis H test followed by Dunnett’s correction was used to analyze GSI, TC, HDL-C, LDL-C, dehydroepiandrosterone, 17 alpha hydroxyprogesterone, androstenedione, estrone, 3-beta-HSD, 11-beta-HSD2, 20-beta-HSD, P450scc, P450c17, P45011beta, and StAR data for an abnormal distribution or heteroscedasticity. A one-way analysis of variance (ANOVA) followed by Tukey’s test was adopted for statistical analyses of effectual hormones and the other two intermediates, as well as steroidogenesis enzymes 17-beta-HSD1 and P450arom, because all assumption were met for parametric analyses. Effect size was also considered during statistical analysis. η2 was calculated as η2 = SSeffect/SStotal for parametric analysis and η2 = H/(N−1) for Kruskal-Wallis tests. By convention, 0.01, 0.06, and 0.15 are considered “small”, “medium”, and “large”-effect sizes, respectively.
References
Liu, G. Moncrotophos pesticide residue were detected from snow peas samples in Wuhan, China. Pesticide Market News 8, 12 (2010).
Swarnam, T. P. & Velmurugan, A. Pesticide residues in vegetable samples from the Andaman Islands, India. Environ Monit Assess. 185, 6119–6127, doi:10.1007/s10661-012-3012-3 (2013).
Anjum, R. & Malik, A. Evaluation of mutagenicity of wastewater in the vicinity of pesticide industry. Environ Toxicol Pharmacol. 35, 284–291, doi:10.1016/j.etap.2012.12.013 (2013).
Tian, H., Ru, S. G., Wang, Z. Y., Cai, W. T. & Wang, W. Estrogenic effects of monocrotophos evaluated by vitellogenin mRNA and protein induction in male goldfish (Carassius auratus). Comp Biochem Physiol C Toxicol Pharmacol. 150, 231–236, doi:10.1016/j.cbpc.2009.04.014 (2009).
Tian, H., Ru, S. G., Bing, X. & Wang, W. Effects of monocrotophos on the reproductive axis in the male goldfish (Carassius auratus): potential mechanisms underlying vitellogenin induction. Aquat Toxicol. 98, 67–73, doi:10.1016/j.aquatox.2010.01.011 (2010).
Becker, M., Staab, D. & Bergmann, K. V. Treatment of severe familial hypercholesterolemia in childhood with sitosterol and sitostanol. J Pediatr. 122, 292–296, doi:10.1016/S0022-3476(06)80136-8 (1993).
Gilman, C. I., Leusch, F. D., Breckenridge, W. C. & MacLatchy, D. L. Effects of a phytosterol mixture on male fish plasma lipoprotein fractions and testis P450scc activity. Gen Comp Endocrinol. 130, 172–184, doi:10.1016/S0016-6480(02)00590-7 (2003).
Sharpe, R. L., Drolet, M. & MacLatchy, D. L. Investigation of de novo cholesterol synthetic capacity in the gonads of goldfish (Carassius auratus) exposed to the phytosterol beta-sitosterol. Reprod Biol Endocrinol. 4, 60–70, doi:10.1186/1477-7827-4-60 (2006).
Sharpe, R. L., Woodhouse, A., Moon, T. W., Trudeau, V. L. & MacLatchy, D. L. β-Sitosterol and 17β-estradiol alter gonadal steroidogenic acute regulatory protein (StAR) expression in goldfish, Carassius auratus. Gen Comp Endocrinol. 151, 34–41, doi:10.1016/j.ygcen.2006.11.005 (2007).
Govoroun, M., Mcmeel, O. M., Mecherouki, H., Smith, T. J. & Guiguen, Y. 17β-Estradiol treatment decreases steroidogenic enzyme messenger ribonucleic acid levels in the rainbow trout testis. Endocrinology. 142, 1841–1848, doi:10.1210/endo.142.5.8142 (2001).
Simard, J. et al. Molecular biology and genetics of the 3 beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. J Endocrinol. Suppl, S189–207 (1996).
Guan, G. et al. Cloning and expression of two carbonyl reductase-like 20beta-hydroxysteroid dehydrogenase cDNAs in ovarian follicles of rainbow trout (Oncorhynchus mykiss). Biochem Biophys Res Commun. 255, 123–128, doi:10.1006/bbrc.1998.0127 (1999).
Wang, Y. J. & Ge, W. Cloning of zebrafish ovarian carbonyl reductase-like 20β-hydroxysteroid dehydrogenase and characterization of its spatial and temporal expression. Gen Comp Endocrinol. 127, 219–216 (2002).
Zhuo, Q. et al. Cloning, characterization, sequence analysis and expression patterns in vivo of testicular 20β-hydroxysteroid dehydrogenase cDNA in yellow catfish (Pelteobagrus fulvidraco). Comp Biochem Physiol B Biochem Mol Biol. 159, 172–182, doi:10.1016/j.cbpb.2011.03.005 (2011).
Deb, S. & Bhattacharya, S. Circulatory cholesterol as an important source of substrate for piscine ovarian steroidogenesis. Indian J Exp Biol. 24, 71–76 (1986).
Fremont, L. & Marion, D. A comparison of the lipoprotein profiles in male trout (Salmo gairdneri) before maturity and during spermiation. Comp Biochem Physiol B. 73, 849–855, doi:10.1016/0305-0491(82)90328-5 (1982).
Babin, P. J. & Vernier, J. M. Plasma lipoproteins in fish. J Lipid Res. 30, 467–489 (1989).
Kusakabe, M., Nakamura, I., Evans, J., Swanson, P. & Young, G. Changes in mRNAs encoding steroidogenic acute regulatory protein, steroidogenic enzymes and receptors for gonadotropins during spermatogenesis in rainbow trout testes. J Endocrinol. 189, 541–554, doi:10.1677/joe.1.06684 (2006).
Zhang, W. L. et al. Molecular cloning of two isoforms of 11beta-hydroxylase and their expressions in the Nile tilapia, Oreochromis niloticus. Gen Comp Endocrinol. 165, 34–41, doi:10.1016/j.ygcen.2009.05.018 (2010).
Rajakumar, A. Endosulfan and flutamide impair testicular development in the juvenile Asian catfish. Clarias batrachus. Aquat Toxicol. 110, 123–132, doi:10.1016/j.aquatox.2011.12.018 (2012).
Yokota, H. et al. Effects of 4-tert-pentylphenol on the gene expression of P450 11β-hydroxylase in the gonad of medaka (Oryzias latipes). Aquat Toxicol. 26, 121–132, doi:10.1016/j.aquatox.2004.10.017 (2005).
Kusakabe, M., Nakamura, I. & Young, G. 11β-Hydroxysteroid dehydrogenase complementary deoxyribonucleic acid in rainbow trout: cloning, sites of expression, and seasonal changes in gonads. Endocrinology. 144, 2534–2545, doi:10.1210/en.2002-220446 (2003).
Liu, J. China farmed fish reproduction physiology 34 (Beijing, China 1993).
Senthilkumaran, B. Pesticide- and sex steroid analogue-induced endocrine disruption differentially targets hypothalamo-hypophyseal-gonadal system during gametogenesis in teleosts - A review. Gen Comp Endocrinol. 219, 136–142, doi:10.1016/j.ygcen.2015.01.010 (2015).
Yu, M. et al. Anti-estrogenic effect of semicarbazide in female zebrafish (Danio rerio) and its potential mechanisms. Aquat Toxicol. 170, 262–270, doi:10.1016/j.aquatox.2015.11.025 (2016).
Odermatt, A., Strajhar, P. & Engeli, R. T. Disruption of steroidogenesis: Cell models for mechanistic investigations and as screening tools. J Steroid Biochem Mol Biol. 158, 9–21, doi:10.1016/j.jsbmb.2016.01.009 (2016).
Tian, H., Wu, P., Wang, W. & Ru, S. G. Disruptions in aromatase expression in the brain, reproductive behavior, and secondary sexual characteristics in male guppies (Poecilia reticulata) induced by tributyltin. Aquat Toxicol. 162, 117–125, doi:10.1016/j.aquatox.2015.03.015 (2015).
Hutchinson, T. H., Ankley, G. T., Segner, H. & Tyler, C. R. Screening and testing for endocrine disruption in fish—biomarkers as “Signposts,” not “Traffic Lights,” in risk assessment. Environ Health Perspect. 114(Suppl 1), 106–114 (2006).
Seki, M. et al. Effect of ethinylestradiol on the reproduction and induction of vitellogenin and testis-ova in medaka(Oryzias latipes). Environ Toxicol Chem. 21, 1692–1698, doi:10.1002/etc.v21:8 (2002).
Pawlowski, S., van Aerle, R., Tyler, C. R. & Braunbeck, T. Effects of 17α-ethinylestradiol in a fathead minnow (Pimephales promelas) gonadal recrudescence assay. Ecotoxicol Environ Saf. 57, 330–345, doi:10.1016/j.ecoenv.2003.07.019 (2004).
Van den Belt, K., Verheyen, R. & Witters, H. Comparison of vitellogenin responses in zebrafish and rainbow trout following exposure to environmental estrogens. Ecotoxicol Environ Saf. 56, 271–81, doi:10.1016/S0147-6513(03)00004-6 (2003).
Van den Belt, K., Berckmans, P., Vangenechten, C., Verheyen, R. & Witters, H. Comparative study on the in vitro/in vivo estrogenic potencies of 17β-estradiol, estrone, 17α-ethynylestradiol, and nonylphenol. Aquat Toxicol. 66, 183–195, doi:10.1016/j.aquatox.2003.09.004 (2004).
Fenske, M., van Aerle, R., Brack, S., Tyler, C. R. & Segner, H. Development and validation of a homologous zebrafish vitellogenin ELISA and its application for studies on estrogenic chemicals. Comp Biochem Physiol C Toxicol Pharmacol. 129, 217–232, doi:10.1016/S1532-0456(01)00194-6 (2001).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, 1–7, doi:10.1186/gb-2002-3-7-research0034 (2002).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31202001).
Author information
Authors and Affiliations
Contributions
Hua Tian and Shaoguo Ru designed the experiment and wrote the manuscript. Yang Sun and Hui Wang undertook the experimental tasks and data processing work. Xin Bing and Wei Wang offered advice on this study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, H., Sun, Y., Wang, H. et al. Monocrotophos pesticide affects synthesis and conversion of sex steroids through multiple targets in male goldfish (Carassius auratus). Sci Rep 7, 2306 (2017). https://doi.org/10.1038/s41598-017-01935-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01935-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.