Abstract
Citrus black spot (CBS) caused by Phyllosticta citricarpa, is the most recent introduction of an exotic citrus pathogen into Florida and has been a challenge to control to date. Understanding the dispersal pattern of the disease within affected groves is vital in developing effective control strategies to limit the spread of the disease. The spatial pattern of CBS-affected trees was studied in two commercial ‘Valencia’ orange groves over three consecutive citrus seasons. Cluster analyses based on nearest-neighbor distance (F, G and J-functions) and pairwise distances between points (Ripley’s K function, Besag’s L function and the pair correlation function, g) were used to test the hypothesis of complete spatial randomness (CSR) of CBS infected trees within the groves. In both groves, the hypothesis of CSR was rejected for all tests performed including quadrats testing (2 × 2 trees up to 10 × 10 trees). The relationship between tree age and disease was assessed at one experimental site. Citrus trees bearing fruit for the first time accounted for approximately 13% of trees positive for disease and were located within areas of heavy disease pressure. These findings support short distance movement of inoculum as the main spread of disease in the groves studied.
Similar content being viewed by others
Introduction
Phyllosticta citricarpa the causal agent of citrus black spot (CBS) was first discovered in southwest Florida in April 20101, Uganda in 20062, Cuba in 20103 and more recently in Ghana in 20124. Under favorable conditions new infections can be initiated by either the sexual or asexual spore (ascospore or conidia, respectively). Ascospores are considered the major source of inoculum5, 6 and are released from pseudothecia typically formed in infected leaf litter following periods of wetting and drying. The sexual spores are released during wetting events, ejected into the air and dispersed into the canopy and beyond by wind, constituting long distance dispersal7. Conidia are the asexual spore produced in the leaf litter and on infected fruits, twigs and other infected citrus tissue. Conidia rely on splash dispersal to reach susceptible tissue, typically infecting susceptible tissue below its site of origin. Conidia produced in the leaf litter can only reach susceptible tissue through splash dispersal and hence has historically been considered to have a minor role in the disease cycle6. Once spores land on susceptible tissue, they penetrate the cuticle and epidermis to form a quiescent infection. Symptom expression is usually not seen in citrus leaves with the exception of lemons but occurs on the fruit as the fruit ripens.
In Florida however, mating type locus studies have not been able to find both mating types necessary for the production of ascospores8. This may indicate an atypical disease cycle in Florida. Based on this criterion it is believed that new infections are due to short range dispersal of the asexual spore, the conidia within the grove. Recent studies done in the laboratory suggest that conidia can be spread by splash and wind9 within tree-to-tree distances currently found in Florida groves.
Southwest Florida’s climate is divided into two distinct seasons based on rainfall pattern. The rainy season begins in June and extends to October accounting for approximately 70% of annual rainfall. The dry season runs from November through to May. Annual temperatures range from 15–18 °C to the high 20 s °C, with average temperatures between 23 and 24 °C, encompassing the optimum temperature for ascomata formation (21–28 °C)7, 10. In addition to the rainfall duality, Florida has a history of hurricanes and tropical storms. The latter is of much concern as conditions associated with these events have been associated with long-distance dissemination of citrus pathogens such as Xanthomonas citri subsp. citri, the causal agent of citrus canker11,12,13,14,15. It is possible that these events may play a significant role in future movement of P. citricarpa in the state of Florida.
The environmental conditions conducive for infection by P. citricarpa are temperatures between 24 and 30 °C16 and a minimum leaf wetness of 8 to 12 hours at optimal temperatures9, 17. Ascospore release occurs within a minimum of 20 min of leaf wetting10 and infection and colonization of susceptible tissue have been estimated at a minimum of 15 h in the presence of free standing water18. Our hypothesis was that the disease exhibits a clustered pattern in support of the conidia as the major source of inoculum within an infected grove in Florida rather than the ascospore.
Results
Weather Data and Susceptibility
Between January 1, 2010 and December 31, 2015 there were a total of 97, 44 and 139 days where ‘Valencia’ citrus fruit were susceptible to infection by the fungus and weather parameters were conducive to infection for IN1, IN2 and IN3 respectively (Table 1). Based on predicated flush of ‘Valencia’ sweet oranges in the study area, susceptible fruit were on the tree between April and August of each year. In 2011, 2012, 2013 and 2015, susceptible fruit were on the trees as early as the latter portion of February. In 2010, 2011, 2013, 2014 and 2015, susceptible fruit were expected to be on the tree into the early portions of September. Rainfall data for susceptible months is given in Table 2.
Spatial Pattern
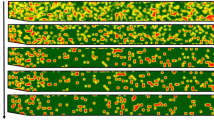
In Grove I, the disease progressed eastward as reflected in the increase in the disease incidence and enlarging of the plots in an easterly direction as shown in Fig. 1A–C, from 100 meters (2013–2014 citrus season) to 150 meters (2015–2016 citrus season). Incidence increased from approximately 2.7% at the beginning of the study to 9.3% in the third year of study. Intensity increased with each season from 0.0010 (2013–2014) to 0.0013 trees per m2 (2014–2015) and finally 0.0034 trees per m2 during 2015–2016 citrus season. In Grove II, the disease incidence in the study area increased from 6.95% during the 2013–2014 citrus season to 26.57% in the 2015–2016 citrus season. Most of the infected fruit could be found in the northern region of the study area (Fig. 2A–C). The average intensity increased from 0.0029 to 0.012 CBS positive trees per m2 between the 2013–2014 and 2015–2016 citrus seasons. Quadrat analysis indicated clustering of diseased trees in both groves from the smallest quadrat size (2 trees × 2 trees) to the largest (10 trees × 10 trees; Table 3).
Grove I point pattern analyses. Comparisons of point pattern analyses for Grove I for three contiguous citrus seasons (Panel A) 2013–2014, (Panel B) 2014–2015 and (Panel C) 2015–2016. Each panel consists of a plot map of CBS positive trees (open circles; left), edge-corrected F- and G-functions (middle) and edge-corrected Ripley’s K-function (right). Positive trees contain at least one fruit with a single hard-spot lesion. Values of FCBS(r) < FCSR(r), and of GCBS(r) > GCSR(r) that falls outside of the Monte Carlo envelopes, indicate statistically significant clustering. For each graph, the red line corresponds to a Poisson process representing complete spatial randomness (CSR) and the blue shaded area is the Monte Carlo envelope that corresponds to the 5th to 95th percentiles of CSR, F(r), G(r) and Ripley’s K(r) respectively for values based on randomizations of the original data.
Grove II point pattern analyses. Comparisons of point pattern analyses for Groves II for three contiguous citrus seasons (Panel A) 2013–2014, (Panel B) 2014–2015 and (Panel C) 2015–2016. Each panel consists of a plot map of CBS positive trees (open circles; left), edge-corrected F- and G-functions (middle) and edge-corrected Ripley’s K-function (right). Positive trees contain at least one fruit with a single hard-spot lesion. Values of FCBS(r) < FCSR(r), and of GCBS(r) > GCSR(r) that falls outside of the Monte Carlo envelopes, indicate statistically significant clustering. For each graph, the red line corresponds to a Poisson process representing complete spatial randomness (CSR) and the blue shaded area is the Monte Carlo envelope that correspond to the 5th to 95th percentiles of CSR, F(r), G(r) and Ripley’s K(r) respectively for values based on randomizations of the original data.
The three nearest-neighbor functions, F, G and J indicated that CBS positive trees were clustered in both Grove I and Grove II for all 3 years (Figs 1 and 2 respectively). Ripley’s K function, Besag’s L function and the pair correlation function also confirmed clustering of CBS positive trees. For Grove I and II, Ripley’s K indicated statistically significant clustering above 4.08 m and 6.5 m respectively regardless of year.
Grove II: Tree Age and Citrus Black Spot
In the 2014–2015 citrus season, there were 1499 trees classified as resets and of these 98% (1471) fruited in the following citrus season. One hundred and twenty-two (8.3%) of these newly fruiting resets contained fruit that was positive for CBS in the 2015–2016 citrus season. The vast majority of these positive fruiting resets were located within the northern portions of the study area where disease pressure was highest (Fig. 3).
Grove II: Fruiting resets for the 2015–2016 citrus season. (A) – Spatial distribution of fruiting resets (open circles). Quadrat analysis of fruiting resets regardless of CBS status indicated random distribution of fruiting resets for 2 × 2 tree quadrats (dispersion index, D = 1.02; p-value = 0.347). For quadrat sizes 3 × 3 trees to 10 × 10 trees fruiting resets were statistically significantly clustered with dispersion indices, D ranging from 1.32 to 4.15; p < 0.001 for all quadrats. (B) – Spatial distribution of fruiting resets with fruit positive for citrus black spot (open circles). Quadrat analysis indicated disease clustering in fruiting resets with fruits positive for citrus black spot for all quadrat sizes tested (2 × 2 trees to 10 × 10 trees; D ranging from 1.25 to 11.03, p < 0.001 for all quadrat sizes). (C) – Column chart comparing the proportion of trees classified as fruiting resets and fruiting trees containing at least one fruit with a single hard-spot lesion in the 2014–2015 and 2015–2016 citrus season. Fruiting resets represent resets fruiting for the first time in their respective seasons.
During the 2015–2016 citrus season, trees were categorized as new resets, resets, fruiting resets and fruiting trees. There were 1153 trees with fruit positive for CBS, of these 148 were fruiting resets, accounting for approximately 13% of positives and 1005 were fruiting trees (87.2%). Fruiting resets made up 36.4% (1330/3656) of fruiting citrus within the study, while fruiting trees made up the remaining 63.6% of which 43.2% contained fruit positive for CBS. For trees with CBS positive fruit, there were significantly less resets fruiting for the first time (Category 3) in the 2015–2016 citrus season than fruiting trees (Category 4; 12.8% vs 87.2%; χ 2 p < 0.0001).
The average intensity for fruiting reset (Category 3) was 0.013475 per m2 in the 2015–2016 citrus season within the study area. Second-order characteristics, Ripley’s K, Besag’s L and G functions all indicate complete spatial randomness above 10 meters. For fruiting resets with CBS positive fruit, the average intensity was 0.001499 per m2 during the 2015–2016 citrus season. The nearest-neighbor, F, G and J functions all indicate significant clustering between 4 and 15 meters. While the second-order characteristics, Ripley’s K and Besag’s L functions indicate significant clustering between 4 and 35 meters.
Sample Size
Using the observed annual proportions of infected trees as p in eq. (2), the minimum number of citrus trees needed to estimate disease incidence within each grove with 20% relative precision and 95% confidence was 1331, 1184 and 647 for Grove I for the 2013–2014, 2014–2015 and 2015–2016 citrus seasons respectively. For Grove II, minimum sample size estimates were 979, 413 and 251 for citrus seasons 2013–2014, 2014–2015 and 2015–2016 respectively. Using the observed annual proportion of infected quadrats as p (Table 3), the number of 2 by 2 quadrats required was 385, 347 and 217 for Grove I and 290, 124 and 74 from Grove II for citrus seasons 2013–2014, 2014–2015 and 2015–2016 respectively. The number of quadrats required to estimate the incidence of CBS with 95% confidence and within 20% of the true incidence in Grove I is shown for each quadrat size (2 by 2 to 10 by 10) in Fig. 4.
For the 2014–2015 citrus season, a total of 60,391,000 bearing citrus trees (orange, grapefruit and tangerine) were reported on a total of 456,000 acres in Florida, with an average of 132 trees per acre. At the lowest incidence rate surveyed of 2.7% (Grove I, 2013–2014 citrus season) there is a 95% chance that a positive tree will be observed in 83 or fewer surveyed trees within an average acre planting of 132 trees. At the highest incidence rate surveyed of 26.6% (Grove II, 2015–2016 citrus season) there is a 96% chance that a positive tree will be observed in 10 or fewer surveyed trees within an average acre planting of 132 trees. As the percent incidence of disease decreases the number of trees needed to be surveyed before finding a positive tree will increase.
Discussion
The objective of this study was to determine the spatial distribution patterns within two CBS positive groves over time. The results indicate that the spatial pattern is non-random, i.e. evidence of CBS is clustered at all levels of analysis, similar to the situation found in Brazil19. A caveat to this study is the fact that symptom expression is required to positively identify a CBS infected tree. This does not mean that trees with no symptoms are negative as the organism has an extensive latent period6, 20.
This finding of clustering of the disease symptoms within affected groves may indicate that the spread of disease occurs over very short distances, which may be attributed to the asexual spores (conidia). Inoculum sources of P. citricarpa are the conidia and ascospore (the sexual spore). The conidia are produced on infected deadwood, twigs, leaves and fruits and are distributed by water (irrigation or rainfall). Once pycnidia mature, moisture events initiate the release of conidia as a mucilaginous mass from the osteole. These can then either be splash dispersed to nearby susceptible tissue or splash–wind driven. Splash dispersal of conidia from infected material is most likely the source of infection within the tree and between neighboring trees within rows in this study. Wind driven rain is likely the conveyor of conidia between rows (across swales and roadways, approximately 6.7 m or less). Recent findings suggests that rain combined with wind speeds of 7 m/s (25.2 km/h) has the potential of carrying aerosolized droplets across a distance of 8 m and to heights of 75 cm9. In addition, droplets ≤1 mm were likely to contain 0 to 4 conidia9. Together this may indicate in theory the potential for single conidia to travel greater distances that previously thought.
Field studies in Brazil indicate short distance dispersal of <0.8 m when symptomatic fruits and twigs were used as inoculum sources in a CBS–free experimental grove21. This distance is well below the average spacing in Grove II (6.70 m × 3.36 m) but within the canopy distances among trees (Category 4) where branches may overlap to form a continuous canopy. In this study where newly fruiting resets have been planted consecutively in rows (3.36 m spacing) and across rows (6.70 m spacing) well outside the 0.8 m of splash dispersed conidial influence, a large portion of these within the northern area of the study were found to have fruit with CBS symptoms. This along with published data suggesting that the sexual spore, which is thought to be responsible for long distance dissemination of the disease has not been found in Florida8, 22 suggest that the conidia is travelling greater distances than previously attributed to it. Whether this is on infected twigs and leaves from diseased trees or by wind driven rain/irrigation is not known. One could speculate that the sheer numbers of conidia present in the northern portion of the study area increases the likelihood of wind driven rain/irrigation containing conidial spores. It is more likely that under hurricane or tropic storm conditions, diseased twigs and branches (with or without fruit) may disseminate the disease over greater distances as seen with citrus canker11,12,13,14,15.
A combination of events is required for the spread of disease. For citrus black spot these are the presence of the disease causing agent – Phyllosticta citricarpa; the correct environmental conditions for infection and establishment of the disease; and, the presence of susceptible tissue – citrus (fruit, leaves and twigs). All three exist in Florida. Published data taken from other regions where citrus black spot is endemic have been used in lieu of local information, due to the lack thereof, to estimate environmental conditions conducive to infection. In southwest Florida between 2010 and 2015, suspect favorable environmental conditions for CBS infection occurred consistently between April and August of each year. In some years, these conditions appeared to start as early as February and extended until October. This was due to a combination of total daily rainfall ≥0.25 mm, temperatures between 24 and 40 °C for a minimum of 8 hr and the presence of susceptible fruit (fruit set to 5 months post fruit set) occurring within the same time frame and geographical location. These conditions increase the likelihood for conidia maturation and release, splash dispersal onto susceptible tissue and infection of said tissues. A caution to the strict definitions used here for assigning periods of total daily rainfall ≥0.25 mm is the consideration that extended periods of rainfall or heavy rainfall have the potential to wash inoculum out of the trees and away from susceptible tissues. Secondly, although the data for weather conditions are relevant to the Immokalee area, within grove weather data would have allowed the definitions for IN1 thru IN3 to be more accurately determined, as irrigation events and tree cover tends to increase and maintain high humidity for longer periods of time than in an open field. Even with these considerations, the environmental conditions above may have played a role in the increase incidence of disease seen over the three year study.
The incidence of CBS increased from 2.7% to 9.3% in Grove I and 7% to 26.6% in Grove II over the three seasons. This increase is not unique to Florida, as a doubling of disease incidence within a single season (10.7% to 22.7%) has been documented in ‘Valencia’ groves in Brazil19. And when considering ‘Valencia’ groves where there is an overlap of fruit between the current (for example the 2013–2014 crop) and the following season (2014–2015 crop) there is more likelihood of infected fruits contributing to the following season’s disease incidence. Using IN2 to define days when environmental conditions were conducive for infection, there were on average 125 days (range 107–136) where the humidity was high enough (>90% for 8 consecutive hours) to maintain standing water on leaves and fruits, perhaps even to runoff. Timmer and colleagues noted that, “In Florida, leaf wetness durations exceed 3 h nearly every night and are often 10 to 12 h”23. Guignardia spp, such as G. psidii which causes black spot symptoms on guava required as little as 6 hr of wetting for germination and appressorium formation24 and to establish disease25. Similarly conidia of G. bidwellii, which causes black rot of grapes, requires a minimum of 6 hr to cause infection on leaves at 26.5 °C and 9 hr at 29 °C26. It is plausible to speculate that the environmental conditions defined by IN1, IN2 and IN3 contributed to the increase in diseased incidence from one citrus season to the next.
A number of cultural practices have been suggested to reduce and/or control CBS. These include the removal of infected fruit to reduce inoculum load especially where there is an overlap of fruit as occurs in ‘Valencia’ groves. Harvest of ‘Valencia’ oranges took place between March and April of each year of study. Despite the removal of infected fruit during harvest, the incidence of CBS still increased over the three seasons. It is possible that a portion of these fruit were infected during the first 20 to 40 days post fruit set due to inoculum from ripening fruit. Additionally splash dispersal from other infected citrus tissues, such as dead twigs within the canopy27 may have also contributed to the increase in incidence of the disease over the three years of study as has been suggested by research in Brazil21. Failure to remove off season infected fruit, trimming and removal of dead tree limbs and twigs also contributes to sustaining inoculum sources in the grove.
Long distance dispersal of the disease has been attributed to the ascospore in South African, Asia and Australia. However, in Brazil whose micro-climates are closest to that of Florida, the conidia are considered the major source of disease spread21. Additionally, there is no evidence at this time that both mating types are present in Florida8 and multi-locus studies of P. citricarpa suggest a clonal lineage22. These studies could indicate the introduction and spread of the disease in Florida may be attributed solely to the asexual spores – the conidia. However the potential for long and short distance spread due to personnel and equipment moving inoculum must be considered. At this time, there is no evidence in the literature that the inoculum can be spread on clothing, equipment and/or machinery.
Rapid turnover and replanting of citrus trees within Grove II allowed for the investigation into the relationship between tree age and disease, unlike Grove I where there was little to no replanting. In Grove II, while mature fruiting trees were the predominant trees with CBS positive fruit, newly fruiting resets accounted for 13% of the positives finds during the 2015–2016 season. Previous literature has suggested and demonstrated that CBS infected dead twigs serve as a sources of inoculum6, 21 and this has been interpreted by some that only dead twigs can be colonized and serve as an inoculum source. Younger trees tend to have less woody branches and are less likely to contain dead twigs for P. citricarpa colonization. However, recent research had demonstrated that healthy green twigs are colonized by the fungus and once detached from the tree can produce viable inoculum within the first 45 days27.
The approach to sample size calculation was much different than formerly proposed by Sposito and colleagues28. Under the assumption that trees are evaluated at random, the type of spatial distribution of the CBS infection becomes irrelevant to estimating the proportion of CBS infected citrus trees. Of importance here is obtaining a sample size large enough to give an estimate within ±20% of the true population incidence of CBS. The sample sizes used in Groves I and II for each season of study proved more than adequate, especially as the incidence of CBS increased from year to year. The caveat to this is at very low or very high incidence of disease, simple random sampling may be inadequate to access the true disease incidence29, especially when individual trees are examined. However to overcome this inadequacy, quadrats can be established throughout the grove, with random quadrats as the sampling unit, this is the equivalent to cluster sampling29. In that case, all citrus trees within a randomly selected quadrat are examined and thus using a quadrat approach may be more efficient than randomly sampling individual trees in the grove.
Citrus black spot disease is clustered in the groves evaluated in this study in Florida, indicating short distance movement of the inoculum. The disease has the ability to progress rapidly under optimal environmental conditions of which southwest Florida exemplifies. Under heavy disease pressure, newly fruiting resets are more likely to have fruit exhibiting disease symptoms. Further understanding of the movement of inoculum in the grove and inoculum sources are required to devise better management systems to reduce spread of this disease.
Methods
Data collection
The incidence of trees with fruit exhibiting citrus black spot lesions was assessed in two commercial citrus groves in Florida between 2013 and 2016. In Florida, citrus is grown with irrigation ditches (swale) or a road (drivable surface) between alternating rows – row-ditch-row-road-row-ditch. Preliminary grove maps were prepared to assess the planting block. Next, fruits were evaluated between November and April of each citrus season following color break for the presence of hard spots. The hard spot lesion was chosen due to its ease of recognition and distinctiveness as a symptom of CBS. The exposed surfaces of fruits on each tree were observed for hard spots while walking along the swale and road19. If a single fruit on a tree was positive for blackspot, a thorough search of neighboring trees was carried out. The location of each tree with symptomatic fruit was plotted on their respective grove maps. Grove characteristics are given in Table 4.
Weather Data
Groves are located in and around the Immokalee, FL area. Grove I (26°32′55.7″N 81°28′54.2″W) and Grove II (26°21′58.5″N 81°24′29.5″W) are approximately 10.46 km NNW and 11.08 km SSE of the Florida Automated Weather Network’s (FAWN) Immokalee station (26°27′43.5″N 81°26′25.9″W). The FAWN Immokalee station was used to gather 15 minute data on soil temperature, air temperature at 0.6, 2 and 10 m; relative humidity (RH) at 2 m (%), dew point at 2 m, rainfall (mm), wind speed at 10 m and solar radiation between January 1, 2010 and January 1, 2016. A 24-hr period was defined as starting at 12:00 am extending to 11:59 pm the following night. A rainy day was defined as a day when the measured rainfall was greater-than or equal to 0.25 mm within a 24 h period. A wet canopy (leaves and fruit) was defined as a day when the RH was equal to and greater than 90% for at least 8 consecutive hours30. Data on fruit set was extrapolated and estimated from data on flower bloom on the Flower Bud Induction Overview and Advisory web page (http://www.crec.ifas.ufl.edu/extension/flowerbud/index.shtml) and from the Citrus Flowering Monitor model at http://disc.ifas.ufl.edu/bloom. Search parameters were set to: Weather Station: Immokalee, Cultivar: Valencia, Expected Yield: Average/Low, Tree age: 4 or more years, Soil type: Deep sands and Date: according to year (2010–2015). Average duration of bloom in individual groves has been estimated at 12 to 20 days31. Bloom does not occur simultaneously, as such, estimated dates assumed to contain blooms were set to 9-days prior and –post bloom dates. Fruit set in ‘Valencia’ oranges is reliant on pollination. The number of days from pollination to fruit set was estimated as 14 days, based on work done by Mesejo and colleagues32. These data were used to estimate periods when conditions for infection were likely in the study groves. Fruit are susceptible to infection beginning at fruit set and extending up to 5 months following fruit set. From these data, three separate categories of potential for infection were defined:
IN1 = RH ≥ 90% for 8 hr + Total daily rainfall ≥0.25 mm + Temperature between 24 and 40 °C + susceptible fruit (fruit set to 5 months post fruit set).
IN2 = RH ≥ 90% for 8 hr + Temperature between 24 and 40 °C + susceptible fruit (fruit set to 5 months post fruit set).
IN3 = Total daily rainfall ≥0.25 mm + Temperature between 24 and 40 °C + susceptible fruit (fruit set to 5 months post fruit set).
Spatial and Statistical analysis
The spatial pattern of trees with CBS positive fruit in each grove was analyzed using PROC SPP (SAS v9.4, SAS Institute Inc., Cary, NC). Briefly, a density-based approach was used to describe the first-order spatial properties of CBS in the mapped groves for each citrus season. Statistics based on nearest-neighbor distance and pairwise distances between points were used to test the hypothesis of complete spatial randomness (CSR) of disease (citrus black spot), that is, whether the diseased trees were clustered or not, in the study area of Grove I and Grove II.
Three nearest-neighbor functions, F, G and J, were used to assess clustering or regularity of trees with CBS positive fruit in each grove. These functions compare the empirical distribution function (EDF) calculated from the observed disease in the grove to the expected EDF for a pattern of CSR, in this case a homogenous Poisson process that has a first-order intensity λ, using a Monte Carlo simulation approach. The first-order intensity is computed from the number of trees with CBS positive fruit within the study area. The F-function is also known as the empty-space function, that is, it quantifies spatial interaction based on an event (tree with CBS positive fruit) proximity to voids. For distance r, when values of FCBS(r) < FCSR(r), and fall outside of the Monte Carlo envelopes, then the spatial pattern of trees with CBS positive fruit is statistically significantly clustered. The G-function quantifies spatial interactions based on event to nearest event distances, that is, from a tree with CBS positive fruit to the nearest trees with CBS positive fruit. In the case of the G-function, for distance r, when values of GCBS(r) > GCSR(r), and falls outside of the Monte Carlo envelopes, then the spatial pattern of trees with CBS positive fruit is statistically significantly clustered. Finally, the J-function compares the F-function and G-function and is written as:
A homogenous Poisson process has a theoretical value of 1, and clustering is indicated when JCBS(r) < 1.
Second-order characteristics, that is, pairwise distances between diseased trees were used to assess clustering versus regularity. Ripley’s K function, Besag’s L function and the pair correlation function were used to assess spatial patterns of citrus black spot in the study area of Grove I and Grove II. Ripley’s K function quantifies interaction in spatial point patterns by assessing the number of trees with CBS positive fruit within a radius, r, around each diseased tree. For Ripley’s K function, when values of KCBS(r) > KP(r) = πr2, for a homogeneous Poisson process within a distance of r from an arbitrary diseased tree, then the spatial pattern of CBS is clustered. In the case of Besag’s L function, which is a transformation of the K function, when values of LCBS(r) > LP(r), and falls outside of the Monte Carlo envelopes, then the spatial pattern of CBS is statistically significantly clustered. Besag’s L function was utilized for ease of interpretation as it has the properties of stabilizing the variance of the K-function and is relatively constant under CSR. Finally, the pair correlation function for a homogenous Poisson process has a theoretical value of 1, and clustering is indicated when g CBS(r) > 1 at distance r. Additionally, because all distance functions are influenced by edge effects, border edge correction is accounted for in the F, K and G functions33. All test were considered statistically significant at p < 0.05.
Tree Age and Citrus Black Spot
During the 2013–2014 citrus season, at the beginning of the study, trees were either classified as resets (trees that were newly planted but had not begun to fruit) and fruiting trees – trees which contained citrus fruit at the time of the survey. In the 2014–2015 and 2015–2016 citrus seasons trees were further categorized into five groups based on approximate age and fruiting status. Tree were classified as indicated: 4 = trees > 4 years in the grove, fruiting during the 2013–2014 citrus season at the beginning of the study; 3 = fruiting resets, resets fruiting for the first time in the 2014–2015 citrus season (2–4 years); 2 = Resets, not yet fruiting (1.5–2 years); 1 = new resets and newly planted resets (1–1.5 years). Statistics based on nearest-neighbor distance and pairwise distances between points were used to test the hypothesis of complete spatial randomness (CSR) for resets bearing fruit for the first time in Grove II during the 2015–2016 citrus season. Additionally, CSR for fruiting resets with citrus black spot positive fruit during the 2015–2016 citrus season was analysed using PROC SPP (SAS v9.4, SAS Institute Inc., Cary, NC). The nearest-neighbor functions, F, G and J, and the second-order characteristics, Ripley’s K, Besag’s L and G functions were used to assess spatial patterns as previously described above. The distribution of trees (Category 3 and 4) among trees with CBS positive fruit were calculated and measures of association determined using the χ2-statistic (PROC FREQ; SAS v9.4, SAS Institute Inc., Cary, NC). All tests were considered statistically significant at p < 0.05.
Sample Size Estimation
Data on the observed proportion of CBS positive events, P, either individual trees or quadrats with varying number of trees (2 × 2 to10 × 10; Table 3) from Grove I and II for each citrus season and overall incidence were used to generate estimates of sample size. Sample size estimation was made in order to answer two fundamental questions. (1) How many trees or quadrats need to be surveyed in order to accurately predict the incidence of disease within a FL grove with a relative precision of 20% and (2) how many citrus trees have to be surveyed in order to find fruit symptoms of the disease with 95% probability ? For both approaches the following assumptions were made:
-
(i)
Sampling is carried out after color break when symptoms of citrus black spot are evident on the fruit.
-
(ii)
Sampling of trees or quadrats is random without replacement, i.e. quadrats or individual trees within the grove are sampled randomly and once sampled will not be reassessed for disease within a single citrus season.
Given that sampling is completely random and without replacement, the number of infected sampling units, m, in a random sample of n infected sampling units has a hypergeometric distribution. Hence, based on large-sample theory for the sample proportion, the number of citrus trees or quadrats (sample size), n, needed to estimate disease incidence within a grove with (1−α)100% confidence and a relative precision of ε (%) is given by
where N is the total number of citrus trees in the grove, p is the incidence of CBS and \({z}_{1-\frac{\alpha }{2}}\) is the standard normal distribution quantile associated with (1−α)100% confidence (e.g. z = 1.96 for α = 0.05).
The value used in eq. (2) for p should be based on prior knowledge or expert opinion as to the likely true incidence of disease in the grove. We calculated the required sample size needed for sampling individual trees and for sampling quadrats of size 2 × 2 to 10 × 10.
To estimate the number of trees that must be scouted in order to be (1−α)100% sure that the grove is infected, that is, number of trees assessed until the first CBS positive tree is observed we used the cumulative distribution function for the negative hypergeometric distribution
where N is the total number of citrus trees in the grove and M is the number of trees with CBS. The number of infected trees to be observed before scouting is stopped and thus the grove is labelled as infected is set to r = 1 and the number of trees surveyed before disease is found is given by y, where y = (r, r + 1, r + 2, …, r + N − M).
The average number of trees per acre was calculated from the Florida Citrus Statistics 2014–2015 data, based on the total number of bearing orange, grapefruit and tangerine trees for that period, and the total estimated acreage. Calculations are based on the estimated trees per acre.
References
Schubert, T. S. et al. First report of Guignardia citricarpa associated with citrus black spot on sweet orange (Citrus sinensis) in North America. Plant Dis 96, 1225–1225, doi:10.1094/Pdis-01-12-0101-Pdn (2012).
Reeder, R., Kelly, P. L. & Harling, R. First confirmed report of citrus black spot caused by Guignardia citricarpa on sweet oranges (Citrus sinensis) in Uganda. Plant Pathol 58, 399–399, doi:10.1111/j.1365-3059.2008.01966.x (2009).
Hidalgo Góngora, E. I. & Pérez Vicente, L. Diferenciación morfológica, cultural y biológica de Guignardia citricarpa y Guignardia mangiferae en frutos cítricos de Cuba. Fitosanidad 14, 141–152 (2010).
Brentu, F. C. et al. Crop loss, aetiology, and epidemiology of citrus black spot in Ghana. Eur J Plant Pathol 133, 657–670, doi:10.1007/s10658-012-9944-1 (2012).
McOnie, K. C. Germination and infection of citrus by ascospores of Guignardia citricarpa in relation to control of black spot. Phytopathology 57, 743–746 (1967).
Kotze, J. M. Epidemiology and control of citrus black spot in South Africa. Plant Dis 65, 945–950 (1981).
Huang, C. S. & Chang, S. L. Leaf infection with citrus black spot and perithecial development in relation to ascospore discharge of Guignardia citricarpa Kiely. Journal of Taiwan Agricultural Research 21, 256–263 (1972).
Wang, N. Y., Zhang, K., Huguet-Tapia, J. C., Rollins, J. A. & Dewdney, M. M. Mating type and simple sequence repeat markers indicate a clonal population of Phyllosticta citricarpa in Florida. Phytopathology 106, 1300–1310, doi:10.1094/Phyto-12-15-0316-R (2016).
Perryman, S. A. M., Clark, S. J. & West, J. S. Splash dispersal of Phyllosticta citricarpa conidia from infected citrus fruit. Sci Rep-Uk 4, doi:10.1038/srep06568 (2014).
Lee, Y. S. & Huang, C. S. Effect of climatic factors on the development and discharge of ascospores of the citrus black spot fungus. Journal of Taiwan Agricultural Research 22, 135–144 (1973).
Bock, C. H., Parker, P. E. & Gottwald, T. R. Effect of simulated wind-driven rain on duration and distance of dispersal of Xanthomonas axonopodis pv. citri from canker infected citrus trees. Plant Dis 89, 71–80, doi:10.1094/Pd-89-0071 (2005).
Graham, J. H., Gottwald, T. R., Cubero, J. & Achor, D. S. Xanthomonas axonopodis pv. citri: Factors affecting successful eradication of citrus canker. Mol Plant Pathol 5, 1–15, doi:10.1046/J.1364-3703.2003.00197.X (2004).
Gottwald, T. R., Graham, J. H. & Egel, D. S. Analysis of foci of Asiatic citrus canker in a Florida citrus orchard. Plant Dis 76, 389–396 (1992).
Gottwald, T. R., Graham, J. H. & Schubert, T. S. An epidemiological analysis of the spread of citrus canker in urban Miami, Florida, and synergistic interaction with the Asian citrus leafminer. Fruits (Paris) 52, 383–390 (1997).
Gottwald, T. R., Hughes, G., Graham, J. H., Sun, X. & Riley, T. The citrus canker epidemic in Florida: The scientific basis of regulatory eradication policy for an invasive species. Phytopathology 91, 30–34, doi:10.1094/Phyto.2001.91.1.30 (2001).
Yonow, T., Hattingh, V. & de Villiers, M. CLIMEX modelling of the potential global distribution of the citrus black spot disease caused by Guignardia citricarpa and the risk posed to Europe. Crop Prot 44, 18–28, doi:10.1016/j.cropro.2012.10.006 (2013).
Noronha, M. D. A. Escala diagramatica para avaliacao da mancha preta em folhas de citros e efeito da temperatura e da duracao do molhamento na pre-penetracao de conidios de Guignardia citricarpa Kiely [Phyllosticta citricarpa (McAlp.) Van der Aa] Masters Dissertation. thesis, University of São Paulo (USP) (2002).
Kotze, J. M. Studies on the black spot disease of citrus caused by Guignardia citricarpa Kiely, with particular reference to its epiphytology. Thesis 148 (1963).
Sposito, M. B., Amorim, L., Ribeiro, P. J., Bassanezi, R. B. & Krainski, E. T. Spatial pattern of trees affected by black spot in citrus groves in Brazil. Plant Dis 91, 36–40, doi:10.1094/Pd-91-0036 (2007).
Aguiar, R. L., Scaloppi, E. M. T., Goes, Ad, Sposito, M. B. & de Goes, A. Incubation period of Guignardia citricarpa at the different phenological stages in sweet orange ‘Valencia’ Periodo de incubacao de Guignardia citricarpa em diferentes estadios fenologicos de frutos de laranjeira ‘Valencia’. Trop Plant Pathol 37, 155–158, doi:10.1590/s1982-56762012000200010 (2012).
Sposito, M. B. et al. Relative importance of inoculum sources of Guignardia citricarpa on the citrus black spot epidemic in Brazil. Crop Prot 30, 1546–1552, doi:10.1016/j.cropro.2011.08.007 (2011).
Zavala, M. G. M. et al. Genetic variation among Phyllosticta strains isolated from citrus in Florida that are pathogenic or nonpathogenic to citrus. Trop Plant Pathol 39, 119–128 (2014).
Timmer, L. W., Zitko, S. E., Gottwald, T. R. & Graham, J. H. Phytophthora brown rot of citrus: Temperature and moisture effects on infection, sporangium production, and dispersal. Plant Dis 84, 157–163, doi:10.1094/Pdis.2000.84.2.157 (2000).
Escanferla, M. E., Moraes, S. R. G., Salaroli, R. B. & Massola Junior, N. S. Prepenetration stages of Guignardia psidii in guava: Effects of temperature, wetness duration and fruit age. J Phytopathol 157, 618–624, doi:10.1111/j.1439-0434.2008.01505.x (2009).
Soares-Colletti, A. R., Fischer, I. H., Lourenco, Sd. A. & de A. Lourenco, S. The effects of temperature and wetness duration on the development of Guignardia psidii in guava fruit naturally infected. Australas Plant Path 44, 413–418 (2015).
Spotts, R. A. Effect of leaf wetness duration and temperature on infectivity of Guignardia bidwellii on grape leaves. Phytopathology 67, 1378–1381 (1977).
de Oliveira Silva, A. et al. Epidemiological aspects of Phyllosticta citricarpa colonization and viability in Citrus sinensis. J Plant Dis Protect, 1–8, doi:10.1007/s41348-016-0046-8 (2016).
Sposito, M. B., Amorim, L., Bassanezi, R. B., Filho, A. B. & Hau, B. Spatial pattern of black spot incidence within citrus trees related to disease severity and pathogen dispersal. Plant Pathol 57, 103–108, doi:10.1111/j.1365-3059.2007.01705.x (2008).
Madden, L. V. & Hughes, G. Sampling for plant disease incidence. Phytopathology 89, 1088–1103, doi:10.1094/Phyto.1999.89.11.1088 (1999).
Rowlandson, T. et al. Reconsidering leaf wetness duration determination for plant disease management. Plant Dis 99, 310–319, doi:10.1094/pdis-05-14-0529-fe (2015).
Simanton, W. A. Seasonal patterns of citrus bloom. Proceedings of the Florida State Horticultural Society 82, 96–98 (1970).
Mesejo, C., Munoz-Fambuena, N., Reig, C., Martinez-Fuentes, A. & Agusti, M. Cell division interference in newly fertilized ovules induces stenospermocarpy in cross-pollinated citrus fruit. Plant Sci 225, 86–94, doi:10.1016/j.plantsci.2014.05.019 (2014).
Szmyt, J. Spatial statistics in ecological analysis: from indices to functions. Silva Fenn 48, doi:10.14214/sf.1008 (2014).
Acknowledgements
This project was partially funded by the Citrus Research and Development Foundation (grant number 716) and by the Specialty Crop Block Grant Program (project number 15SCBGPFL0038).
Author information
Authors and Affiliations
Contributions
P.R. designed initial experiments and contributed to data collection and analysis. K.H. conducted the research, compiled, analyzed the data, wrote the main manuscript text and prepared the figures and tables unless otherwise stated. M.C. conducted the sample size analysis. All authors critically reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hendricks, K.E., Christman, M. & Roberts, P.D. Spatial and Temporal Patterns of Commercial Citrus Trees Affected by Phyllosticta citricarpa in Florida. Sci Rep 7, 1641 (2017). https://doi.org/10.1038/s41598-017-01901-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01901-2
This article is cited by
-
Climate suitability of the Mediterranean Basin for citrus black spot disease (Phyllosticta citricarpa) based on a generic infection model
Scientific Reports (2022)
-
Polyphasic identification and MAT1-2 isolates of Phyllosticta citricarpa in Cuba
European Journal of Plant Pathology (2022)
-
The Effect of Weather and Location of Fruit within the Tree on the Incidence and Severity of Citrus Black Spot on Fruit
Scientific Reports (2020)
-
Overcoming randomness does not rule out the importance of inherent randomness for functionality
Journal of Biosciences (2019)
-
Spatial and climatic factors associated with the geographical distribution of citrus black spot disease in South Africa. A Bayesian latent Gaussian model approach
European Journal of Plant Pathology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.