Abstract
A gold standard for diagnosis of periprosthetic joint infection (PJI) has not yet been established. The objective of this study was to evaluate the diagnostic value of serum and synovial fluid interleukin (IL)-6 levels for PJI. The MEDLINE and EMBASE databases were searched for studies describing PJI diagnosis using serum and synovial fluid IL-6 and published between January 1990 and October 2016. Seventeen studies were included in the analysis. The pooled sensitivities of serum and synovial fluid IL-6 were 0.72 (95% confidence interval [CI]: 0.63–0.80) and 0.91 (95% CI: 0.82–0.96), respectively. The pooled specificities of serum and synovial fluid IL-6 were 0.89 (95% CI: 0.77–0.95) and 0.90 (95% CI: 0.84–0.95), respectively. The pooled diagnostic odds ratios (DORs) of serum and synovial fluid IL-6 were 20 (95% CI: 7–58) and 101 (95% CI: 28–358), respectively, and the pooled areas under the curve (AUCs) were 0.83 (95% CI: 0.79–0.86) and 0.96 (95% CI: 0.94–0.98), respectively. Synovial fluid IL-6 had high diagnostic value for PJI. Although serum IL-6 test was less sensitive than synovial fluid IL-6 test, it may be regularly prescribed for patients with prosthetic failure owing to its high specificity.
Similar content being viewed by others
Introduction
Periprosthetic joint infection (PJI) is a severe complication of arthroplasty and causes unrelieved pain and joint dysfunction. Although standardised perioperative management has lowered the risk of hip and knee PJI to less than 2%, PJI is still a significant cause of revision surgery1,2,3,4,5. In contrast to aseptic loosening, PJI is often associated with a two-stage revision procedure and long-term antibiotic therapy. Given these adverse implications, an accurate diagnosis method for PJI is valuable in arthroplasty surgery. In the past decades, various diagnostic methods and guidelines have been established for diagnosing PJI. Unfortunately, in the absence of a gold standard test, it is still challenging to differentiate PJI from aseptic loosening6. A previous prospective cohort study reported that 25% of PJI patients were misdiagnosed with aseptic loosening in the first year after arthroplasty7. Periprosthetic tissue culture and histological examination are reliable diagnostic methods for PJI detection8; however, neither method can guide the operative decision before revision surgery.
Inflammatory biomarkers in the serum and synovial fluid can be evaluated before surgery8. Traditional biomarkers such as serum white cell count (WCC) and C-reactive protein (CRP) have limited diagnostic accuracy for PJI detection9, 10. Previous meta-analysis indicated that the sensitivity of serum WCC for PJI detection is 0.459. The pooled sensitivity and specificity of serum CRP are 0.82 and 0.77, respectively10. Recently, the diagnostic value of interleukin-6 (IL-6) for PJI detection was investigated. IL-6 is produced by lymphoid and non-lymphoid cells, and it participates in the inflammatory response11. Serum IL-6 levels increase with trauma, infection, and surgery11,12,13. In patients with aseptic prosthetic loosening, IL-6 levels decrease to the normal level within 48 h after arthroplasty13. However, following infection, IL-6 activates the release of CRP14. Therefore, the increase of IL-6 precedes that of CRP after infection; thus, IL-6 may be a more sensitive marker for PJI.
The previous meta-analysis summarised the results of three studies and showed that high serum IL-6 level strongly indicates PJI9. Following this study, the diagnostic capacity of serum and synovial fluid IL-6 for PJI has been widely evaluated; however, there were some discrepancies in the results15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31. The objective of the present meta-analysis was to estimate the value of serum and synovial fluid IL-6 assessments for PJI diagnosis.
Results
Search Results
A total 323 articles were identified following the database and bibliography search. After further screening, 257 articles were excluded, and 33 articles were excluded after full-text evaluation (Fig. 1). Seventeen articles were included in the current analysis (Table 1). Nine studies were conducted in the United States, three in Germany, and one study each in Sweden, Argentina, Austria, United Kingdom, and Egypt. Nine articles described the diagnostic accuracy of serum IL-6 for PJI, and six articles focused on synovial fluid IL-6 test. Two additional studies investigated both serum and synovial fluid IL-6 assessments for PJI diagnosis. All studies included in the current meta-analysis were prospective studies. Consecutive patient enrolment was used in four studies, while the others did not indicate the type of patient enrolment. Three studies included patients with shoulder arthroplasty, while the others focused on knee and/or hip arthroplasty. The number of participants ranged from 35 to 120, and the mean age ranged from 58 to 72 years. The cut-off values of serum and synovial fluid IL-6 levels for PJI detection in the selected studies ranged from 2.6 to 10.4 pg/mL and 359.3 to 13,350 pg/mL, respectively. All studies were evaluated using the QUADAS tool, and showed moderate to high quality.
Diagnostic Accuracy
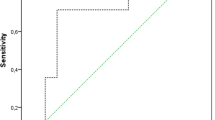
Pooled sensitivity, specificity, area under the curve (AUC), and diagnostic odds ratio (DOR) results are shown in Fig. 2. The pooled sensitivity and specificity for PJI diagnosis using serum IL-6 were 0.72 (95% CI: 0.63–0.80) and 0.89 (95% CI: 0.77–0.95), respectively. The pooled sensitivity and specificity for PJI diagnosis using synovial fluid IL-6 were 0.91 (95% CI: 0.82–0.96) and 0.90 (95% CI: 0.84–0.95), respectively. The pooled DORs for PJI diagnosis using serum and synovial fluid IL-6 were 20 (95% CI: 7–58) and 101 (95% CI: 28–358), respectively. The pooled AUCs for serum and synovial fluid IL-6 tests were 0.83 (95% CI: 0.79–0.86) and 0.96 (95% CI: 0.94–0.98), respectively. Heterogeneity was evaluated by I2. The I2 of serum IL-6 was 81, indicating substantial variation among the included studies. No heterogeneity was found for synovial fluid IL-6 test (I2 = 0).
Evaluation of the Clinical Utility
The positive likelihood ratio (PLR) and negative likelihood ratio (NLR) of serum IL-6 for PJI diagnosis were 6.4 (95% CI: 2.9–14.1) and 0.31 (95% CI: 0.22–0.44), respectively, while those of synovial fluid IL-6 were 9.5 (95% CI: 5.3–17.2) and 0.09 (95% CI: 0.04–0.21), respectively (Fig. 3). Likelihood ratios and pre-test probabilities were used to calculate post-test probabilities. Based on the low prevalence of PJI, 20% pre-test probabilities were used in current study. The post-test probability of PJI was 7% and 2% for serum and synovial IL-6 tests, respectively, indicating negative results (Fig. 3).
Subgroup Analysis
Results of the subgroup analysis are presented in Table 2. Both serum and synovial fluid IL-6 tests showed reliable diagnostic accuracy for PJI after hip and knee arthroplasty. The sensitivities of serum and synovial fluid IL-6 for hip and/or knee PJI diagnosis were 0.74 (95% CI: 0.63–0.80) and 0.92 (95% CI: 0.82–0.97), respectively. The specificities of serum and synovial fluid IL-6 for hip and/or knee PJI diagnosis were 0.92 (95% CI: 0.79–0.97) and 0.91 (95% CI: 0.83–0.95), respectively. The current analysis also showed that the cut-off value could influence the diagnostic accuracy of both serum and synovial fluid IL-6 tests. When the cut-off value was greater than 10 and 2,300 pg/mL for serum and synovial fluid IL-6 tests, respectively, the diagnostic accuracy was improved. The diagnostic accuracy of synovial fluid IL-6 was not affected by the presence of inflammatory diseases. In studies that included patients with inflammatory diseases, the pooled sensitivity and specificity of synovial fluid IL-6 were 0.92 and 0.93, respectively.
Assessment of Publication Bias
No potential publication bias was identified for studies investigating serum (p = 0.98) and synovial fluid IL-6 (p = 0.57) tests. The results are presented in Fig. 4.
Discussion
The current meta-analysis indicated that synovial fluid IL-6 can be used for the diagnosis of PJI after joint arthroplasty. Although serum IL-6 is less sensitive than synovial fluid IL-6, it is one of the best serum biomarkers for PJI detection. Because of its reliable post-test probability, serum IL-6 assessment should be included as a regular test for patients with prosthetic failure.
Treatment for PJI involves intensive debridement, one- or two-stage implantation, and long-term antimicrobial therapy; in contrast, a generic revision procedure is typically sufficient to treat aseptic loosening6. Thus, a precise diagnosis is necessary for surgeons to lay out the operation plan. However, PJI is difficult to diagnose before revision surgery in the absence of uniform criteria and a gold standard test. In the past decade, several meta-analyses have been performed to assess the diagnostic accuracy of various established tests for PJI detection (Table 3 and Fig. 5)9, 10, 32,33,34,35,36,37,38,39,40,41,42,43. Traditionally, periprosthetic tissue culture, which has a positive detection rate of 0.70–0.90, is regarded as the gold standard test for PJI diagnosis8, 44,45,46. Two prior meta-analyses have demonstrated that sonication and polymerase chain reaction can increase the diagnostic accuracy of prosthetic biopsy41, 42. Unfortunately, these intraoperative tests cannot be performed before revision surgery.
Radiography is the most basic test prescribed for patients with joint pain after arthroplasty. Implant migration with transcortical sinus tracts is a reliable indicator for PJI6. However, septic hip prosthetic loosening typically shows normal radiographic findings47. Because metal-related artefacts are used in these patients, computed tomography and magnetic resonance imaging cannot be used for PJI diagnosis. Nuclear medicine appears to have a high sensitivity for PJI detection. The pooled sensitivity and specificity of leukocyte scintigraphy were 0.88 and 0.92, respectively, and those for fluorodeoxyglucose positron emission tomography were 0.86 and 0.93, respectively. The disadvantages of nuclear medicine for PJI diagnosis are high cost and radiation dose8.
Serum biomarkers are commonly utilised to detect infections worldwide. According to the guidelines specified by the Infectious Diseases Society of America (IDSA), the erythrocyte sedimentation rate and CRP should be evaluated for all patients suspected as having PJI8. However, serum biomarker levels can be easily influenced by antibiotic therapy, systemic infection, trauma, and surgery48; thus, these biomarkers may indicate inflammation rather than infection. According to a previous study, procalcitonin (PCT) is the most specific serum biomarker of PJI with a specificity of 0.9232.
Recently, several studies have investigated the diagnostic capacity of serum and synovial fluid IL-6 for PJI. IL-6 is a 212-amino acid interleukin encoded by a single gene mapped to chromosome 7 in humans49. The correlation between high levels of IL-6 in body fluids and local acute bacterial infection was reported in 198950. The level of serum IL-6 is typically less than 10 pg/mL in healthy adults51. IL-6 is an anti-inflammatory cytokine and a mediator of infection response52. It is considered the major mediator of acute phase protein production by hepatocytes53. Serum CRP level increases after 4–6 h of IL-6 stimulation54, indicating that IL-6 is a relatively sensitive biomarker of early-stage immune response. As reported in a previous meta-analysis that assessed the findings of three studies, the pooled sensitivity and specificity of serum IL-6 were 0.97 and 0.91, respectively for PJI detection9. This remarkable diagnostic accuracy is inconsistent with the evidence presented in the current report. In the present meta-analysis, 11 studies that assessed serum IL-6 levels from 2005 to 2015 were included. The present study demonstrated that low detection sensitivity (0.72) was a major limiting factor of using serum IL-6 for PJI diagnosis. Notably, two prior studies that included 103 patients reported poor sensitivity (0.13–0.14) for serum IL-6 to detect periprosthetic shoulder infections26, 28. The poor sensitivity reported in these studies may be related to the high proportion of low-grade infections in shoulder PJI. As has been established previously, different microorganisms cause PJI of different joints6. Low-virulent bacteria such as Propionibacterium acnes are frequently detected in patients with periprosthetic shoulder infection55. Nevertheless, Ettinger et al. reported a promising result for serum IL-6 testing to predict low-grade PJI with a sensitivity of 0.8029. Additional large-scale studies are necessary to determine the diagnostic value of serum IL-6 for shoulder PJI.
Joint aspiration is conventionally performed in patients with suspected PJI. Synovial fluid culture is an accurate diagnosis method with a sensitivity and specificity of 0.72 and 0.95, respectively33. Thus, results of aspiration culture can be used to guide treatment plans. In recent years, synovial fluid biomarkers have received significant attention for their diagnostic value for PJI detection. The pooled sensitivities of synovial fluid WCC, polymorphonuclear leukocytes, CRP, α-defensin, and leukocyte esterase are 0.88, 0.90, 0.92, 1.00, and 0.81, respectively, and their pooled specificities are 0.93, 0.88, 0.90, 0.96, and 0.97, respectively32, 34,35,36. Synovial fluid α-defensin appears to be the most valuable test to diagnose PJI (Fig. 5); however, it is not a universal test and is expensive (760 USD per test)36. The use of the α-defensin test for PJI diagnosis was first reported in 201456, 57. To our knowledge, it is not yet used in clinical practice in China. The synovial fluid IL-6 test is considerably more common and economical than the synovial fluid α-defensin test. The present meta-analysis indicated that abnormally high synovial fluid IL-6 level is a strong indicator of PJI, with an AUC of 0.96. Notably, our subgroup analysis indicated the high diagnostic value of the synovial fluid IL-6 test was not influenced by the presence of inflammatory diseases, which renders the test more advantageous for PJI detection in clinical practice. However, as with the serum IL-6 test, the optimal cut-off value of synovial fluid IL-6 is yet to be determined (range: 359.3–13,350 pg/mL).
The current meta-analysis has several strengths: 1) to the best of our knowledge, this is the first meta-analysis to report the diagnostic capacity of synovial fluid IL-6 for PJI detection; and 2) when compared to a prior meta-analysis, the current analysis included more studies (11 articles) assessing serum IL-6 test for PJI diagnosis, and showed that serum IL-6 was less sensitive than synovial fluid IL-6. We believe that this meta-analysis may aid orthopaedists to improve the diagnostic accuracy for PJI in clinical practice.
However, this meta-analysis has several limitations. First, the result of statistical analysis indicated significant heterogeneity for the serum IL-6 test. Two studies reported exceedingly low diagnostic values for the serum IL-6 test in the detection of periprosthetic shoulder infections26, 28. The heterogeneity was still present after the two studies on shoulder PJI were excluded (I2 = 79), which lowered the reliability of the findings. Second, a gold standard test for PJI diagnosis could not be proposed because multiple reference standards were used in the studies included in this meta-analysis. Third, antibiotic therapies and systemic inflammatory diseases may affect the diagnostic accuracy of serum IL-6. Only few studies excluded patients with prior antibiotic therapy or other infections. Fourth, the current study only included articles published between January 1990 and October 2016. Lastly, we did not register a protocol for the current study in PROSPERO.
The results of this meta-analysis showed that synovial fluid IL-6 has significant diagnostic value for PJI diagnosis, particularly in periprosthetic knee and hip infections. Although serum IL-6 test is less sensitive than synovial fluid IL-6 test, it can be regularly prescribed for patients with prosthetic failure owing to its high specificity.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used to guide methods for the current study58.
Search Strategy
The MEDLINE and EMBASE databases were searched for studies that utilised the serum or synovial fluid IL-6 test to diagnose PJI between January 1990 and October 2016. The search was based on the following terms: interleukin, IL-6, inflammatory, arthroplasty or replacement, serum or synovial fluid, sensitivity or specificity, septic or septically, prosthesis infection, infectious or infected, and diagnose or diagnostic. Additional entries were identified through the bibliographies of the selected studies and reviews.
The following inclusion criteria were used to evaluate the articles: (1) articles indicated the date of serum or synovial fluid IL-6 assessment of patients undergoing revision arthroplasty; (2) the diagnosis of PJI was made based on the IDSA guideline, Musculoskeletal Infection Society (MSIS) criteria, or American Academy of Orthopaedic Surgeons (AAOS) guideline8, 45, 46; (3) sufficient data were described for the calculation of the true-positive (tp), false-negative (fn), false-positive (fp), and true-negative (tn) values; (4) the number of participants were more than 10; and (5) the article was written in English.
When the same patients were included in multiple articles, findings from the most detailed study were used. When an article described more than one cut-off value, the most efficient value was used in the present analysis. After a full-text assessment, article quality was assessed using the QUADAS tool59. Two independent reviewers performed the evaluation, and a third reviewer was solicited to resolve disagreements.
Data Extraction
Two reviewers independently completed data extraction from eligible studies. The characteristics of each study were recorded in a standardised form to include the following details: name of the first author, publication year, number of patients, mean age of patients, study design, type of patient enrolment, location of the operation, cut-off value, and reference standards. A third reviewer was solicited to check for discrepancies and resolve conflicting findings of the first two reviewers.
Statistical Analysis
Statistical analysis was performed by two independent reviewers. The tp, fn, fp, and tn values were calculated according to the statistical data extracted from eligible articles. Quantitative indicators, including sensitivity, specificity, AUC, and DOR, were calculated to estimate the diagnostic value of the IL-6 test for PJI detection. The PLR, NLR, and post-test probability were used to calculate the clinical utility of the IL-6 test. I2 was calculated to evaluate study heterogeneity60. Studies with I2 values of >50% were considered heterogeneous. For the heterogeneous studies, the diagnostic accuracy of the IL-6 test was calculated using the random effects model61. Subgroup analysis was performed to determine the effect of various characteristics on the diagnostic accuracy of the IL-6 test for PJI detection. Publication bias was evaluated using Deeks’ funnel plot asymmetry test62. STATA version 14 (StataCorp, College Station, TX, USA) was used for other statistical analyses.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Bozic, K. J. et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am 91, 128–133, doi:10.2106/JBJS.H.00155 (2009).
Clohisy, J. C., Calvert, G., Tull, F., McDonald, D. & Maloney, W. J. Reasons for Revision Hip Surgery. Clinical Orthopaedics and Related Research 429, 188–192, doi:10.1097/01.blo.0000150126.73024.42 (2004).
Bozic, K. J. et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 468, 45–51, doi:10.1007/s11999-009-0945-0 (2010).
Namba, R. S., Inacio, M. C. & Paxton, E. W. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am 95, 775–782, doi:10.2106/JBJS.L.00211 (2013).
NIH consensus conference: Total hip replacement. NIH Consensus Development Panel on Total Hip Replacement. JAMA 273, 1950–1956, doi:10.1001/jama.1995.03520480070043 (1995).
Zimmerli, W., Trampuz, A. & Ochsner, P. E. Prosthetic-joint infections. N Engl J Med 351, 1645–1654, doi:10.1056/NEJMra040181 (2004).
Fernandez-Sampedro, M. et al. 26Postoperative diagnosis and outcome in patients with revision arthroplasty for aseptic loosening. BMC Infect Dis 15, 232, doi:10.1186/s12879-015-0976-y (2015).
Osmon, D. R. et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56, e1–e25, doi:10.1093/cid/cis803 (2013).
Berbari, E. et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am 92, 2102–2109, doi:10.2106/JBJS.I.01199 (2010).
Yuan, K., Chen, H. L. & Cui, Z. M. Diagnostic accuracy of C-reactive protein for periprosthetic joint infection: a meta-analysis. Surg Infect (Larchmt) 15, 548–559, doi:10.1089/sur.2013.066 (2014).
Song, M. & Kellum, J. A. Interleukin-6. Crit Care Med 33, S463–465, doi:10.1097/01.CCM.0000186784.62662.A1 (2005).
van der Poll, T. et al. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis 176, 439–444, doi:10.1086/jid.1997.176.issue-2 (1997).
Wirtz, D. C., Heller, K. D., Miltner, O., Zilkens, K. W. & Wolff, J. M. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop 24, 194–196, doi:10.1007/s002640000136 (2000).
Selberg, O. et al. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit Care Med 28, 2793–2798, doi:10.1097/00003246-200008000-00019 (2000).
Di Cesare, P. E., Chang, E., Preston, C. F. & Liu, C. J. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am 87, 1921–1927, doi:10.2106/JBJS.D.01803 (2005).
Bottner, F. et al. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br 89, 94–99, doi:10.1302/0301-620X.89B1.17485 (2007).
Nilsdotter-Augustinsson, A. et al. Inflammatory response in 85 patients with loosened hip prostheses: a prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop 78, 629–639, doi:10.1080/17453670710014329 (2007).
Buttaro, M. A., Tanoira, I., Comba, F. & Piccaluga, F. Combining C-reactive protein and interleukin-6 may be useful to detect periprosthetic hip infection. Clin Orthop Relat Res 468, 3263–3267, doi:10.1007/s11999-010-1451-0 (2010).
Deirmengian, C. et al. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res 468, 2017–2023, doi:10.1007/s11999-010-1298-4 (2010).
Worthington, T. et al. Serum procalcitonin, interleukin-6, soluble intercellular adhesin molecule-1 and IgG to short-chain exocellular lipoteichoic acid as predictors of infection in total joint prosthesis revision. Br J Biomed Sci 67, 71–76, doi:10.1080/09674845.2010.11730294 (2010).
Jacovides, C. L., Parvizi, J., Adeli, B. & Jung, K. A. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty 26, 99–103 e101, doi:10.1016/j.arth.2011.03.025 (2011).
Abou El-Khier, N. T., El Ganainy Ael, R., Elgeidy, A. & Rakha, S. A. Assessment of interleukin-6 and other inflammatory markers in the diagnosis of Egyptian patients with periprosthetic joint infection. Egypt J Immunol 20, 93–99 (2013).
Glehr, M. et al. Novel biomarkers to detect infection in revision hip and knee arthroplasties. Clin Orthop Relat Res 471, 2621–2628, doi:10.1007/s11999-013-2998-3 (2013).
Gollwitzer, H. et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am 95, 644–651, doi:10.2106/JBJS.L.00205 (2013).
Deirmengian, C. et al. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res 472, 3254–3262, doi:10.1007/s11999-014-3543-8 (2014).
Grosso, M. J. et al. Poor utility of serum interleukin-6 levels to predict indolent periprosthetic shoulder infections. J Shoulder Elbow Surg 23, 1277–1281, doi:10.1016/j.jse.2013.12.023 (2014).
Randau, T. M. et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One 9, e89045, doi:10.1371/journal.pone.0089045 (2014).
Villacis, D. et al. Serum interleukin-6 as a marker of periprosthetic shoulder infection. J Bone Joint Surg Am 96, 41–45, doi:10.2106/JBJS.L.01634 (2014).
Ettinger, M. et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis 61, 332–341, doi:10.1093/cid/civ286 (2015).
Frangiamore, S. J. et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am 97, 63–70, doi:10.2106/JBJS.N.00104 (2015).
Frangiamore, S. J. et al. Synovial Cytokines and the MSIS Criteria Are Not Useful for Determining Infection Resolution After Periprosthetic Joint Infection Explantation. Clin Orthop Relat Res 474, 1630–1639, doi:10.1007/s11999-016-4710-x (2016).
Xie, K., Qu, X. & Yan, M. Procalcitonin and alpha-Defensin for Diagnosis of Periprosthetic Joint Infections. J Arthroplasty 32, 1387–1394, doi:10.1016/j.arth.2016.10.001 (2016).
Qu, X. et al. Preoperative aspiration culture for preoperative diagnosis of infection in total hip or knee arthroplasty. J Clin Microbiol 51, 3830–3834, doi:10.1128/JCM.01467-13 (2013).
Qu, X. et al. Evaluation of white cell count and differential in synovial fluid for diagnosing infections after total hip or knee arthroplasty. PLoS One 9, e84751, doi:10.1371/journal.pone.0084751 (2014).
Wang, C., Wang, Q., Li, R., Duan, J. Y. & Wang, C. B. Synovial Fluid C-reactive Protein as a Diagnostic Marker for Periprosthetic Joint Infection: A Systematic Review and Meta-analysis. Chin Med J (Engl) 129, 1987–1993, doi:10.4103/0366-6999.187857 (2016).
Wyatt, M. C. et al. The Alpha-Defensin Immunoassay and Leukocyte Esterase Colorimetric Strip Test for the Diagnosis of Periprosthetic Infection: A Systematic Review and Meta-Analysis. J Bone Joint Surg Am 98, 992–1000, doi:10.2106/JBJS.15.01142 (2016).
Ouyang, Z., Li, H., Liu, X., Zhai, Z. & Li, X. Prosthesis infection: diagnosis after total joint arthroplasty with three-phase bone scintigraphy. Ann Nucl Med 28, 994–1003, doi:10.1007/s12149-014-0899-5 (2014).
Xing, D. et al. Use of anti-granulocyte scintigraphy with 99mTc-labeled monoclonal antibodies for the diagnosis of periprosthetic infection in patients after total joint arthroplasty: a diagnostic meta-analysis. PLoS One 8, e69857, doi:10.1371/journal.pone.0069857 (2013).
Verberne, S. J., Raijmakers, P. G. & Temmerman, O. P. The Accuracy of Imaging Techniques in the Assessment of Periprosthetic Hip Infection: A Systematic Review and Meta-Analysis. J Bone Joint Surg Am 98, 1638–1645, doi:10.2106/JBJS.15.00898 (2016).
Tsaras, G. et al. Utility of intraoperative frozen section histopathology in the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am 94, 1700–1711, doi:10.2106/JBJS.J.00756 (2012).
Qu, X. et al. PCR-based diagnosis of prosthetic joint infection. J Clin Microbiol 51, 2742–2746, doi:10.1128/JCM.00657-13 (2013).
Zhai, Z. et al. Meta-analysis of sonication fluid samples from prosthetic components for diagnosis of infection after total joint arthroplasty. J Clin Microbiol 52, 1730–1736, doi:10.1128/JCM.03138-13 (2014).
Ouyang, Z. et al. Limitations of Gram staining for the diagnosis of infections following total hip or knee arthroplasty. Exp Ther Med 9, 1857–1864, doi:10.3892/etm.2015.2315 (2015).
Parvizi, J., Fassihi, S. C. & Enayatollahi, M. A. Diagnosis of Periprosthetic Joint Infection Following Hip and Knee Arthroplasty. Orthop Clin North Am 47, 505–515, doi:10.1016/j.ocl.2016.03.001 (2016).
Della Valle, C. et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am 93, 1355–1357, doi:10.2106/JBJS.9314ebo (2011).
Parvizi, J. et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 469, 2992–2994, doi:10.1007/s11999-011-2102-9 (2011).
Tigges, S., Stiles, R. G. & Roberson, J. R. Appearance of septic hip prostheses on plain radiographs. AJR Am J Roentgenol 163, 377–380, doi:10.2214/ajr.163.2.8037035 (1994).
Hausfater, P. Biomarkers and infection in the emergency unit. Med Mal Infect 44, 139–145, doi:10.1016/j.medmal.2014.01.002 (2014).
Ferguson-Smith, A. C. et al. Regional localization of the interferon-beta 2/B-cell stimulatory factor 2/hepatocyte stimulating factor gene to human chromosome 7p15–p21. Genomics 2, 203–208, doi:10.1016/0888-7543(88)90003-1 (1988).
Helfgott, D. C. et al. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol 142, 948–953 (1989).
Sakamoto, K. et al. Elevation of circulating interleukin 6 after surgery: factors influencing the serum level. Cytokine 6, 181–186, doi:10.1016/1043-4666(94)90040-X (1994).
Barton, B. E. IL-6: insights into novel biological activities. Clin Immunol Immunopathol 85, 16–20, doi:10.1006/clin.1997.4420 (1997).
Gauldie, J., Richards, C., Harnish, D., Lansdorp, P. & Baumann, H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA 84, 7251–7255, doi:10.1073/pnas.84.20.7251 (1987).
Povoa, P. C-reactive protein: a valuable marker of sepsis. Intensive Care Med 28, 235–243, doi:10.1007/s00134-002-1209-6 (2002).
Sperling, J. W., Kozak, T. K., Hanssen, A. D. & Cofield, R. H. Infection after shoulder arthroplasty. Clin Orthop Relat Res 206–216, doi:10.1097/00003086-200101000-00028 (2001).
Deirmengian, C. et al. Combined measurement of synovial fluid alpha-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am 96, 1439–1445, doi:10.2106/JBJS.M.01316 (2014).
Bingham, J. et al. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res 472, 4006–4009, doi:10.1007/s11999-014-3900-7 (2014).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62, e1–34, doi:10.1016/j.jclinepi.2009.06.006 (2009).
Whiting, P., Rutjes, A. W., Reitsma, J. B., Bossuyt, P. M. & Kleijnen, J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3, 25, doi:10.1186/1471-2288-3-25 (2003).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560, doi:10.1136/bmj.327.7414.557 (2003).
Gardiner, J. C., Luo, Z. & Roman, L. A. Fixed effects, random effects and GEE: what are the differences? Stat Med 28, 221–239, doi:10.1002/sim.3478 (2009).
Deeks, J. J., Macaskill, P. & Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58, 882–893, doi:10.1016/j.jclinepi.2005.01.016 (2005).
Acknowledgements
This work was supported by the National Natural Science Foundation for Youths (No. 81401852), the National Natural Science Foundation of China (No. 51631009), the Natural Science Foundation of Shanghai (No. 14ZR1424000), and ‘Chen Guang’ Project of the Shanghai Municipal Education Commission and the Shanghai Education Development Foundation (No. 14CG14).
Author information
Authors and Affiliations
Contributions
K.X. searched the database, performed the meta-analysis, and helped draft the manuscript. K.D. participated in the design of the study. X.Q. searched the database and performed the meta-analysis. M.Y. participated in the design of the study and drafted the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, K., Dai, K., Qu, X. et al. Serum and Synovial Fluid Interleukin-6 for the Diagnosis of Periprosthetic Joint Infection. Sci Rep 7, 1496 (2017). https://doi.org/10.1038/s41598-017-01713-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01713-4
This article is cited by
-
Diagnostic accuracy of sonication fluid cultures from prosthetic components in periprosthetic joint infection: an updated diagnostic meta-analysis
Journal of Orthopaedic Surgery and Research (2023)
-
Joint fluid interleukin-6 combined with the neutral polymorphonuclear leukocyte ratio (PMN%) as a diagnostic index for chronic periprosthesis infection after arthroplasty
Journal of Orthopaedics and Traumatology (2023)
-
Emerging Technologies in Diagnosing Periprosthetic Joint Infections
Indian Journal of Orthopaedics (2023)
-
Meta-analysis of synovial fluid polymerase chain reaction for diagnosing periprosthetic hip and knee infection
Journal of Orthopaedic Surgery and Research (2022)
-
Serum versus synovial fluid interleukin-6 for periprosthetic joint infection diagnosis: a systematic review and meta-analysis of 30 diagnostic test accuracy studies
Journal of Orthopaedic Surgery and Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.