Abstract
Excessive daytime sleepiness (EDS) is highly prevalent in the general population; however little is known about its evolution and predictors. Our objectives were to document its natural history, provide estimates of its prevalence, incidence and persistence rates, and to identify predictors of increased daytime sleepiness (DS) in a longitudinal community study of 2157 adults over 5 years. Participants completed postal assessment at baseline and at each yearly follow-up. DS was evaluated by the Epworth Sleepiness scale (ESS). At baseline, 33% reported EDS (ESS > 10) with 33% of them reported persistent EDS. Of those without EDS at baseline, 28% developed incident EDS (15% were persistent) and 31% increased DS (augmentation ≥4-points between two consecutive evaluations). Younger age and depression were independent predictors of incident EDS and DS increase while lower coffee consumption, smoking, insomnia, tiredness and chronic pain were associated with incident EDS, and living alone with DS increase only. Persistent vs transient EDS or DS showed association with poor general health including metabolic diseases. Thus, sleepiness fluctuated over time and it was predicted by common lifestyle and psychological factors potentially modifiable. However, persistent sleepiness was associated with chronic medical diseases thus highlighting a homogeneous group at risk requiring a dedicated management.
Similar content being viewed by others
Introduction
Excessive daytime sleepiness (EDS) is characterized by a difficulty to stay awake and alert during the major waking episodes of the day, with sleep occurring unintentionally or at inappropriate times of the wake period1. EDS is often associated with a wide range of illnesses including metabolic, cardiovascular, neurological, psychiatric diseases2,3,4,5 but also with voluntary behaviors reflecting poor sleep and sleep debt, leading to disability and increased risk of mortality6. EDS is also commonly associated with social and economic consequences thus constituting a significant public health problem. The prevalence of EDS in the general population varies from 9 to 28%5, 7,8,9,10. This wide range can be explained by differences according to demographic and cultural/geographical factors (e.g. higher prevalence in young11 and older adults3, higher prevalence in northern countries12) but also inconsistencies in study design, sample size, EDS assessment and definition13. Survey studies used either single questions to evaluate excessive sleepiness, sleep propensity during wakefulness and unrefreshing sleep, self-report questionnaires to measure daytime sleepiness (DS)13, or a more complex structured interview using criteria proposed for the DSM-510. The most commonly used instrument in clinical and research settings is the Epworth Sleepiness Scale (ESS), a standardized self-report questionnaire14. A cut-off greater than 10 was used to indicate a clinically significant EDS in clinical and epidemiological studies with a prevalence ranging from 8.5% to 22.2% in the general population5, 8, 11, 15, 16. In addition to this cut-off, a change of four points or more on ESS scores between any two evaluations was considered clinically relevant and was frequently used as a primary endpoint in pharmacological studies on central hypersomnias17, 18.
To our knowledge, factors predicting EDS are not well understood and those triggering DS increase remain unknown. Longitudinal studies of factors associated with EDS onset are scarce and give inconsistent results. Using single questions to evaluate EDS with Likert-type scales or dichotomized responses, only three studies reported that early morning awakening and anxiety increase the risk of EDS in young adults19, insomnia, anxiety/depression and smoking predict incident EDS in women20, and obesity and depression are associated with the occurrence of EDS in middle-aged adults21. However, none of these cohorts used the ESS scale to define EDS and despite long follow-ups (10 years or more), they neither fully took into account changes in covariates nor the fluctuation of sleepiness course over time.
The objectives of the present study were to document the natural history of EDS and provide estimates of prevalence, incidence, and persistence rates of EDS using the ESS, and to identify predictors of increased DS using time-dependent covariates over a 5-year follow-up in a longitudinal community study of adults.
Results
Subject characteristics
The final sample included 2167 participants with a median baseline age of 51 years (range, 18–89) of whom 64.1% were women. As detailed in the flow-chart diagram (Fig. 1), these subjects were free of central hypersomnia disorders, day workers, and with ESS completed at baseline and at least at one of the five annual follow-up evaluations. Participants excluded from the study (N = 261) had a significantly lower education level, were younger, current smoker and more depressed. No significant differences were found for sleep duration, number of naps, insomnia, EDS, or associated chronic diseases.
Regarding other baseline sleep characteristics, 13.1% slept less than 6 hours per night, 13.7% took three naps or more during the week, and 16.1% had a moderate to severe level of insomnia (ISI > 14). Only 14.1% took hypnotics (50.2% BZD, 19.6% BZD-like compounds), 12.6% antidepressants, and 29.4% OTC medication (23.8% antihistaminics and 5.6% melatonin).
Description of the natural history of EDS
The Cronbach’s coefficient alpha for the 8 items of ESS scale was around 0.80–0.81 at baseline and at each year-follow-up. The inter-rater agreement measures between two assessment points were good ranging from 0.64 95% CI = [0.62–0.67] to 0.79 95% CI = [0.77–0.81].
At baseline, 32.9% (95% CI = [31.0–34.9]) (n = 714) of participants reported EDS (ESS > 10), of whom 25.4% were severely sleepy (ESS ≥ 16). During the follow-up visits, prevalences of EDS were slightly lower ranging from 28.7 (95% CI = [26.8–30.8]) to 26.4% (95% CI = [24.3–28.5]). However, trajectories of EDS vary considerably with approximately 8% of new cases each year, between 8 and 13% of remitted cases since the previous year and approximately 19% of recurrent cases since the previous year (Fig. 2). Regarding the persistence of EDS during the follow-up, 32.6% (n = 233) reported a persistent EDS at baseline and at all 5 subsequent assessments, 25 developed EDS after 1-year of follow-up with a persistence at all subsequent assessments, 14 after two years, 13 after three years and 11 after four years.
Determinants of EDS at baseline
Participants with EDS were younger, slept less than eight hours per night, had a higher frequency of naps, and took hypnotics less frequently (Table 1). They were more likely to complain insomnia, fatigue, depressive and anxiety symptoms, were obese and less physically active, and reported higher rates of diagnoses of sleep apnea, chronic pain, neurological or other diseases (p ≤ 0.02 for all comparisons) (Table 1). To identify which determinants were independently associated with EDS, gender and other characteristics associated with EDS at p < 0.15 were introduced into a multivariate model. Younger age, shorter sleep duration, higher frequency of naps, fatigue, levels of trait-anxiety, sleep apnea and the absence of hypnotic consumption were associated with EDS, but not insomnia, depressive symptoms or chronic diseases (Table 1).
To examine whether determinants could be differentially associated with EDS severity, subjects were categorized into three groups: no EDS (ESS ≤ 10, n = 1453), EDS (ESS between 11 and 15, n = 533) and severe EDS (ESS ≥ 16, n = 181). Compared to those without EDS, those with EDS or severe EDS were independently more tired, trait-anxious, had frequent naps and consumed no hypnotic whereas those with non-severe EDS only were younger and reported more short sleep duration, and those with severe EDS only had more sleep apnea.
Predictors of EDS over 5-year follow-up
Among the 1453 subjects without EDS at baseline, 411 (28.3%) met criteria for incident EDS at least in one of the subsequent follow-ups. The median occurrence of the first EDS event was 2 years [range of 0.40–5.71].
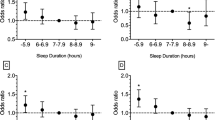
In bivariate Cox models including time-dependent variables, the risk of incident EDS was higher in subjects living alone, smoking occasionally, consuming less coffee, sleeping less than eight hours, taking frequently naps, practicing physical activities less than 4 times per week, being insomniac, tired and depressed, less anxious, and with chronic diseases (restless legs syndrome, chronic pain, endocrine, metabolic neurological and other diseases) (Table 2). BMI category or a change in BMI categories was not significantly associated with incident EDS. In a Cox multivariate model including gender, ESS baseline and characteristics associated with a p < 0.15, being younger (borderline significant), consuming less coffee, smoking occasionally, being insomniac, depressed, with fatigue and having chronic pain were independent predictors of the incidence of EDS (Table 3). To test whether a potential multicollinearity of fatigue, depression symptoms and insomnia, several multivariate models were rerun including all covariates associated at p < 0.15 1) without the covariate fatigue 2) without the depression symptoms 3) without insomnia severity. The results of associations between potential predictors and incidence of EDS during the 5-year follow-up remained unchanged.
Among the participants with incident EDS, only 15.3% (n = 63) reported a persistent EDS in all subsequent assessments. Compared to participants with transient EDS, participants with persistent EDS reported a higher frequency of diabetes (HR = 3.23 95% CI = [1.51;6.91], p = 0.003) in bivariate analysis and this result remained unchanged after adjustment for gender, age and ESS baseline (HR = 3.10 95% CI = [1.40;6.85], p = 0.005).
Predictors of increased DS over 5-year follow-up
Over the 5-year follow-up, 658 (31.3%) participants had increased DS (≥4 points on ESS) with a median delay between baseline and follow-up augmentation of 2.16 years [range of 0.25–5.59].
In bivariate Cox models including time-dependent variables, participants with increased DS were significantly more likely to have a low educational level, live alone, sleep less than eight hours per night, practice physical activities less than 4 hours per week and reported more severe insomnia, fatigue, and depressive symptoms. They were more obese and reported more chronic diseases (chronic pain, diabetes, cardiovascular, endocrine and metabolic, neurological and other diseases) (Table 2). In a multivariate model, participants with increased DS were independently younger, lived alone and more depressed (Table 3). Among participants with increased DS, 35.6% reported a persistent DS through the study. In bivariate analysis, compared to participants with transient DS increase, those with persistent increase DS had more diabetes (HR = 1.61 95% CI = [1.05;2.45], p = 0.03), endocrine and metabolic diseases (HR = 1.41 95% CI = [1.06;1.92], p = 0.03) and other diseases (HR = 1.39 95% CI = [1.05;1.84], p = 0.02). In multivariate analysis, including these three diseases, age, gender and ESS baseline, only other diseases were independently associated with a persistent increase DS (HR = 1.36 95% CI = [1.01;1.83], p = 0.04).
Discussion
In this study of community-dwelling middle aged adults, 33% of the participants met the criteria for self-reported EDS at baseline (ESS > 10) (of whom 25% had severe EDS), with 33% of them reporting persistent EDS over 5 years. During the follow-up, 28% had incident EDS and 31% had increased DS (≥4-point on ESS). However, the course of EDS fluctuates over time with only 15% having persistent EDS and 36% persistent DS. We found that younger age and depressive symptoms were independent predictors of incident EDS and DS increase while lower coffee consumption, smoking, insomnia, tiredness and chronic pain were associated with incident EDS, and living alone with DS increase only. Only poor general health with somatic diseases, especially metabolic diseases, was associated with persistent EDS or DS increased.
We evaluated DS using the ESS which is based more on observable behaviors than subjective experience, reflecting real-life experience, with a good correlation found between the scores obtained by patients and their partners. The score is reliable and reproducible with good test-retest reliability (r = 0.82) between two administrations of the ESS over a time period of 5-months22. Prevalence rates for EDS were in the same range at baseline and at each point of the follow-up. These estimations were, however, higher than previous studies using the same questionnaire, ranging from 8.5 to 22.2%5, 8, 11, 15, 16. Reasons for such estimations may be due to the administration mode of the ESS scale, being here self-reported and completed via a postal mailing thus without clear face-to-face information on how to answer it. It has been reported higher score when the questionnaire was only self-administrated23. Consequently, we cannot excluded a certain degree of misclassification, with some overlapping with fatigue.
In our study, no gender difference was found while EDS slightly decreased with age as previously reported8, 15. In contrast, shorter sleep duration, higher frequency of naps, fatigue, higher levels of trait-anxiety and the absence of hypnotic consumption were associated with EDS. Only sleep apnea was related to severe EDS (ESS > 16).
We also found a high frequency of incident EDS (28%) over the 5-year period compared to previous studies8, 15. However, among participants who developed EDS in our study only 15% were persistent which suggests instability, with frequent waxing and waning in the natural course of EDS in the general population. This finding could not be assessed in previous studies, as no data on DS were available between baseline and endpoint follow-up8, 15. Low persistent EDS rate was, however, in agreement with a recent study measuring EDS using two single questions21. Among participants with EDS at baseline, 21% only persisted at the 10-year follow-up21. Thus, EDS appears to be an unstable symptom in the general population and cannot be considered as a disorder per se 24.
In addition to changes in the trajectory of sleepiness propensity with time, we investigated the test-retest reliability of the tool. Despite its widespread use, and its good psychometric properties in students22, a systematic review of the ESS showed poor test-retest reliability especially in middle-aged populations and after a time period greater than one year25. Furthermore, despite validated norms in healthy subjects with scores between 0 and 10, a grey zone for pathological scores existed with doubt for some conditions due to potential difficulties in understanding the questions and the quality of the subject’s perception of their sleepiness. Moreover, significant changes within the scores may occur even under the threshold (i.e. the “presymptomatic stage”), or already above the threshold referring to an aggravation of the condition. Accordingly, we reanalyzed the ESS scores when there was an increase of 4-points or more between two consecutive evaluations. As already reported in the literature17, 18 such an important difference in DS may be considered as clinically meaningful and should therefore alert the clinician. Results showed that 33% had increased DS but only 36% were persistent; although this is low, it is twice as high as for persistent EDS.
Other important findings of our study concerned the risk factors associated with incident EDS and DS increase. We found that younger age was an independent predictor of incident EDS and DS increase but did not observe a U-shaped relationship as previously described for EDS21. We replicated the association between depressive symptoms and incident EDS and DS increase shown in others studies20, 21. We also reported that lifestyle factors (i.e. lower coffee consumption and smoking) were associated with incident EDS and living alone with DS increase only, all factors being modifiable on a behavioral level. Smoking as previously reported associated with the development of EDS20. These two lifestyle factors were related to EDS with mechanisms of action being potentially related to the stimulant effect of caffeine and the non-restorative sleep effect of nicotine26. In the same way, insomnia, fatigue and chronic pain, being markers of poor health status, were associated with incident EDS. Fatigue and insomnia were already reported as potential risk factors for EDS but to our knowledge we reported for the first time that chronic pain may be a risk factor for EDS in the general population. The mechanism underlying this association remains unknown but it may involve sleep fragmentation and chronic low-grade inflammation27, 28. Several arguments already suggest a complex relationship between insomnia, hypersomnia and chronic pain29. We failed to report any relationship between change of BMI classes and EDS/DS onset. This discrepancy may relate to the low proportions of subjects with obesity (~20%) and with significant BMI changes in our sample (~15%). Last, typical short sleep duration was associated with incidence of EDS and DS increase in bivariate models with similar tendency for the former outcome in multivariate model. Finally, potential association with long sleep duration defined as >9 hours in the literature30 could not be assessed here as it corresponds to only 6% (n = 88) of the population.
We also identify predictors of persistent vs transient EDS or DS increase that showed associations with poor general health including somatic diseases and especially metabolic diseases. None of lifestyle and psychological factors were associated with persistent sleepiness. Findings focusing on subjects with persistent sleepiness are of major interest as they highlight a more homogeneous group potentially at risk for serious medical consequences such as cardiovascular and neurodegenerative disorders. Interventional studies exploring whether the management of sleep complaint improves or prevents the medical condition, and whether the management of the medical condition improves sleep symptoms, are warranted in these subjects with persistent sleepiness.
The strengths of this present study are the prospective design, the size of the cohort and the large number of potential risk factors including socio-demographic, lifestyle, health and psychological variables. The study had also a long follow-up of five years with yearly evaluations allowing assessment of potential fluctuation of sleepiness course and changes in covariates over time. Furthermore, the evaluation of EDS was assessed using the ESS which is the most commonly used tool in sleep research and clinical setting.
This study has some limitations. Selection bias may exist even if random selection was used for the recruitment of the participants. The data are self-reported with possible recall and common method bias. The responses when filling the questionnaire may depend on the participant’s perceptual experience. Objective measures of sleep quality and DS by polysomnographic (PSG) and multiple sleep latency test (MSLT) recordings were not available for this study; however, performing PSG and MSLT were very difficult within the context of a large epidemiological survey. Moreover the agreement between objective and subjective EDS was always unsatisfactory.
In conclusion, our study reports a high fluctuation of EDS and increased DS over time in the general population. Sleepiness is predicted by a similar set of lifestyle and psychological factors that are potentially modifiable. In contrast, persistent EDS or increased DS are associated with chronic medical diseases thus individualizing a homogeneous group at risk of serious medical consequences requiring a dedicated management.
Methods
Study population
Subjects included were recruited as part of a larger epidemiological study aiming to assess prevalence and incidence, risk factors, natural history, and burden of insomnia in Canada. Detailed design and sampling procedures for this study have been reported elsewhere31. Briefly, subjects aged ≥18 years were recruited from a random selection of more than 12000 subjects who completed a telephone interview about their sleep between 2007 and 2008. Participants were then asked if they wanted to take part in the longitudinal phase of the study, which involved completion of seven postal evaluations over a 5-year period: the baseline evaluation was sent one month after the telephone interview, the second at 6 months and the remaining evaluations scheduled every years. Overall, 3006 completed baseline postal assessment. All participants provided written informed consent to participate in the study, prior to the study protocol and approved by the ethical committee of the Université Laval-Quebec, Canada. The methods in the current study were carried out in accordance with the approved guidelines.
EDS and DS increase during the 5-year follow-up
EDS was assessed at baseline and at each follow-up wave (after 6 months, 1, 2, 3, 4 and 5 years) by the ESS14. This scale comprises eight items evaluating sleepiness on a Likert scale from 0 to 3; total scores vary between 0 and 24. A total score >10 indicates EDS, and 15 severe EDS.
New cases of EDS between two consecutive visits were defined as participants with EDS at year ‘t’ but without EDS at year ‘t-1’. Remitted EDS cases were defined as participants without EDS at year ‘t’ but with EDS at year ‘t-1’, and recurrent EDS cases as participants with EDS at year ‘t-1’ and at year ‘t’.
In longitudinal analyses, the incidence of EDS was identified from participants without EDS at baseline but who subsequently developed EDS in at least one the follow-up evaluations. Persistent EDS cases were defined as participants reporting EDS at “t”-year follow-up that persists until the end of follow-up. Transient EDS cases was defined as participants reporting EDS at “t”-year follow-up without persistence until the end of follow-up.
A significant increase of DS during the follow-up was defined by at least a 4-point augmentation on the ESS scale between two consecutive evaluations that can or not cross the cut-off of 10. A 4-point change was considered clinically-relevant in previous pharmacological research studies on hypersomnia17, 18 and statistically, it corresponds to the lowest decile of all differences between two consecutive ESS scores. However, an augmentation of 4 points from previous ESS score equal to 0, 1, 2 and 3 was ruled out as considered non-clinically significant (N = 42). Persistent DS increase was defined as the absence of ESS decrease of more than 3-point in the subsequent follow-ups.
Other sleep-related measures
At each evaluation, sleep duration during the last month was self-reported by participant and divided into 3 categories: <6.0 hours/night, between 6.0 and 7.9, ≥8 based on published literature10. The number of naps during the last month was recorded as none; <1 per week; 1 or 2; ≥3. The severity of insomnia was evaluated using the Insomnia Severity Index (ISI)32. This seven-item questionnaire assesses the nature, severity, and impact of sleep difficulties yielding a total score ranging from 0 to 28. The total score is interpreted as follows: absence of insomnia (0–7); sub-threshold insomnia (8–14); moderate insomnia (15–21); and severe insomnia (22–28).
A standardized questionnaire was completed relative to diagnosis made by healthcare professionals on sleep disorders including central hypersomnias, insomnia, sleep apnea syndrome, and restless legs syndrome.
Sleep medication including prescriptions and over-the-counter (OTC) drugs used in the previous month were systematically detailed and classified as benzodiazepine (BZD), BZD-like compounds (zolpidem, zopiclone, zaleplon), antidepressants and miscellaneous medications (including barbiturates, OTC, and neuroleptics).
Socio-demographic, lifestyle, health and psychological measures
Standardized evaluation at baseline and at each follow-up included questions related to demographic characteristics, level of education (high school/college; university level), marital status (categorized as being single, divorced/separated or widowed; married, common-law couple), daily life habits such as alcohol consumption (categorized as none; 1 drink per day; ≥2 drinks), caffeine intake (categorized as none; 1–2 cups per day; ≥3), smoking status (classified as never; present or past users), and physical activity (categorized as never or less than one per week; between 1 and 3 per week, more than 4 times per week) and anthropometric data with height and weight to calculate body mass index (BMI) (classified as <25 kg/m2: normal; 25–30: overweight; ≥30: obese).
History of diabetes, endocrine and metabolic disorders (e.g. cholesterol, dysthyroidism), hypertension, cardio-vascular diseases, chronic pain, neurological diseases (epilepsy, headache, migraine) and other diseases including allergies, cancer, digestive, bone, lung, otorhinolaryngology, skin, urinary or genital problems was established at baseline and at each follow-up using standardized questions.
Standardized questionnaires were also completed at baseline and at each follow-up. Depressive symptoms were evaluated using the Beck Depression Inventory II (BDI-II)33. This scale contains 21 questions, with a total score ranging from 0 to 63, with higher scores suggesting more severe depressive symptoms (≤13: minimal; 14–19: mild, 20–28: moderate and 29–69: severe depression). State-Trait Anxiety Inventory (STAI) was used to measure state and trait anxiety symptoms34. The two subscales are composed of 20 items with overall scores ranging from 20 to 80. In the absence of validated cut-off score, STAI trait and state scores were divided into tertiles. General fatigue was assessed by the multidimensional fatigue inventory (MFI)35. The dimension of General Fatigue was used with a score varying between 4 and 20. The score was also divided into tertiles.
Statistical analysis
Associations between the characteristics of participants and EDS (ESS > 10 vs. ≤10) at baseline were quantified with odds ratios (OR) and their 95% confidence intervals (CI). Age, gender and other sociodemographic, health behavior and psychological variables associated with EDS at p < 0.15 in bivariate analysis were entered in a logistic regression model to determine which characteristics were independently associated with the presence of EDS. To study which factors were independently associated with EDS severity, three groups of subjects with ESS score ≤10, between 11 and 15 and ≥16 were compared by multinomial logistic regression model with age, gender and factors associated with the severity at p < 0.15. The p-value cut-off choice of 0.15 was based on the recommendations of several authors to use a significance level as high as 0.15, for variable selection in the bivariate analysis because such as 0.05 can fail to identify variables known to be important36.
Cox proportional hazard models were used to estimate hazard ratios (HR) and their 95% CI for the associations between potential predictors of a first event of EDS in bivariate analysis. Covariables that appeared or changed during the follow-up were taken into account as time-dependent covariates in the Cox proportional hazard models. The covariate values the year prior to the event time was taken but if this covariate was not updated at an annual visit, then the prior value was carried forward in the time-dependent model. In the case of multiple events during the follow-up, the first event was considered in the analysis. The multivariate analysis, which included age, gender and variables associated at p < 0.15 in the bivariate analysis, was performed using Cox proportional hazard model to evaluate which predictors were independently associated with the incidence of EDS. Similar methodology was performed for the study of predictors of incident DS during the 5-year follow-up. Significance level was set at p < 0.05. Analyses were performed using SAS-version 9.4 (SAS Inc, Cary, NC, USA).
References
American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. (American Academy of Sleep Medicine, 2014).
Akbaraly, T. N. et al. Sleep complaints and metabolic syndrome in an elderly population: the Three-City Study. Am J Geriatr Psychiatry 23, 818–828, doi:10.1016/j.jagp.2014.10.001 (2015).
Bixler, E. O. et al. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab 90, 4510–4515, doi:10.1210/jc.2005-0035 (2005).
Ohayon, M. M. & Vecchierini, M. F. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med 162, 201–208, doi:10.1001/archinte.162.2.201 (2002).
Tsuno, N. et al. Determinants of excessive daytime sleepiness in a French community-dwelling elderly population. J Sleep Res 16, 364–371, doi:10.1111/jsr.2007.16.issue-4 (2007).
Empana, J. P. et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke 40, 1219–1224, doi:10.1161/STROKEAHA.108.530824 (2009).
Jaussent, I. et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep 34, 1103–1110, doi:10.5665/SLEEP.1170 (2011).
Joo, S. et al. Prevalence of excessive daytime sleepiness and associated factors in the adult population of Korea. Sleep Med 10, 182–188, doi:10.1016/j.sleep.2008.03.017 (2009).
Klink, M. & Quan, S. F. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest 91, 540–546, doi:10.1378/chest.91.4.540 (1987).
Ohayon, M. M., Dauvilliers, Y. & Reynolds, C. F. 3rd Operational definitions and algorithms for excessive sleepiness in the general population: implications for DSM-5 nosology. Arch Gen Psychiatry 69, 71–79, doi:10.1001/archgenpsychiatry.2011.1240 (2012).
Pallesen, S. et al. Prevalence and risk factors of subjective sleepiness in the general adult population. Sleep 30, 619–624, doi:10.1093/sleep/30.5.619 (2007).
Ohayon, M. M., Priest, R. G., Zulley, J., Smirne, S. & Paiva, T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology 58, 1826–1833, doi:10.1212/WNL.58.12.1826 (2002).
Ohayon, M. M. From wakefulness to excessive sleepiness: what we know and still need to know. Sleep Med Rev 12, 129–141, doi:10.1016/j.smrv.2008.01.001 (2008).
Johns, M. W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–545, doi:10.1093/sleep/14.6.540 (1991).
Souza, J. C., Magna, L. A. & Reimao, R. Excessive daytime sleepiness in Campo Grande general population, Brazil. Arq Neuropsiquiatr 60, 558–562, doi:10.1590/S0004-282X2002000400008 (2002).
Wu, S. et al. Excessive daytime sleepiness assessed by the Epworth Sleepiness Scale and its association with health related quality of life: a population-based study in China. BMC public health 12, 849, doi:10.1186/1471-2458-12-849 (2012).
Dauvilliers, Y. et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. The Lancet. Neurology 12, 1068–1075, doi:10.1016/S1474-4422(13)70225-4 (2013).
US Modafinil in Narcolepsy Multicenter Study Group. Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy: US Modafinil in Narcolepsy Multicenter Study Group. Neurology 54, 1166–1175, doi:10.1212/WNL.54.5.1166 (2000).
Hasler, G. et al. Excessive daytime sleepiness in young adults: a 20-year prospective community study. J Clin Psychiatry 66, 521–529, doi:10.4088/JCP.v66n0416 (2005).
Theorell-Haglow, J., Akerstedt, T., Schwarz, J. & Lindberg, E. Predictors for Development of Excessive Daytime Sleepiness in Women: A Population-Based 10-Year Follow-Up. Sleep 38, 1995–2003, doi:10.5665/sleep.5258 (2015).
Fernandez-Mendoza, J. et al. Natural history of excessive daytime sleepiness: role of obesity, weight loss, depression, and sleep propensity. Sleep 38, 351–360, doi:10.5665/sleep.4488 (2015).
Johns, M. W. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 15, 376–381, doi:10.1093/sleep/15.4.376 (1992).
Kaminska, M. et al. The Epworth Sleepiness Scale: self-administration versus administration by the physician, and validation of a French version. Can Respir J 17, e27–34, doi:10.1155/2010/438676 (2010).
Dauvilliers, Y., Lopez, R., Ohayon, M. & Bayard, S. Hypersomnia and depressive symptoms: methodological and clinical aspects. BMC Med 11, 78, doi:10.1186/1741-7015-11-78 (2013).
Kendzerska, T. B., Smith, P. M., Brignardello-Petersen, R., Leung, R. S. & Tomlinson, G. A. Evaluation of the measurement properties of the Epworth sleepiness scale: a systematic review. Sleep Med Rev 18, 321–331, doi:10.1016/j.smrv.2013.08.002 (2014).
Soldatos, C. R., Kales, J. D., Scharf, M. B., Bixler, E. O. & Kales, A. Cigarette smoking associated with sleep difficulty. Science 207, 551–553, doi:10.1126/science.7352268 (1980).
Irwin, M. R., Olmstead, R. & Carroll, J. E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry 80, 40–52, doi:10.1016/j.biopsych.2015.05.014 (2016).
Vgontzas, A. N., Bixler, E. O. & Chrousos, G. P. Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Annals of the New York Academy of Sciences 1083, 329–344, doi:10.1196/annals.1367.023 (2006).
Roehrs, T. & Roth, T. Sleep and pain: interaction of two vital functions. Seminars in neurology 25, 106–116, doi:10.1055/s-2005-867079 (2005).
Ohayon, M. M., Reynolds, C. F. 3rd & Dauvilliers, Y. Excessive sleep duration and quality of life. Ann Neurol 73, 785–794, doi:10.1002/ana.23818 (2013).
Morin, C. M. et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med 169, 447–453, doi:10.1001/archinternmed.2008.610 (2009).
Bastien, C. H., Vallieres, A. & Morin, C. M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2, 297–307, doi:10.1016/S1389-9457(00)00065-4 (2001).
Beck, A. T., Steer, R. A. & Brown, G. K. Inventaire de dépression de Beck- deuxième édition Toronto: Pyschological Corporation edn (1996).
Spielberger, C. Manual for the State-Trait anxiety Inventory (Form Y) (Palo Alto, CA: Consulting Psychologists Press, 1983).
Smets, E. M., Garssen, B., Bonke, B. & De Haes, J. C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 39, 315–325, doi:10.1016/0022-3999(94)00125-O (1995).
Hosmer, D. & Lemeshow, S. Applied Logistic Regression Second Edition (Wiley inter-science, 2000).
Acknowledgements
Dr. C. Morin received research support from Novartis and served as consultant for Merck. Prof. Dauvilliers has received funds for seminars, board engagements and travel to conferences by UCB Pharma, Jazz, Theranexus, Flamel and Bioprojet.
Author information
Authors and Affiliations
Contributions
I.J., C.M., and Y.D. participated in the conception and design of the study. I.J. conducted the analyses and wrote the first draft of the manuscript. I.J., C.M., Y.D. participated in the interpretation of the data. I.J., C.M. and Y.D. contributed to the writing of the manuscript. C.M., and H.I. participated in the acquisition of the data.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jaussent, I., Morin, C.M., Ivers, H. et al. Incidence, worsening and risk factors of daytime sleepiness in a population-based 5-year longitudinal study. Sci Rep 7, 1372 (2017). https://doi.org/10.1038/s41598-017-01547-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01547-0
This article is cited by
-
Machine learning polysomnographically-derived electroencephalography biomarkers predictive of epworth sleepiness scale
Scientific Reports (2023)
-
Effectiveness of non-pharmacological interventions on sleep characteristics among adults with musculoskeletal pain and a comorbid sleep problem: a systematic review
Chiropractic & Manual Therapies (2021)
-
The neurophysiological basis of excessive daytime sleepiness: suggestions of an altered state of consciousness
Sleep and Breathing (2020)
-
Wanted: a better cut-off value for the Epworth Sleepiness Scale
Wiener klinische Wochenschrift (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.