Abstract
We hypothesised that the established association of endothelial lipase (EL) plasma levels with atherogenic lipid profile is altered in acute heart failure (AHF) and additionally affected by overlapping metabolic syndrome (MetS). We examined the association of EL plasma levels and lipid/lipoprotein plasma levels in AHF patients without and with overlapping MetS. The study was performed as a single-centre, observational study on 152 AHF patients, out of which 85 had overlapping MetS. In the no-MetS group, EL plasma levels were significantly positively correlated with plasma levels of atherogenic lipids/lipoproteins, including total cholesterol, low-density lipoprotein (LDL)-cholesterol, total LDL particles and triglycerides, but also with plasma levels of antiatherogenic high-density lipoprotein (HDL)-cholesterol, total HDL particles and small HDL particles. In the MetS group, EL plasma levels were positively correlated with triglyceride and small LDL-particle levels, and significantly negatively correlated with plasma levels of large HDL particles as well as with LDL- and HDL-particle size, respectively. EL- and lipid/lipoprotein- plasma levels were different in the no-MetS patients, compared to MetS patients. The association of EL with atherogenic lipid profile is altered in AHF and additionally modified by MetS, which strongly modulates EL- and lipid/lipoprotein-plasma levels in AHF.
Similar content being viewed by others
Introduction
Endothelial lipase (EL) is a member of the triglyceride lipase gene family, expressed primarily by vascular endothelial cells (ECs) and to a lesser extent smooth muscle cells (SMCs) and macrophages1, 2. EL is a phospholipase which has a high affinity for high-density lipoprotein-phospholipids. EL expression can be induced by tumor necrosis factor-α, interleukin-1β and biomechanical forces in vascular EC3, 4, by angiotensin II and hypertension in vascular SMCs5 as well as by lipopolysaccharide in macrophages6. EL concentrations in human plasma have been found to be strongly associated with inflammatory markers, such as C-reactive protein (CRP), interleukin-6 (IL-6) and secretory phospholipase A2 type IIA levels7. EL plasma levels are increased in metabolic syndrome (MetS) and associated with subclinical atherosclerosis, measured as coronary artery calcification8. In contrast to EL plasma levels, the EL activity in post-heparin plasma was not significantly increased in humans with MetS, but was dependent on the degree of insulin resistance9. EL is expressed in human and mouse atherosclerotic plaques mainly by macrophages and to a lesser extent by SMCs10,11,12. EL modulates HDL plasma levels and functionality13,14,15,16, and its plasma levels are associated with a proatherogenic lipid profile8, 17. Moreover, EL was recognized as an important provider of lipoprotein-derived fatty acids to cardiac tissue of mice with pressure overload-induced heart failure (HF), a pathophysiological condition accompanied by an increased energy demand and a decreased cardiac lipoprotein lipase (LPL) expression; EL deficient mice presented more severe HF than wild type mice due to decreased cardiac uptake of fatty acids18.
Decreased lipid synthesis and decreased intestinal lipid absorption due to venous congestion, a consequence of right-sided heart failure, underlie the low lipid plasma levels and deranged lipid metabolism in HF19, 20. Considering the disturbed lipid metabolism in HF and metabolic perturbations with upregulation of EL in MetS8, 21,we hypothesised that the established association of EL plasma levels and atherogenic lipid profile, including increased triglycerides, total cholesterol and apoB-containing lipoproteins, as well as decreased HDL-cholesterol plasma levels8, 15,16,17, is altered in acute HF (AHF) and additionally affected by overlapping MetS.
Results
Patients’ clinical characteristics
Of the152 AHF patients included in the study, 85 (55.9%) had MetS (Table 1). The MetS and the no-MetS groups did not differ significantly regarding gender, age, mean arterial pressure (MAP), New York Heart Association (NYHA) classification, time of AHF onset, EF, smoking and final clinical presentation (Table 1). As obvious from patients’ age none of the groups contained premenopausal women. In line with the MetS definition, the MetS group had significantly higher body weight, body mass index (BMI) and waist circumference, as well as a higher incidence of hypertension, type 2 diabetes mellitus (T2DM), hyperlipidaemia/hypertriglyceridaemia and hypercholesterolaemia (Table 1). Additionally, the MetS group had a higher incidence of chronic obstructive pulmonary disease (COPD) (Table 1). The no-MetS and MetS groups had a similar incidence of enlarged liver, ascites and peripheral oedema, the signs implying venous overload due to right-sided heart failure (Table 1).
Laboratory parameters
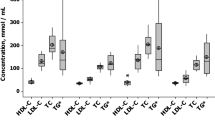
In accordance with previous reports8, 17, EL plasma levels were higher in the MetS group compared with the no-MetS group (Table 2). Total cholesterol and low-density lipoprotein (LDL)-cholesterol were similar in the MetS and the no-MetS groups (Table 2). In line with the MetS definition, the triglyceride plasma levels were significantly higher, and HDL-cholesterol plasma levels were significantly lower in the MetS compared to the no-MetS group (Table 2). Additionally, we found significantly higher plasma levels of large very low-density lipoprotein particles (LVLDL-p) and significantly lower plasma levels of large HDL particles (LHDL-p) in the MetS compared with the no-MetS group (Table 2). Furthermore, the size of HDL particles (HDL-s) was significantly smaller in the MetS compared with the no-MetS group (Table 2). The concentrations of CRP and interleukin-6 (IL-6) were similar in both groups (Table 2).
Correlation of EL plasma levels and plasma lipids/lipoproteins
In the no-MetS group, EL plasma levels were significantly positively correlated with total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, total low-density lipoprotein particle concentrations (TLDL-p), total (T) HDL-p and small (S) HDL-p (Table 3). In the MetS group, similarly to the no-MetS group, EL plasma levels were positively correlated with triglyceride levels (Table 3). In contrast to the no-MetS group, in the MetS group EL was significantly positively correlated with small (S)LDL-particles and significantly negatively correlated with plasma levels of LHDL-particles as well as with LDL-s and HDL-s (Table 3). As shown by partial correlation analysis, T2DM did not affect the correlation between EL and lipids/lipoproteins (Supplementary Table S1).
Impact of venous overload on EL and lipid/lipoprotein plasma levels
Considering the role of venous congestion in the deranged lipid metabolism in HF, together with the increased EL and metabolic perturbations in MetS, we examined whether venous volume overload affects EL- and lipid/lipoprotein- plasma levels, and whether the impact of venous volume overload is modulated by MetS. For this purpose we compared EL- and lipid/lipoprotein- plasma levels in the no-MetS and the MetS patients, having none vs. any one or more of the three signs implying volume overload (enlarged liver, peripheral oedema or ascites), as a consequence of right- sided HF. Within the no-MetS group, plasma levels of EL, total cholesterol, LDL-cholesterol, HDL-cholesterol and LLDL-p were significantly decreased in patients with sign(s) implying volume overload (Table 4). This was not observed in the MetS group (Table 4). Triglyceride-, THDL-p- and SHDL-p- plasma levels were significantly decreased in both the no-MetS and MetS groups, and plasma levels of SLDL-p were decreased only in the MetS group with sign(s) implying volume overload, compared to patients with no sign(s) (Table 4).
Discussion
We hypothesised that the reported association of EL and increased plasma triglycerides, total cholesterol and apoB-containing lipoproteins, as well as with decreased HDL-cholesterol plasma levels8, 15,16,17, is altered in AHF and additionally affected by MetS. To test our hypothesis, we examined the association of EL plasma levels with plasma lipids/lipoproteins in AHF patients without and with overlapping MetS. To our knowledge, this is the first study on EL and its association with plasma lipids/lipoproteins in AHF.
Disturbed haemodynamics and related volume overload with concomitant venous congestion have been found to be associated with impaired renal function, impaired absorption of water, electrolytes, and glucose, as well as decreased plasma levels of lipids and lipoproteins in HF patients19, 22. Decreased lipid synthesis or decreased absorption, likely due to venous congestion may underlie the reportedly decreased lipid and lipoprotein levels in HF19.
We found a strong positive correlation between EL plasma levels and plasma levels of lipids and lipoproteins primarily in the no-MetS group (Table 3). Positive associations with total cholesterol, LDL-cholesterol and triglycerides are in accordance with previous findings8. However, a strong positive association of EL with HDL-cholesterol-, THDL-p- and SHDL-p- concentrations was in sharp contrast to previous studies reporting either no7, 23 or a negative association of EL with HDL-cholesterol- and HDL-p- plasma concentrations8, 15,16,17. A possible explanation for this finding might be that the observed relationship does not reflect the enzyme-substrate interaction but rather a concomitant regulation of EL- and HDL- levels into the same direction by the underlying AHF pathophysiology. Indeed, the levels of EL, HDL-cholesterol, THDL-p and SHDL-p, but not LHDL-p, were decreased by venous volume overload in the no-MetS group, in which the levels of SHDL-p were slightly affected by HF severity (Supplementary Table S2). However, partial correlation analyses revealed only a weak overall impact of venous volume overload (Supplementary Table S3) and HF severity (Supplementary Table S4) on the correlation between EL- and lipid/lipoprotein plasma levels in the no-MetS group. Observed positive correlation between EL and SHDL-p plasma levels, but no correlation with LHDL-p levels and HDL-s in the no-MetS group (Table 3) suggests a disruption of the established relationship between EL and HDL, whereby EL, by acting on HDL, converts LHDL-p into SHDL-p16, 24. This finding is in line with a recent report on metabolic studies in humans, suggesting that complex multiple-sized HDL-p are simultaneously secreted from liver cells, whereby extracellular HDL remodelling represents a minor contribution to HDL-class diversity25. Notably, the EL mass determined in the present study may not necessarily be related to EL activity, which has been shown in a recent study to be negatively correlated with HDL-cholesterol in both healthy and MetS subjects9. Furthermore, since EL presents mainly phospholipase activity, the association of EL levels with HDL cholesterol content and size does not exclude the catabolic role of the enzyme. In the MetS group, where plasma levels of neither EL nor LHDL-p were significantly modulated by venous volume overload, EL was significantly negatively correlated with LHDL-p (Table 3). This is in line with increased HDL particle plasma concentrations in patients with partial and complete loss-of-function mutations in the EL gene as well as in EL genetic variants exhibiting decreased EL activity15, 16. Although the positive correlation between EL and SHDL-p was not significant a significant negative correlation between EL plasma levels and HDL-s in the MetS group (Table 3) corroborates the established role of EL in the generation of SHDL-p16.
It is important to note that besides EL, LPL and hepatic lipase (HL) shape the serum lipoprotein profile in healthy subjects and MetS patients9. Considering the decreased LPL activity in MetS together with its role in the HDL biogenesis26, 27 it is possible that the negative correlation of EL and LHDL-p is, at least in part, a consequence of the decreased LPL activity in MetS patients. Similarly, the strong positive correlation between EL and triglycerides in the MetS group is most likely the reflection of the concomitant increase in EL and triglycerides, the latter due to decreased LPL activity28, a consequence of low-grade inflammation in MetS. A previous study revealed no association between EL levels and HDL subclasses in healthy subjects29. The discrepancy between those results and ours might partly be due to different methodologies used for the evaluation of HDL subclasses namely NMR-spectroscopy used in our study, and the lipoprotein precipitation method30 used in theirs29.
Previous studies showed altered SN1 lipase activities in obesity and a pronounced influence of the degree of insulin resistance on EL activity9. Furthermore, the association of EL plasma levels with HDL-cholesterol- and HDL-phospholipid- levels, that is present in non-obese subjects, was not observed in subjects with excess adipose tissue levels17. Considering the influence of adiposity on lipoprotein size and subclass concentrations31, as well as on EL plasma levels17 together with increased EL phospholipase activity in obese and insulin resistant subjects9, we assumed that a higher BMI and the pronounced positive correlation between EL and BMI in the MetS group (unadjusted: rho = 0.29, p = 0.008; adjusted for T2DM: rho = 0.27, p = 0.016) might, at least in part, explain the different associations between EL- and lipid/lipoprotein- plasma levels in the MetS and no-MetS groups. However, partial correlation analyses showed only a weak impact of BMI (Supplementary Table S5), waist circumference (Supplementary Table S6) and T2DM (Supplementary Table S1) on the correlation between EL- and lipid/lipoprotein plasma levels in both the no-MetS and the MetS group.
Increased EL levels and altered lipid/lipoprotein levels in MetS might be responsible for a less pronounced impact of venous volume overload on EL- and lipid/lipoprotein- levels in the MetS patients than in the no-MetS patients. The significant positive association of EL- and SLDL-p- levels and the significant negative association of EL and LDL-s in the MetS group is in accordance with the promotion of the catabolism of apoB-lipoprotein- containing lipoproteins observed in a hyperlipidaemic mouse model32. The results of our study regarding EL can be compared with a previous study, which revealed a positive association of HL and small, dense LDL-p33.
While we found no correlation between EL and IL-6 plasma levels (not shown), a significant positive correlation was established between EL and CRP, but only in the MetS group (rho: 0.24; p = 0.032). The absence of correlation in the no-MetS group could be due to the pathophysiology of the underlying volume overload, causing a pronounced significant increase in CRP (Supplementary Fig. S1) with concomitantly decreased EL (Table 4).
This study has several limitations: The insufficient quality of NMR-spectra obtained for some lipoprotein subfractions resulted in differing numbers of patients in which particular lipoprotein subfractions could be analysed. Although previous studies showed that pre- and post-heparin EL plasma levels are highly correlated8 or similar34, the possibility cannot be excluded that post-heparin EL plasma levels would be differently affected by AHF-associated pathophysiological conditions and MetS compared to our findings with pre-heparin EL plasma levels. Furthermore, considering EL polymorphisms associated with decreased EL enzyme activity15, 16, together with possibly altered plasma levels of EL inhibitors, such as angiopoetin-like protein 335 or protein convertases36 in AHF, the association of EL activity with plasma lipids/lipoproteins9 might be different from what was found for the EL mass in this study. The study examined only the relationship between EL and HDL levels without addressing HDL functionality, a factor that is more important than HDL levels37, and can be studied by a variety of available experimental methods38. Based on our results, we conclude that the association of EL and atherogenic lipid profile is altered in AHF and additionally modified by MetS, which also strongly modulates EL- and lipid/lipoprotein- plasma levels in AHF.
Methods
Study design and patients
The AHF study was performed as an observational single-centre study and included consecutive white adult hospitalised AHF patients. The study was approved by the Ethics Committee of the University Hospital Centre Sisters of Charity, Zagreb, and of the Medical University of Graz. The investigation conforms with the principles outlined in the Declaration of Helsinki principles39, and informed consent was obtained in compliance with Good Clinical Practice. 152 patients were recruited from November 2013 to February 2015 as described40. Categorisation of the patients was performed according to the European Society of Cardiology (ESC) and ACCF/AHA Guidelines for HF41,42,43 and patients were treated by the standard ESC Guidelines for AHF42, 43. MetS was defined as the manifestation of three or more of the following five abnormalities: central obesity, hypertriglyceridaemia, lowered HDL-cholesterol, glucose intolerance or elevated fasting glucose and hypertension44. Hypertension was diagnosed according to the ESC criteria45. Diabetes was diagnosed in patients with dietary treatment, antidiabetic medication or current fasting plasma glucose levels higher than 7.0 mmol/L46. Hypercholesterolaemia was defined as LDL-cholesterol levels higher than 3.5 mmol/L, or taking a lipid-lowering drug and hypertriglyceridaemia was defined as triglyceride levels higher than 1.7 mmol/L. Patients were also classified according to three signs implying volume overload (enlarged liver, peripheral oedema or ascites) as a consequence of right-sided HF into those having none of the signs (no sign(s)) and those having at least one sign (sign(s)). Patients with severe renal failure (serum creatinine ≥400 mmol/L), renal replacement therapy, hepatic cirrhosis, malignancy, trauma, surgical diseases, pregnancy, major systemic disease or younger than 18 years were not included in the study.
Laboratory assays
For routine laboratory assays blood was obtained at admission to the hospital. It was collected in 6 mL tubes, VACUETTE® Z Serum Clot Activator (Greiner Bio-one GmbH, Kremsmuenster, Austria). The serum aliquots were stored at −80 °C. The Beckman Coulter instrument AU 2700, 2007 (Brea, CA, USA) and Architect c8000, Abbott 2013 (Chicago, IL, USA) were used to measure total plasma cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides and CRP. A specific chemiluminescent ELISA (QuantiGlo; R&D Systems, Wiesbaden-Nordenstadt, Germany) was used to measure IL-6 concentrations and with Human Endothelial Lipase Assay Kit (TaKaRa, Takara Bio Europe S.A.S., Saint-Germain-en-Laye, France) were measured pre-heparin EL protein levels, according to the manufacturer’s instructions.
Lipoprotein profiling by Nuclear Magnetic Resonance (NMR) spectroscopy
The lipoprotein profiles of 138 serum samples were analysed with the AXINON ® lipoFIT ® -S100 test system (Numares Health, Regensburg, Germany). NMR spectra were recorded at a temperature of 310 K on a shielded 600 MHz Bruker Avance III HD spectrometer as described47. To ensure data quality only spectra meeting defined quality criteria were used for analyses. The number (n) of analysed samples is indicated in Table 2.
Statistical analysis
Categorical data are shown as absolute and relative frequencies, and continuous data are presented as mean and SD or as median and range (minimum to maximum) depending on distribution. The differences between patients with and without metabolic syndrome were assessed by t-test or Mann-Whitney U test for continuous parameters and by Fisher’s exact test for categorical parameters. In addition, the differences between patients with and without sign(s) implying volume overload were assessed within the groups using Mann-Whitney U test. The Spearman correlation coefficients were calculated to evaluate the correlation of EL plasma levels and laboratory parameters. In addition, the differences between patients with and without sign(s) implying venous volume overload (enlarged liver, peripheral oedema or ascites), were assessed within the metabolic syndrome groups using Mann-Whitney U test. Furthermore, the impact of T2DM, sign(s) implying venous volume overload, BMI, waist circumference and NYHA classes (2/3 vs. 4) on the correlations between EL and lipid parameters was assessed by means of partial correlations. A p-value < 0.05 was considered statistically significant and results are to be interpreted in an exploratory fashion. All data were analysed using R version 3.3.1.
References
Hirata, K. et al. Cloning of a unique lipase from endothelial cells extends the lipase gene family. J. Biol. Chem. 274, 14170–14175, doi:10.1074/jbc.274.20.14170 (1999).
Jaye, M. et al. A novel endothelial-derived lipase that modulates HDL metabolism. Nat. Genet. 21, 424–428, doi:10.1038/7766 (1999).
Jin, W. et al. Endothelial cells secrete triglyceride lipase and phospholipase activities in response to cytokines as a result of endothelial lipase. Circ. Res. 92, 644–650, doi:10.1161/01.RES.0000064502.47539.6D (2003).
Hirata, K., Ishida, T., Matsushita, H., Tsao, P. S. & Quertermous, T. Regulated expression of endothelial cell-derived lipase. Biochem. Biophys. Res. Commun. 272, 90–93, doi:10.1006/bbrc.2000.2747 (2000).
Shimokawa, Y. et al. Increased expression of endothelial lipase in rat models of hypertension. Cardiovasc. Res. 66, 594–600, doi:10.1016/j.cardiores.2005.01.013 (2005).
Yasuda, T. et al. Endothelial lipase is increased by inflammation and promotes LDL uptake in macrophages. J. Atheroscler. Thromb. 14, 192–201, doi:10.5551/jat.E502 (2007).
Paradis, M. E. et al. Endothelial lipase is associated with inflammation in humans. J. Lipid Res. 47, 2808–2813, doi:10.1194/jlr.P600002-JLR200 (2006).
Badellino, K. O., Wolfe, M. L., Reilly, M. P. & Rader, D. J. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PLoS Med. 3, e22, doi:10.1371/journal.pmed.0030022 (2006).
Miksztowicz, V. et al. Role of SN1 lipases on plasma lipids in metabolic syndrome and obesity. Arterioscler. Thromb. Vasc. Biol. 34, 669–675, doi:10.1161/ATVBAHA.113.303027 (2014).
Azumi, H. et al. Immunohistochemical localization of endothelial cell-derived lipase in atherosclerotic human coronary arteries. Cardiovasc. Res. 58, 647–654, doi:10.1016/S0008-6363(03)00287-6 (2003).
Bartels, E. D. et al. Endothelial lipase is highly expressed in macrophages in advanced human atherosclerotic lesions. Atherosclerosis 195, e42–49, doi:10.1016/j.atherosclerosis.2007.05.002 (2007).
Trbusic, M. et al. Increased expression of endothelial lipase in symptomatic and unstable carotid plaques. J. Neurol. 259, 448–456, doi:10.1007/s00415-011-6198-3 (2012).
Gauster, M. et al. Endothelial lipase-modified high-density lipoprotein exhibits diminished ability to mediate SR-BI (scavenger receptor B type I)-dependent free-cholesterol efflux. Biochem. J. 382, 75–82, doi:10.1042/BJ20031882 (2004).
Ishida, T. et al. Endothelial lipase is a major determinant of HDL level. J. Clin. Invest. 111, 347–355, doi:10.1172/JCI16306 (2003).
Edmondson, A. C. et al. Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J. Clin. Invest. 119, 1042–1050, doi:10.1172/JCI37176 (2009).
Singaraja, R. R. et al. The impact of partial and complete loss-of-function mutations in endothelial lipase on high-density lipoprotein levels and functionality in humans. Circ Cardiovasc. Genet. 6, 54–62, doi:10.1161/CIRCGENETICS.111.962613 (2013).
Paradis, M. E. et al. Visceral adiposity and endothelial lipase. J. Clin. Endocrinol. Metab. 91, 3538–3543, doi:10.1210/jc.2006-0766 (2006).
Nakajima, H. et al. Endothelial lipase modulates pressure overload-induced heart failure through alternative pathway for fatty acid uptake. Hypertension 61, 1002–1007, doi:10.1161/HYPERTENSIONAHA.111.201608 (2013).
Chen, Y. et al. Relationship between lipids levels and right ventricular volume overload in congestive heart failure. J. Geriatr. Cardiol. 11, 192–199, doi:10.11909/j.issn.1671-5411.2014.03.011 (2014).
Giamouzis, G. & Butler, J. Relationship between heart failure and lipids: the paradigm continues to evolve. J. Card. Fail. 13, 254–258, doi:10.1016/j.cardfail.2007.03.009 (2007).
Devaraj, S., Singh, U. & Jialal, I. Human C-reactive protein and the metabolic syndrome. Curr. Opin. Lipidol. 20, 182–189, doi:10.1097/MOL.0b013e32832ac03e (2009).
Joles, J. A., Bongartz, L. G., Gaillard, C. A. & Braam, B. Renal venous congestion and renal function in congestive heart failure. J. Am. Coll. Cardiol. 54, 1632–1633, doi:10.1016/j.jacc.2009.05.068 (2009).
Fujii, H. et al. Putative role of endothelial lipase in dialysis patients with hypoalbuminemia and inflammation. Am. J. Nephrol. 28, 974–981, doi:10.1159/000144025 (2008).
Nijstad, N. et al. Scavenger receptor BI-mediated selective uptake is required for the remodeling of high density lipoprotein by endothelial lipase. J. Biol. Chem. 284, 6093–6100, doi:10.1074/jbc.M807683200 (2009).
Mendivil, C. O., Furtado, J., Morton, A. M., Wang, L. & Sacks, F. M. Novel Pathways of Apolipoprotein A-I Metabolism in High-Density Lipoprotein of Different Sizes in Humans. Arterioscler. Thromb. Vasc. Biol. 36, 156–165, doi:10.1161/ATVBAHA.115.306138 (2016).
Strauss, J. G. et al. Adenovirus-mediated rescue of lipoprotein lipase-deficient mice. Lipolysis of triglyceride-rich lipoproteins is essential for high density lipoprotein maturation in mice. J. Biol. Chem. 276, 36083–36090, doi:10.1074/jbc.M104430200 (2001).
Tani, M. et al. High-density lipoprotein subpopulation profiles in lipoprotein lipase and hepatic lipase deficiency. Atherosclerosis 253, 7–14, doi:10.1016/j.atherosclerosis.2016.08.014 (2016).
van Wijk, J. P., Halkes, C. J., Erkelens, D. W. & Castro Cabezas, M. Fasting and daylong triglycerides in obesity with and without type 2 diabetes. Metabolism 52, 1043–1049, doi:10.1016/S0026-0495(03)00106-9 (2003).
Kilic, H. et al. High-density lipoprotein subfractions and influence of endothelial lipase in a healthy Turkish population: a study in a land of low high-density lipoprotein cholesterol. Scand. J. Clin. Lab. Invest. 74, 278–284, doi:10.3109/00365513.2013.879992 (2014).
Burstein, M., Scholnick, H. R. & Morfin, R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 11, 583–595 (1970).
Goff, D. C. Jr., D’Agostino, R. B. Jr., Haffner, S. M. & Otvos, J. D. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the Insulin Resistance Atherosclerosis Study. Metabolism 54, 264–270, doi:10.1016/j.metabol.2004.09.002 (2005).
Broedl, U. C. et al. Endothelial lipase promotes the catabolism of ApoB-containing lipoproteins. Circ. Res. 94, 1554–1561, doi:10.1161/01.RES.0000130657.00222.39 (2004).
Murdoch, S. J., Carr, M. C., Kennedy, H., Brunzell, J. D. & Albers, J. J. Selective and independent associations of phospholipid transfer protein and hepatic lipase with the LDL subfraction distribution. J. Lipid Res. 43, 1256–1263 (2002).
Ishida, T. et al. ELISA system for human endothelial lipase. Clin. Chem. 58, 1656–1664, doi:10.1373/clinchem.2012.187914 (2012).
Jin, W. et al. Hepatic proprotein convertases modulate HDL metabolism. Cell Metab. 6, 129–136, doi:10.1016/j.cmet.2007.07.009 (2007).
Gauster, M., Hrzenjak, A., Schick, K. & Frank, S. Endothelial lipase is inactivated upon cleavage by the members of the proprotein convertase family. J. Lipid Res. 46, 977–987, doi:10.1194/jlr.M400500-JLR200 (2005).
Santos-Gallego, C. G. HDL: Quality or quantity? Atherosclerosis 243, 121–123, doi:10.1016/j.atherosclerosis.2015.08.027 (2015).
Santos-Gallego, C. G., Giannarelli, C. & Badimon, J. J. Experimental models for the investigation of high-density lipoprotein-mediated cholesterol efflux. Curr. Atheroscler. Rep. 13, 266–276, doi:10.1007/s11883-011-0177-0 (2011).
World Medical, A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310, 2191–2194, doi:10.1001/jama.2013.281053 (2013).
Potocnjak, I. et al. Metrics of High-Density Lipoprotein Function and Hospital Mortality in Acute Heart Failure Patients. PLoS One 11, e0157507, doi:10.1371/journal.pone.0157507 (2016).
Dickstein, K. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. Heart J 29, 2388–2442, doi:10.1093/eurheartj/ehn309 (2008).
McMurray, J. J. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J 33, 1787–1847, doi:10.1093/eurheartj/ehs104 (2012).
Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 62, e147–239, doi:10.1016/j.jacc.2013.05.019 (2013).
Alberti, G, Z. P. & Shaw, J. G. S. The International Diabetes Federation consensus worldwide definition of the metabolic syndrome. Int. Diabetes Fed. 9491 (2006).
Mancia, G. et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 25, 1105–1187, doi:10.1097/HJH.0b013e3281fc975a (2007).
American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 34(Suppl 1), S62–69, doi:10.2337/dc11-S062 (2011).
Kaess, B. M. et al. Large-scale candidate gene analysis of HDL particle features. PLoS One 6, e14529, doi:10.1371/journal.pone.0014529 (2011).
Acknowledgements
We thank Margarete Lechleitner for her expert technical assistance. We also wish to thank Aleksandra Žmegač Horvat, University of Zagreb School of Medicine, for language editing the text. This work was supported by the Austrian Science Foundation [P27166-B23 to SF; P22976-B18 to GM] and the Jubilee Foundation of the Austrian National Bank [15858 to S.F.], which had no roles in the study design, collection, analysis and interpretation of data, report writing or submission of the article.
Author information
Authors and Affiliations
Contributions
Experiments conceived and designed by: I.P., V.D., S.F., G.M. Experiments carried out by: I.P., V.D., M.T., S.D.T., B.R. Contribution of reagents/materials/analysis tolls: B.T., S.F., G.M. Contributed significantly to the execution, and/or analysis and interpretation of data: I.P., M.T., S.D.T., B.R., G.P., A.B., B.T., G.M., V.D., S.F. Paper written by: I.P., V.D., A.B., G.P., G.M., S.F. Participated in reviewing, and/or revising the manuscript for intellectual content: I.P., M.T., S.D.T., B.R., G.P., A.B., B.T., G.M., V.D., S.F. Approved the manuscript for submission: I.P., M.T., S.D.T., B.R., G.P., A.B., B.T., G.M., V.D., S.F.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Potočnjak, I., Trbušić, M., Terešak, S.D. et al. Metabolic Syndrome Modulates Association between Endothelial Lipase and Lipid/Lipoprotein Plasma Levels in Acute Heart Failure Patients. Sci Rep 7, 1165 (2017). https://doi.org/10.1038/s41598-017-01367-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01367-2
This article is cited by
-
LIPG endothelial lipase and breast cancer risk by subtypes
Scientific Reports (2021)
-
Impact of Endothelial Lipase on Cholesterol Efflux Capacity of Serum and High-density Lipoprotein
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.