Abstract
Diabetes duration, diabetic retinopathy (DR), and a diagnostic model have been proposed as clinical parameters favoring the presence of diabetic nephropathy (DN) in biopsied patients with diabetic kidney disease. DN, compared with non-diabetic renal disease, had poorer renal outcomes. We tested whether these clinical parameters favoring DN are associated with poorer renal outcomes in non-biopsied patients. In this study, 1330 patients with type 2 diabetes and chronic kidney disease stages 1–4 were included and divided according to diabetes mellitus (DM) duration >8 years, DR, or a diagnostic model for DN. These clinical parameters favoring DN were found in 62–77% of patients and associated with higher levels of proteinuria. In a Cox survival analysis, DR and the diagnostic model favoring DN were associated with an increased risk for end-stage renal disease with adjusted hazard ratios of 1.69 (95% CI: 1.16–2.45, P = 0.006) and 1.66 (95% CI: 1.05–2.61, P = 0.029), respectively. DR was associated with an increased risk for rapid renal disease progression. DM >8 years was not associated with renal outcome. Propensity score-matched analyses also showed similar results. In conclusion, DR and the diagnostic model favoring DN were associated with poorer renal outcomes.
Similar content being viewed by others
Introduction
The incidence and prevalence of type 2 diabetes mellitus (referred to as diabetes hereafter) are increasing worldwide1. Diabetes is the leading cause of chronic kidney disease (CKD) in many countries, and approximately 40% of patients subsequently develop diabetic kidney disease (DKD)2. DKD is the leading cause of end-stage renal disease (ESRD) in many developed countries3. The classic presentation of DKD is diabetic nephropathy (DN) characterized by albuminuria and nodular glomerulosclerosis. However, non-diabetic renal disease (NDRD) can present in biopsied patients with DKD, with immunoglobulin A (IgA) nephropathy and focal segmental glomerulosclerosis (FSGS) as the leading causes4, 5. In this study, we apply the term “DN” or “NDRD” to biopsy-proven cases and “DKD” to non-biopsied cases. Because NDRD is treatable and has a more favorable prognosis than that of DN6,7,8, renal biopsy is indicated in patients suspected to have NDRD.
Clinical differentiation between DN and NDRD is difficult. Several parameters, especially longer diabetes duration, diabetic retinopathy (DR), and absence of hematuria have been proposed as predictors for DN in recent meta-analyses9, 10. However, the pooled positive predictive value (PPV) for DN was measured to be only 0.72 in a meta-analysis of its most accurate predictor, DR9. A diagnostic model developed at a single center in China which included diabetes duration, systolic blood pressure, HbA1c, hematuria, hemoglobin and diabetic retinopathy as parameters, showed a PPV of 0.89 for DN11. These biopsy studies included specially selected patients with DKD, who usually presented with heavy proteinuria and were young9, 10, 12. These clinical parameters might not be applicable to non-selected patients with DKD. Thus, we propose an approach to test these parameters: if these predictive parameters can differentiate between DN and NDRD in patients with DKD, the DN patients favored by these parameters should have less favorable renal outcomes. However, in a study to investigate the prognostic value of retinopathy in assessing the risk of developing ESRD, cardiovascular morbidity or death among patients from the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) study, DR was common but not independently associated with ESRD or other endpoints13. The predictive value of these parameters in DKD, especially DR and diabetes duration, is still controversial. We thus hypothesized that longer diabetes duration, DR and the diagnostic model favoring DN are associated with less favorable renal outcomes, cardiovascular events and all-cause mortality in non-biopsied patients with DKD.
Results
Baseline characteristics of patients with diabetic kidney disease by clinical parameters favoring diabetic nephropathy
The baseline characteristics of 1330 diabetic patients with CKD stages 1–4 are shown in Table 1. The mean age was 64.2 ± 12.7 years and the estimated glomerular filtration rate (eGFR) was 33.7 (24.2–46.3) mL/min/1.73 m2, with a urine protein-to-creatinine ratio (UPCR) of 928 (299–2536) mg/g and a glycated hemoglobin (HbA1c) level of 7.6% ± 1.8%. Of the patients, 67.1% had hypertension, 27.4% had cardiovascular (CV) disease, and 22.8% of the patients were treated with insulin. In 98 patients who underwent renal biopsy, 54 of them were diagnosed as DN. In multivariate logistic regression, we found that DR, absence of hematuria, low eGFR, and high HbA1c were associated with DN (Supplemental Table 1).
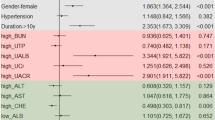
We divided our patients by three clinical parameters favoring DN (diabetes mellitus [DM] > 8 years, DR, and the positive diagnostic model) according to our data and the literature4, 9, 10. The proportions of DM > 8 years, DR, and the positive diagnostic model were 1030 (77.4%), 832 (62.6%), and 860 (64.7%), respectively (P < 0.05). Patients with DM > 8 years, DR, or the positive diagnostic model exhibited a higher prevalence of cardiovascular disease, lower eGFR, higher UPCR (Table 1), lower hemoglobin and albumin level, and higher phosphate level and a higher prevalence of insulin and angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment (Table 2), compared with patients without any of these three parameters (all P < 0.05). However, body mass index (BMI), cholesterol and C-reactive protein (CRP) were not different between groups. Patients with DM > 8 years had higher uric acid; patients with DR and the positive diagnostic model possessed higher HbA1c; and patients with the positive diagnostic model exhibited higher mean blood pressure and a higher prevalence of hematuria and female (all P < 0.05) (Table 1).
Factors associated with DM > 8 years and DR in patients with diabetic kidney disease
In multivariate logistic regression analysis, CV disease was associated with a higher odds ratio (OR) for DM > 8 years, whereas the eGFR and CRP were associated with lower ORs for DM > 8 years (all P < 0.05) (Table 3). By contrast, the UPCR, HbA1c, and BMI were associated with higher ORs for DR, whereas male patients, hemoglobin, and cholesterol were associated with lower ORs for DR (all P < 0.05) (Table 3).
Associations between clinical parameters favoring diabetic nephropathy and renal outcomes in patients with diabetic kidney disease
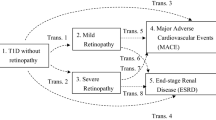
After a median follow-up period of 2.9 years, 24 (8.0%) and 184 (17.9%) patients with DM ≤ 8 years and DM > 8 years progressed to ESRD, respectively (Table 2). In the fully adjusted Cox proportional hazards model, DM > 8 years was associated with a trend of an increased risk for ESRD with a hazard ratio (HR) of 1.54 (95% confidence interval [CI]: 0.99–2.38, P = 0.055), compared with DM ≤ 8 years (Table 4) (Fig. 1). A total of 40 (8.0%) patients without and 168 (20.2%) patients with DR progressed to ESRD, respectively, and 24 (5.1%) patients without and 184 (21.4%) patients with the positive diagnostic model progressed to ESRD, respectively. Both DR and the positive diagnostic model were significantly associated with increased risks for ESRD with HRs of 1.69 (95% CI: 1.16–2.45, P = 0.006) and 1.66 (95% CI: 1.05–2.61, P = 0.029), respectively (Table 4) (Fig. 1). In the fully adjusted multivariate logistic regression, DR was significantly associated with an increased risk for rapid renal progression with an OR of 1.55 (95% CI: 1.5–2.09, P = 0.004) (Table 4).
Association between clinical parameters favoring diabetic nephropathy and clinical outcomes in patients with diabetic kidney disease
In the fully adjusted Cox proportional hazards model, DM > 8 years, DR, and the positive diagnostic model were not associated with all-cause mortality (Table 4) (Fig. 2). A total of 31 (10.3%), 61 (12.2%), and 44 (9.4%) CV events occurred in the patients without DM > 8 years, DR, and the positive diagnostic model, respectively, and 184 (17.9%), 154 (18.5%), and 171 (19.9%) CV events occurred in the patients with DM > 8 years, DR, and the positive diagnostic model, respectively (Table 2). In the fully adjusted Cox proportional hazards model, DR was associated with a significantly increased risk for CV events with an HR of 1.33 (95% CI: 1.01–1.74, P = 0.045) (Table 4) (Fig. 3).
Propensity score-matched analysis
Because these three parameters were associated with CKD stages and other factors, we performed propensity score matching according to all variables listed in supplement Table 2. There were 296 vs 296, 368 vs 368, 308 vs 308 patients in the analyses by DM > 8 years, DR and the diagnostic model, respectively. There was no differences in all these variables between the groups divided by these parameters. The results were similar in renal outcomes. Both DR and the positive diagnostic model were significantly associated with increased risks for ESRD with HRs of 1.80 (95% CI: 1.14–2.86, P = 0.012) and 1.89 (95% CI: 1.02–3.52, P = 0.045), respectively (supplementary Table 3). DR was significantly associated with an increased risk for rapid renal progression with an OR of 1.56 (95% CI: 1.07–2.26, P = 0.020). However, DR was not associated with an increased risk for CV events with an HR of 1.28 (95% CI: 0.85–1.92, P = 0.231).
Discussion
Our study investigated whether DM > 8 years, DR, and the diagnostic model favoring DN are associated with clinical outcomes in patients with diabetic kidney disease. The presence of these clinical parameters, with a prevalence ranging from 62% to 77%, was associated with a higher incidence of CV disease, lower eGFR, and higher UPCR. We further revealed that DR and the diagnostic model favoring DN were significantly associated with an increased risk for ESRD. Diabetes duration was not associated with clinical outcomes.
DN is characterized by the development of albuminuria with renal disease progression. The typical pathological findings include glomerular basement membrane thickening, mesangial expansion, and nodular and global glomerulosclerosis14. NDRD is rarely comorbid with type 1 diabetes mellitus, particularly in patients with a history of diabetes >10 years15; however, reports of the prevalence of NDRD in patients diagnosed with type 2 diabetes mellitus have varied widely among studies, ranging from 17% to more than 50% according to different biopsy indications16,17,18. The most common cause of NDRD is IgA nephropathy in Asian countries and FSGS in Western countries4, 19. Various clinical parameters have been proposed as predictors for DN10 according to different biopsy policies and geographic areas12. A recent meta-analysis found that the presence of DR, longer diabetes duration, higher HbA1c, higher blood pressure (BP), and presence of hematuria could differentiate DN from NDRD; age, urine protein excretion, and serum creatinine could not10. Because DN had less favorable clinical outcomes than did NDRD6,7,8, we tested whether non-biopsied patients with clinical parameters favoring DN had poorer renal outcomes.
Researchers disagree on whether diabetes duration is a reliable predictor for DN. A meta-analysis found that it was reliable10, and a large original study by D’Agati et al. demonstrated that diabetes duration was the strongest predictor for DN in multivariate analysis, and that DM > 8 years was the optimal cutoff for predicting DN4. However, Parving et al. and two large original studies did not find a difference in diabetes duration between DN and NDRD12, 20,21,22. Because diabetes may have developed long before the diagnosis, the known diabetes duration has not been found to accurately predict the presence of DN10, 23. Additionally, in our cohort, more than half of the patients had a junior high school education level and might receive a delayed diagnosis of diabetes. These factors suggest that diabetes duration is not an accurate predictor for DN. Our result further demonstrated that diabetes duration is not a reliable prognostic factor for renal outcomes in patients with DKD.
DR is probably the most accurate single predictor for DN, but it is not perfect9, 10. Retinal microvascular abnormalities are correlated with glomerular lesions in biopsied patients with type 1 diabetes and associated with renal dysfunction in the general population with or without diabetes24. As mentioned previously, a higher incidence of NDRD is observed in cases of type 2 diabetes. Two meta-analyses demonstrated that DR could differentiate DN from NDRD with a pooled sensitivity of 0.65 and a pooled specificity of 0.759, 10. Several original studies have also shown that DR is the most accurate predictor for DN in multivariate analysis7, 8, 15. Both proliferative and non-proliferative DR can be associated with DN25, with proliferative DR possibly being the more sensitive of the two9. However, DR has limitations as an indicator for DN. First, the literature derived from biopsy studies is biased by selection criteria that favor NDRD. Second, many patients with DR did not develop macroalbuminuria within 10 years of follow-up26. New biomarkers for differentiation between DN and NDRD deserve further study27. In addition to these limitations in differentiation, the prognostic value of DR for renal outcomes is controversial. DR has been associated with a faster decline in the eGFR among the general population and the elderly population with or without diabetes24, 28. Reports from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study in patients with early DKD and Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartin (RENAAL) study in patients with advanced DKD have found that DR is associated with renal composite endpoints (ESRD or the doubling of creatinine)29, 30. However, a recent report from the TREAT study did not find a significantly increased risk for ESRD in patients with advanced DKD and anemia13. Our result, demonstrated the association of DR with ESRD, is consistent with the report from the RENAAL study. The discrepancy between these studies of advanced DKD is probably attributable to a higher prevalence of DR and greater proteinuria in the RENAAL study and our study compared with the TREAT study. Future large studies such as DIACORE, a prospective cohort study of incident microvascular and macrovascular complications in 6000 patients with diabetes31, will be helpful for clarifying this relationship.
A single clinical parameter is insufficient to clearly differentiate DN from NDRD32. A differential diagnostic model composed of diabetes duration, blood pressure, HbA1c, hematuria, hemoglobin, and DR was proposed to predict DN11. The authors developed the model from a biopsy cohort with 178 patients and validated their results in another cohort of 55 patients with a sensitivity of 88.5%11. In our study, the prevalence of DN estimated according to DR was similar to that estimated according to this diagnostic model, and the prognostic value of DR for ESRD was similar to that of this diagnostic model (Table 4) (Fig. 1). Our study is the first to apply what we have found in the biopsied patients to non-biopsied patients with DKD. Larger studies are required to establish a more sophisticated diagnostic model for DN.
Hematuria is proposed as a predictive parameter for NDRD but may not be an effective exclusionary parameter for DN. A meta-analysis and several original studies have found that hematuria is a predictor for NDRD10. However, these studies exhibited heterogeneity, especially on the definition of hematuria. Our previous study has shown that hematuria according to ≥5–10 red blood cells per high power field (RBC/HPF), but not 2–5 RBC/HPF, was associated with NDRD33. This is supported by another report11. The prevalence of hematuria was approximately 15% in patients with DKD and ranged widely from 11% to 77% among biopsy studies16,17,18. The absence of hematuria alone is not a sufficient predictor for DN. Thus, we did not include hematuria as a predictive parameter in this study.
Our study findings have several limitations. First, we did not perform biopsies on the kidneys of randomly selected patients with DKD. The association of these clinical parameters with DN was evident only in biopsy studies. However, our purpose is to demonstrate that the evidence we learned from biopsy studies could be tested in non-biopsied patients, but not to substitute renal biopsy. Second, we proved the association between clinical parameters favoring DN and clinical outcomes, but not the direct association between DN and clinical outcomes. Extrapolating these results should be careful. Third, the sensitivity of clinical parameters and the diagnostic model for DN is high but nonetheless flawed. Some patients with DR might also have NDRD. Fourth, the severity of DR was not documented, and proliferative DR might be associated with a higher risk for ESRD. Fifth, diabetes duration was not an accurate predictor in our study. This is related to the low education level of our study population in whom signs of DM can’t be reported early. Sixth, there may be a selection bias involved in recruiting patients from the nephrology outpatient department. DKD patients form this recruitment might have higher percentage of NDRD.
Conclusion
In our study, we tested whether clinical parameters favoring DN could predict clinical outcomes in patients with DKD. DR and the diagnostic model favoring DN were found in two thirds of patients with diabetes and CKD stages 1–4 and were associated with a higher proportion of CV disease, lower eGFR, and higher UPCR. DR and the diagnostic model favoring DN were significantly associated with an increased risk for ESRD. Further biomarker studies on the precise prediction of DN and clinical outcomes are warranted27.
Methods
Participants and measurements
A prospective observation study, the Integrated CKD Care Program Kaohsiung for Delaying Dialysis, was conducted at two hospitals affiliated with Kaohsiung Medical University in southern Taiwan from November 11, 2002 to May 31, 2009 with follow-up until May 31, 201034. The study included patients who were not receiving renal replacement therapy and excluded patients with acute kidney injury defined as a greater than 50% decrease in the eGFR in 3 months. Among 3659 patients with CKD, we included those diagnosed with diabetes as defined by the World Health Organization35 who lacked significant ketonuria and had been receiving insulin treatment for at least 1 y after diagnosis. To observe renal outcomes, we excluded patients diagnosed with CKD stage 5; a final total of 1330 patients were eligible for this study. The study protocol was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUH-IRB-990198). Written informed consent was obtained from the patients and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki.
CKD was defined as abnormalities of kidney structure or function, present for more than 3 months, with implications for health36. DKD was defined as the presence of both diabetes and CKD37. Diabetes duration was defined as the period between the time of diagnosis by a physician and the time of enrollment. DN, as stated in the introduction, referred to the definition by renal pathology37. DR was defined as the presence of background, preproliferative, and proliferative changes determined through fundoscopy or a digital fundus photography examination. Diabetic neuropathy was defined as generalized peripheral neuropathy determined through a nerve conduction velocity test. Microscopic hematuria was defined according to a rate ≥5–10 RBC/HPF. An automated cation-exchange high-performance liquid chromatography method was used to measure HbA1c. A six-parameter diagnostic model for DN including diabetes duration, blood pressure, HbA1c, hematuria, anemia and diabetes retinopathy was used. It was established and validated by Chen et al. as P DN = exp (0.846 + 0.022 DM + 0.033 BP + 2.050 HbA1c − 2.664 Hu − 0.078 Hb + 2.942 DR)/(1 + exp [0.846 + 0.022 DM + 0.033 BP + 2.050 HbA1c − 2.664 Hu − 0.078 Hb + 2.942 DR])11. P DN was the probability of DN diagnosis (P DN ≥ 0.5 as DN). The units of the parameters are listed as follows: diabetes duration (mo); BP (mmHg); HbA1c (1 ≥ 7%, 0 < 7%); hematuria (1 = yes, 0 = no); Hb (g/L); and DR (1 = yes, 0 = no). Three parameters (DM > 8 years4, DR9, 10, and the diagnostic model11) were chosen as predictors for DN in this study. The absence of hematuria was not selected as a predictor for DN because our previous study had already addressed this question.
The baseline comorbidities of the patients, clinical data, and biochemical parameters were studied. The demographic features were recorded at the first visit and the medical history was recorded in a chart review. Hypertension was defined as systolic BP > 140 mmHg, diastolic BP > 90 mmHg, or the use of antihypertensive medication. Normotensive patients who took ACEI/ARB were not classified as hypertension. CV diseases were defined as a clinical diagnosis of heart failure, acute or chronic ischemic heart disease, or cerebrovascular disease. Laboratory data were also obtained at the baseline visit.
Outcomes
Four outcomes were assessed: ESRD, rapid renal progression, all-cause mortality, and CV events. ESRD was defined as the initiation of hemodialysis, peritoneal dialysis, or renal transplantation. The initiation of ESRD was ascertained by reviewing patient charts and the catastrophic illness cards issued by Taiwan National Health Insurance. Kidney function was examined through the simplified Modification of Diet in Renal Disease (MDRD) study equation: eGFR mL/min/1.73 m2 = (186) × (serum creatinine−1.154) × (age−0.203) × 0.742 (if female) × 1.212 (if black). Rapid renal disease progression was defined as an eGFR slope <−5 mL/min/1.73 m2/y according to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline. Survival status and cause of death were determined on the basis of death certificates, patient charts, and the National Death Index. CV events were ascertained by reviewing charts and defined according to hospitalization for acute coronary syndrome (Deyo’s modified Charlson score, ICD-9-CM: 410.x–412.x), acute cerebrovascular disease (430.x–438.x), congestive heart failure (428.x), or peripheral arterial occlusion disease (443.9, 441.x, 785.4, V43.4, procedure 38.48), or death by any of the aforementioned causes.
Statistical analysis
The summarized statistical results of the baseline characteristics of the patients were expressed as counts and percentages for the categorical data. The means with standard deviations and medians with interquartile ranges were determined for continuous variables with approximately normal distributions. Competing risk Cox proportional hazards analysis was used to assess the relationship between parameters and clinical outcomes. Multivariate logistic regression analysis was used to evaluate the relationships between parameters and rapid renal progression. The model was adjusted for age, sex, the eGFR, the log-transformed UPCR, hypertension, CV disease, MBP, HbA1c, hemoglobin, albumin, BMI, log-transformed cholesterol, log-transformed CRP, and phosphorus, according to the literature and our previous publications33, 34.
We estimated the propensity scores for DM > 8 years, DR or positive diagnostic model using a non-parsimonious multivariable logistic regression model including all parameters shown in supplement table. The model was well-calibrated (Hosmer–Lemeshow test: P = 0.155) with reasonable discrimination (c statistic = 0.69). We matched patients between negative and positive groups with similar propensity scores to five, four, three, two and one decimal places in five repeated steps. In the first step, we multiplied the raw propensity scores by 100 000, then rounded it to the nearest value. This was repeated, multiplying by 10000, 1000, 100, and 10. A result of P < 0.05 was considered statistically significant. Statistical analysis was performed using the R 3.3.0 software (R Foundation for Statistical Computing, Vienna, Austria) and the Statistical Package for Social Sciences version 21.0 for Windows (SPSS Inc., Chicago, IL).
References
Danaei, G. et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378, 31–40, doi:10.1016/S0140-6736(11)60679-X (2011).
Kdoqi. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. American journal of kidney diseases: the official journal of the National Kidney Foundation 49, S12–154, doi:10.1053/j.ajkd.2006.12.005 (2007).
Collins, A. J. et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. American journal of kidney diseases: the official journal of the National Kidney Foundation 59(A7), e1–420, doi:10.1053/j.ajkd.2011.11.015 (2012).
Sharma, S. G. et al. The modern spectrum of renal biopsy findings in patients with diabetes. Clinical journal of the American Society of Nephrology: CJASN 8, 1718–1724, doi:10.2215/CJN.02510213 (2013).
Teng, J. et al. Spectrum of renal disease in diabetes. Nephrology 19, 528–536, doi:10.1111/nep.12288 (2014).
Wong, T. Y. et al. Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes care 25, 900–905, doi:10.2337/diacare.25.5.900 (2002).
Chang, T. I. et al. Renal outcomes in patients with type 2 diabetes with or without coexisting non-diabetic renal disease. Diabetes research and clinical practice 92, 198–204, doi:10.1016/j.diabres.2011.01.017 (2011).
Byun, J. M. et al. Renal outcomes and clinical course of nondiabetic renal diseases in patients with type 2 diabetes. The Korean journal of internal medicine 28, 565–572, doi:10.3904/kjim.2013.28.5.565 (2013).
He, F., Xia, X., Wu, X. F., Yu, X. Q. & Huang, F. X. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia 56, 457–466, doi:10.1007/s00125-012-2796-6 (2013).
Liang, S. et al. Identifying parameters to distinguish non-diabetic renal diseases from diabetic nephropathy in patients with type 2 diabetes mellitus: a meta-analysis. PloS one 8, e64184, doi:10.1371/journal.pone.0064184 (2013).
Liu, M. Y. et al. Validation of a differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases and the establishment of a new diagnostic model. Journal of diabetes 6, 519–526, doi:10.1111/1753-0407.12150 (2014).
Mazzucco, G. et al. Different patterns of renal damage in type 2 diabetes mellitus: a multicentric study on 393 biopsies. American journal of kidney diseases: the official journal of the National Kidney Foundation 39, 713–720, doi:10.1053/ajkd.2002.31988 (2002).
Bello, N. A. et al. Retinopathy and clinical outcomes in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia. BMJ open diabetes research & care 2, e000011, doi:10.1136/bmjdrc-2013-000011 (2014).
Tervaert, T. W. et al. Pathologic classification of diabetic nephropathy. Journal of the American Society of Nephrology: JASN 21, 556–563, doi:10.1681/ASN.2010010010 (2010).
Wirta, O. et al. Renal findings and glomerular pathology in diabetic subjects. Nephron 84, 236–242, doi:45583 (2000).
Akimoto, T. et al. Microscopic hematuria and diabetic glomerulosclerosis–clinicopathological analysis of type 2 diabetic patients associated with overt proteinuria. Nephron. Clinical practice 109, c119–126, doi:10.1159/000145454 (2008).
Haider, D. G. et al. Kidney biopsy results versus clinical parameters on mortality and ESRD progression in 2687 patients with glomerulonephritis. European journal of clinical investigation 44, 578–586, doi:10.1111/eci.12274 (2014).
Pham, T. T., Sim, J. J., Kujubu, D. A., Liu, I. L. & Kumar, V. A. Prevalence of nondiabetic renal disease in diabetic patients. American journal of nephrology 27, 322–328, doi:10.1159/000102598 (2007).
Haider, D. G. et al. Kidney biopsy in patients with diabetes mellitus. Clinical nephrology 76, 180–185, doi:10.5414/CN106955 (2011).
Bi, H. et al. Nondiabetic renal disease in type 2 diabetic patients: a review of our experience in 220 cases. Renal failure 33, 26–30, doi:10.3109/0886022X.2010.536292 (2011).
Christensen, P. K., Larsen, S., Horn, T., Olsen, S. & Parving, H. H. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int 58, 1719–1731, doi:10.1046/j.1523-1755.2000.00333.x (2000).
Parving, H. H. et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int 41, 758–762, doi:10.1038/ki.1992.118 (1992).
Tone, A. et al. Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes research and clinical practice 69, 237–242, doi:10.1016/j.diabres.2005.02.009 (2005).
Wong, T. Y. et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. Journal of the American Society of Nephrology: JASN 15, 2469–2476, doi:10.1097/01.ASN.0000136133.28194.E4 (2004).
Oh, S. W. et al. Clinical implications of pathologic diagnosis and classification for diabetic nephropathy. Diabetes research and clinical practice 97, 418–424, doi:10.1016/j.diabres.2012.03.016 (2012).
Lovestam-Adrian, M., Agardh, E. & Agardh, C. D. The incidence of nephropathy in type 1 diabetic patients with proliferative retinopathy: a 10-year follow-up study. Diabetes research and clinical practice 39, 11–17, doi:10.1016/S0168-8227(97)00106-X (1998).
Ryong Cha, D. Where do we stand on human diabetic nephropathy? Kidney research and clinical practice 32, 93–95, doi:10.1016/j.krcp.2013.07.007 (2013).
Edwards, M. S. et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. American journal of kidney diseases: the official journal of the National Kidney Foundation 46, 214–224, doi:10.1053/j.ajkd.2005.05.005 (2005).
Mottl, A. K. et al. The degree of retinopathy is equally predictive for renal and macrovascular outcomes in the ACCORD Trial. Journal of diabetes and its complications 28, 874–879, doi:10.1016/j.jdiacomp.2014.07.001 (2014).
Parving, H. H., Mogensen, C. E., Thomas, M. C., Brenner, B. M. & Cooper, M. E. Poor prognosis in proteinuric type 2 diabetic patients with retinopathy: insights from the RENAAL study. QJM: monthly journal of the Association of Physicians 98, 119–126, doi:10.1093/qjmed/hci017 (2005).
Dorhofer, L. et al. Study design of DIACORE (DIAbetes COhoRtE) - a cohort study of patients with diabetes mellitus type 2. BMC medical genetics 14, 25, doi:10.1186/1471-2350-14-25 (2013).
Gonzalez Suarez, M. L., Thomas, D. B., Barisoni, L. & Fornoni, A. Diabetic nephropathy: Is it time yet for routine kidney biopsy? World journal of diabetes 4, 245–255, doi:10.4239/wjd.v4.i6.245 (2013).
You-Hsien Lin, H. et al. Microscopic Haematuria and Clinical Outcomes in Patients With Stage 3–5 Nondiabetic Chronic Kidney Disease. Scientific reports 5, 15242, doi:10.1038/srep15242 (2015).
Liu, W. C. et al. Association of hyperuricemia with renal outcomes, cardiovascular disease, and mortality. Clinical journal of the American Society of Nephrology: CJASN 7, 541–548, doi:10.2215/cjn.09420911 (2012).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 15(7), 539–53 (1998).
Summary of Recommendation Statements. Summary of Recommendation Statements, Kidney international supplements 3, 5–14, 10.1038/kisup.2012.77 (2013).
Gross, J. L. et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes care 28, 164–176, doi:10.2337/diacare.28.1.164 (2005).
Author information
Authors and Affiliations
Contributions
Research concept and study design: C.C.H., H.Y.H.L., D.Y.H. and L.M.L. Data acquisition: H.Y.H.L., I.C.K., Y.W.C., L.M.L. and C.C.H. Data analysis/interpretation; L.M.L., D.Y.H., H.Y.H.L. and I.C.K.; Statistical analysis: C.C.H. Supervision and mentorship: S.J.H. and H.C.C. Each author contributed crucial intellectual content during manuscript drafting and revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors reviewed the manuscript. Lee-Moay Lim is responsible for the honesty, accuracy, and transparency of the report; ensuring that no important aspects of the study were omitted; and confirming that any discrepancies from the study as planned (and, if relevant, registered) are explained.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hung, CC., Lin, H.YH., Hwang, DY. et al. Diabetic Retinopathy and Clinical Parameters Favoring the Presence of Diabetic Nephropathy could Predict Renal Outcome in Patients with Diabetic Kidney Disease. Sci Rep 7, 1236 (2017). https://doi.org/10.1038/s41598-017-01204-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01204-6
This article is cited by
-
Clinical predictors for nondiabetic kidney diseases in patients with type 2 diabetes mellitus: a retrospective study from 2017 to 2021
BMC Endocrine Disorders (2022)
-
Association of retinopathy severity with cardiovascular and renal outcomes in patients with type 1 diabetes: a multi-state modeling analysis
Scientific Reports (2022)
-
Microvascular Complications and Their Associated Risk Factors Among Rural Type 2 Diabetic Population: A Cross-Sectional Study
SN Comprehensive Clinical Medicine (2021)
-
Pyuria, urinary tract infection and renal outcome in patients with chronic kidney disease stage 3–5
Scientific Reports (2020)
-
Impact of Liver Cirrhosis on Incidence of Dialysis Among Patients with Type 2 Diabetes
Diabetes Therapy (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.