Abstract
Detecting circulating tumour cells (CTCs) is considered as effective and minimally invasive technique to predict the prognosis of patients with metastatic colorectal cancer (CRC), but its clinical validity is still conflicting in patients without metastasis. We performed this meta-analysis to evaluate whether detection of CTCs in the peripheral blood can be used as a prognostic marker for patients with non-metastatic CRC. We performed a comprehensive search of the EMBASE, PubMed, and Web of Science databases (up to September 2016). Meta-analyses were conducted using a random-effects model with the hazard ratio (HR) and 95% confidence interval (95% CI) as the effect measures. Twenty studies including 3,687 patients were eligible for inclusion. Overall analyses demonstrated that the presence of CTCs was significantly associated with aggressive disease progression (HR = 2.57, 95% CI = 1.64–4.02, P heterogeneity < 0.001, I 2 = 81.0%) and reduced disease survival (HR = 2.41, 95% CI = 1.66–3.51, P heterogeneity = 0.002, I 2 = 59.7%). Subgroup analyses further supported the prognostic effect of CTCs based on different subsets, including sampling time, detection method and cancer type. Our findings suggest that detection of CTCs in the peripheral blood has the clinical utility to indicate poor prognosis in patients with non-metastatic CRC.
Similar content being viewed by others
Introduction
Despite tremendous efforts, colorectal cancer (CRC) remains the third most common cancer, with 1.36 million new cases and 694,000 deaths per year worldwide1. Metastasis and recurrence are the primary reasons for the CRC-related deaths. Clinically, approximately 25–50% of patients with early stage CRC develop cancer relapse after radical surgery and adjuvant treatment2. In addition, many patients with non-metastatic CRC (especially patients with stage III disease) undergo unnecessary treatment or overtreatment. Therefore, identifying an effective monitoring method to predict prognosis is quite important for CRC management in order to prevent metastasis and recurrence, as well as overtreatment.
According to the “seed and soil theory”, circulating tumour cells (CTCs) released into the peripheral blood from the primary tumour are crucial for the formation of metastases and recurrence3. In recent years, accumulating evidence has demonstrated the prognostic role of CTCs in the peripheral blood in several human cancers, such as gastric cancer4, head and neck squamous cell carcinoma5, prostate cancer6, and breast cancer7, as well as its subtypes8. However, these studies primarily focused on metastatic cancers. Although the prognostic effect of CTCs on CRC has been reported previously9, 10, whether CTCs could predict poor outcome in patients with non-metastatic CRC is still inconclusive. Some studies reported that patients with CTCs had a worse prognosis than patients without CTCs11,12,13,14,15,16,17,18,19, while other studies did not support the conclusion20,21,22,23,24,25,26,27. Two main detection methods were applied in these studies, namely, reverse transcriptase polymerase chain reaction (RT-PCR) and immunological methods (e.g. CellSearch, Epispot, or CMx platform). For immunological methods, different cut-off values were also used (as shown in Table 1). Moreover, sampling times seem to influence the prognostic effect of CTCs. van Dalum et al. surprisingly found that CTCs detected in the weeks after surgery were not significantly associated with CRC progression whereas CTCs detected 2–3 years after surgery were significantly associated with CRC progression28. In summary, these discrepancies may result from the small sample size of individual studies, different time points of blood collection, or the use of various detection methods.
Therefore, we performed this meta-analysis to comprehensively and quantitatively evaluate the prognostic significance of CTCs detected in the peripheral blood of patients with non-metastatic CRC. Moreover, we investigated the potential role of CTCs in different subgroups based on patient number, sampling time, detection method, detection rate, disease stage, or cancer type. The outcomes of interest were disease progression (including disease-free survival [DFS] and recurrence-free survival [RFS]) and disease survival (including overall survival [OS], cancer-related survival [CRS] and colon cancer related death [CCRD]).
Results
Characteristics of the included studies
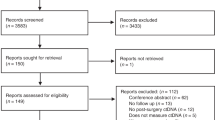
As shown in Fig. 1, a total of 2,301 records were initially identified from the EMBASE, PubMed, and Web of Science databases. By screening the title and abstract, we excluded 712 duplicates and 1,546 unrelated records, and then retrieved 43 relevant full-text articles. Twenty-three studies were further removed because of failure to distinguish data of early stage (M0) and metastatic stage (M1) CRC (n = 16), insufficient information to estimate the insufficient information to estimate the hazard ratio (HR) and 95% confidence interval (95% CI) (n = 4), failure to report CTC data of peripheral blood samples (n = 2), or having populations overlapping with another study (n = 1). Finally, 20 eligible studies were included in this meta-analysis11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30.
The 20 studies including 3,687 patients with non- metastatic CRC were performed in Asia, Europe, North America and Oceania. Eight studies evaluated the effect of CTCs at a “preoperative” time point11, 14, 16, 18,19,20, 24, 25, eight studies evaluated at a “postoperative” time point12, 13, 17, 21, 22, 26, 29, 30, two studies evaluated at “preoperative” and “postoperative” time points27, 28, one study evaluated at “preoperative and postoperative” (persistent CTC-positive before and after surgery)15, and one study evaluated at a “during surgery” time point23. CTCs in these studies were detected by two types of detection methods, RT-PCR and immunological methods (e.g. CellSearch, Epispot, or CMx platform). The detection rates ranged from 8.8% to 74%. Table 1 summarises the characteristics of the 20 included studies. Eighty percent (16/20) of these studies were high-quality (quality score ≥6) according to the Newcastle-Ottawa scale (Supplementary Table S1).
Overall analyses
HRs for disease progression (DFS and RFS) were provided by 16 studies12, 13, 15,16,17,18,19,20,21,22,23,24, 26,27,28,29 including 3,263 patients with non-metastatic CRC. In three studies, more than one HR was collected from each trial by using different disease stages20, research populations (e.g. training set and validation set)16, or sampling time points27. The overall analysis demonstrated that patients who were CTC-positive with non-metastatic CRC had a significant higher risk of disease progression (HR = 2.57, 95% CI = 1.64–4.02, P heterogeneity < 0.001, I 2 = 81.0%; Fig. 2A).
HRs for disease survival (OS, CRS, CCRD) were provided by 12 studies11, 12, 14, 16,17,18, 22, 25,26,27,28,29 including 2,616 patients with non-metastatic CRC. More than one HR was collected in three studies16, 27, 28 because of the same reasons as mentioned above. The overall analysis showed that, compared with patients who were CTC-negative with non-metastatic CRC, patients who were CTC-positive had a two-fold increased risk of worse survival (HR = 2.41, 95% CI = 1.66–3.51, P heterogeneity = 0.002, I 2 = 59.7%; Fig. 2B).
Meta-regression and subgroup analyses
Because of the significant heterogeneity among studies, we conducted a meta-regression analysis to investigate potential sources (Table 2). Results showed that patient number (P = 0.023) and detection rate (P = 0.022) were significant factors affecting heterogeneity for disease progression. Meta-regression analysis also indicated that sampling time (P = 0.001) and detection rate (P = 0.011) were responsible for heterogeneity for disease survival.
Moreover, we performed subgroup analyses to further assess the prognostic value of CTCs in different subsets (Table 3). In the subgroup analysis based on patient number (≥100 or not), a significant prognostic effect of CTC detection was only identified in the analysis of studies with ≥100 cases (Disease progression: HR = 4.40, 95% CI = 2.32–8.35, P heterogeneity < 0.001, I 2 = 88.6%; Disease survival: HR = 2.40, 95% CI = 1.59–3.62, P heterogeneity = 0.004, I 2 = 66.2%). Subgroup analyses based on sampling time confirmed that whether performed before surgery or after, detection of CTCs in the peripheral blood could predict worse disease progression (Preoperative: HR = 2.57, 95% CI = 1.57–4.21, P heterogeneity = 0.003, I 2 = 65.5%; Postoperative: HR = 2.41, 95% CI = 1.24–4.69, P heterogeneity < 0.001, I 2 = 81.1%). A prognostic role of CTCs in disease survival was also shown in the analysis of studies collecting blood samples at the preoperative point (HR = 3.71, 95% CI = 2.78–4.96, P heterogeneity = 0.903, I 2 = 0.0%), as well as at the postoperative point (HR = 1.47, 95% CI = 0.89–2.42, P heterogeneity = 0.051, I 2 = 52.0%), although the latter did not reach statistical significance. Subgroup analyses based on detection rate showed that a CTC-positive detection rate ≥35% tended to indicate an unfavourable prognosis (Disease progression: HR = 1.41, 95% CI = 0.75–2.66, P heterogeneity < 0.001, I 2 = 71.4%; Disease survival: HR = 1.28, 95% CI = 0.65–2.51, P heterogeneity = 0.083, I 2 = 48.6%), although statistical significance was not reached. Similar results were also observed in the subgroup analysis of TNM stage III disease (Disease progression: HR = 1.55, 95% CI = 0.55–4.39, P heterogeneity = 0.004, I 2 = 77.6%; Disease survival: HR = 2.06, 95% CI = 0.38–10.99, P heterogeneity = 0.014, I 2 = 83.4%). In other subgroup analyses, detection of CTCs showed a prognostic value for both disease progression and survival for non-metastatic CRC, under various conditions including different detection methods (RT-PCR or immunological methods), different disease stages (Dukes’ B or Dukes’ C), different cancer types (colon cancer or rectal cancer), or after multivariate adjustment (shown in Table 3).
Sensitivity analyses and publication bias
Sensitivity analyses indicated that our pooled results were quite stable for both disease progression (Supplementary Figure S1) and disease survival (Supplementary Figure S2). Moreover, the funnel plots and the Begg’s and Egger’s tests showed no evidence of publication bias on the pooled analysis of disease progression (Fig. 3A; P Egger’s test = 0.413, P Begg’s test = 0.624) and disease survival (Fig. 3B; P Egger’s test = 0.830, P Begg’s test = 1.000).
Discussion
The prognostic significance of CTCs has been confirmed in several metastatic cancers, but rarely evaluated in early-stage cancers. This meta-analysis comprehensively summarised the relevant studies and provided strong evidence that the presence of CTCs in the peripheral blood could predict a poor disease progression and survival in patients with non-metastatic CRC.
In 2010, Rahbari et al. performed an excellent meta-analysis that demonstrated the unfavourable prognostic role of CTCs in patients with primary CRC9. However, in their subgroup analysis to evaluate CTCs detected in peripheral/central blood from patients with CRC stage I-III, only one study was included for evaluation of RFS (I 2 = 78%) and five studies were included for evaluation of OS (I 2 = 69%). It is difficult to obtain a convincing conclusion based on the limited study number and high heterogeneity. In 2015, Huang et al. conducted an updated meta-analysis to evaluate the prognostic utility of CTCs detected in the peripheral blood by the CellSearch System10. However, their work also did not distinguish stage M0 from M1. Our study addressed these issues and further confirmed the potential clinical utility of CTC detection in patients with localised cancers. Furthermore, we performed subgroup analyses to thoroughly assess the prognostic effect of CTCs based on patient number, sampling time, detection method, detection rate, disease stage and cancer type.
When the studies were divided into two groups based on patient number, the pooled results of the group with lower patient numbers (n < 100) failed to reach statistical significance in terms of both disease progression and survival (Table 3). Patient number was also identified as a potential source of the heterogeneity by meta-regression analyses. This might explain, at least partially, why non-significant results in this research field were often observed in these “small” studies. Preoperative CTCs and postoperative CTCs in the peripheral blood usually indicate different clinical events, respectively. The presence of CTCs before surgery reflect the invasion of the primary tumour into the blood and could determine those subpopulations at high risk for recurrence; the presence of CTCs after surgery could be considered as an early indicator of the undetectable metastasis19, 28. Our results showed a significant association between preoperative CTCs and poor disease progression and survival, suggesting that detecting CTCs before surgery is a promising method to distinguish patients with high-risk CRC at early stages. A significant association was also identified in the analysis of disease progression in the postoperative subgroup, suggesting the prognostic value of postoperative CTCs in patients with non-metastatic CRC. However, in the analysis of disease survival, postoperative CTCs did not significantly indicate a poor survival. A possible explanation was that there were other factors influencing the survival of patients (e.g. death due to accidents or other diseases), and our sample size was not large enough to identify the significance. We also performed subgroup analysis based on detection rate, which was the median of the detection rate of each study included in the overall analysis. We noticed inconsistent results, in that the subset with a detection rate <35% showed a significant association while the subset with detection rate ≥35% only showed a similar trend but failed to reach statistical significance. When we performed subgroup analyses based on disease stage, we observed consistently significant associations without heterogeneity at most disease stages, except for TNM stage III. This included a limited number of studies (n < 5); significant heterogeneity (I 2 = 77.6% for disease progression and I 2 = 83.4% for disease survival) might be responsible for the conflicting results of TNM stage III disease. In addition, other subgroup analyses suggested that CTCs had a significantly prognostic effect in patients with non-metastatic CRC regardless of detection method, cancer type, or multivariate adjustment.
Several limitations should be addressed as follows. Firstly, because of the limited number of studies focusing on separate disease stages, we could not fully evaluate the prognostic value of CTCs in patients with CRC at each clinical stage, especially TNM I or Dukes’ stage A. Secondly, some of the included studies did not provide multivariate adjusted HRs; in this case, we recorded unadjusted HRs or extrapolated them by reported data instead. Thus, our pooled results carry a risk of bias due to potential confounders in the original studies. However, subgroup analysis based on multivariate adjustment showed a significant result for studies with adjusted HRs and a similar trend of results for studies with unadjusted HRs. This suggests that suggested that potential confounders in the original studies might not affect the conclusions of our meta-analysis. Thirdly, significant heterogeneity among studies was observed in the overall analyses. Although meta-regression analyses identified patient number, sampling time, and detection rate as significant heterogeneous factors, subgroup analyses based on these factors also showed obvious heterogeneity, which suggests that there were other potentially influencing factors. Finally, the influence of adjuvant therapies on the prognostic effect of CTCs was not evaluated in our work since few included studies provided such data. In spite of these limitations, our work is the first meta-analysis to assess the prognostic utility of CTCs detected in the peripheral blood for patients with non-metastatic CRC.
In conclusion, our meta-analysis strongly suggests that the detection of CTCs in peripheral blood is a clinically promising predictor of worse disease progression and survival for patients with non-metastatic CRC. More high-quality cohort studies with refined designs are still required to further validate our results.
Methods
Literature search and eligibility criteria
Our meta-analysis was conducted according to the statement of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)31. We performed a comprehensive electronic search in multiple databases including EMBASE, PubMed and Web of Science through September 2016, without any restriction. The search items were combinations of “circulating tumour cells”, “micrometastasis”, “disseminated tumour cells”, “isolated tumour cells”, “occult tumour cells”, “colorectal”, “colon”, “rectal”, “cancer”, “tumour”, “neoplasm”, “malignancy”, “carcinoma”, “prognosis”, “survival” and “recurrence”. We also carefully reviewed the reference lists of the identified articles to retrieve potentially relevant studies. Only articles in English published on peer-reviewed journals were included.
Eligible studies were included if they met the following criteria: (1) cohort studies evaluating the prognostic significance of CTCs detected in patients with non-metastatic CRC; (2) studies reporting HRs and 95% CIs, or providing sufficient data to extrapolate these outcome measures; (3) samples collected from peripheral blood. The exclusion criteria were as follows: (1) studies not distinguishing stage M0 and M1; (2) outcome measures not reporting or impossible to be calculated from originally published data; (3) studies with overlapping data or patients. If a study had overlapping data with other studies, we kept the study with larger sample size. Two independent authors performed the literature search and study selection. Discrepancies were resolved by consensus or consultation of a third party.
Data extraction and quality evaluation
The following items were independently extracted by two authors from each eligible study: first author, publication year, country, patient number, sampling time, the median follow-up, disease stage, cancer type, detection method, detection rate, outcomes, HRs and 95% CIs, and so on. If more than one peripheral blood sample per patient was collected at different points in time, each sampling time point was recorded, and all these results were considered as independent data sets. We used the Newcastle-Ottawa (NOS) scale (Supplemental Table S1)32 to evaluate the quality of each included study. The NOS score ranges from “0” to “9” and a score ≥6 indicates high quality. Discrepancies were resolved through consensus.
Statistical analysis
Multivariate adjusted HR and 95% CI was preferentially chosen from each eligible study, if available. For those studies not reporting HR and 95% CI, we extrapolated the values using the methods of Parmar33 and Tierney34. A random-effects model was performed to pool these HRs and 95% CIs. Heterogeneity was examined by Cochran’s Q test and further quantified by the I 2 index. P < 0.10 or I 2 > 50% suggest significant heterogeneity among the included studies35. We also conducted meta-regression analysis to explore the possible sources of heterogeneity. To further investigate the effect of CTCs on the prognosis of non-metastatic CRC, we carried out subgroup analyses based on potential modifiers including patient number, sampling time, detection method, detection rate, TNM stage, Dukes’ stage, and cancer type. In addition, we assessed the stability of the pooled results by one-way sensitivity analysis and examined the publication bias by Egger’s36 and Begg’s37 tests. All statistical tests were conducted using Stata 12.1 software (College Station, TX, USA). A two-sided P ≤ 0.05 was considered as significant, unless otherwise specified.
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer 136, E359–386, doi:10.1002/ijc.29210 (2015).
Torino, F. et al. Circulating tumor cells in colorectal cancer patients. Cancer treatment reviews 39, 759–772, doi:10.1016/j.ctrv.2012.12.007 (2013).
Fidler, I. J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature reviews. Cancer 3, 453–458, doi:10.1038/nrc1098 (2003).
Huang, X. et al. Clinicopathological and prognostic significance of circulating tumor cells in patients with gastric cancer: a meta-analysis. International journal of cancer 136, 21–33, doi:10.1002/ijc.28954 (2015).
Wu, X. L. et al. Diagnostic and Prognostic Value of Circulating Tumor Cells in Head and Neck Squamous Cell Carcinoma: a systematic review and meta-analysis. Scientific reports 6, 10.1038/srep20210 (2016).
Wang, F. B. et al. A higher number of circulating tumor cells (CTC) in peripheral blood indicates poor prognosis in prostate cancer patients–a meta-analysis. Asian Pacific journal of cancer prevention: APJCP 12, 2629–2635 (2011).
Zhang, L. et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 18, 5701–5710, doi:10.1158/1078-0432.CCR-12-1587 (2012).
Lu, Y. J. et al. The significant prognostic value of circulating tumor cells in triple-negative breast cancer: a meta-analysis. Oncotarget 7, 37361–37369, doi:10.18632/oncotarget.8156 (2016).
Rahbari, N. N. et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology 138, 1714–1726, doi:10.1053/j.gastro.2010.01.008 (2010).
Huang, X. et al. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC cancer 15, 202, doi:10.1186/s12885-015-1218-9 (2015).
Hardingham, J. E. et al. Molecular detection of blood-borne epithelial cells in colorectal cancer patients and in patients with benign bowel disease. International journal of cancer 89, 8–13, doi:10.1002/(ISSN)1097-0215 (2000).
Koch, M. et al. Prognostic impact of hematogenous tumor cell dissemination in patients with stage II colorectal cancer. International journal of cancer 118, 3072–3077, doi:10.1002/ijc.21784 (2006).
Allen-Mersh, T. G. et al. Role of circulating tumour cells in predicting recurrence after excision of primary colorectal carcinoma. The British journal of surgery 94, 96–105, doi:10.1002/bjs.5526 (2007).
Koyanagi, K. et al. Prognostic relevance of occult nodal micrometastases and circulating tumor cells in colorectal cancer in a prospective multicenter trial. Clinical cancer research: an official journal of the American Association for Cancer Research 14, 7391–7396, doi:10.1158/1078-0432.CCR-08-0290 (2008).
Uen, Y. H. et al. Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I–III colorectal cancer after curative resection. Annals of surgical oncology 15, 2120–2128, doi:10.1245/s10434-008-9961-7 (2008).
Iinuma, H. et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 29, 1547–1555, doi:10.1200/JCO.2010.30.5151 (2011).
Lu, C. Y. et al. Molecular detection of persistent postoperative circulating tumour cells in stages II and III colon cancer patients via multiple blood sampling: prognostic significance of detection for early relapse. British journal of cancer 104, 1178–1184, doi:10.1038/bjc.2011.40 (2011).
Bork, U. et al. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. British journal of cancer 112, 1306–1313, doi:10.1038/bjc.2015.88 (2015).
Tsai, W. S. et al. Circulating Tumor Cell Count Correlates with Colorectal Neoplasm Progression and Is a Prognostic Marker for Distant Metastasis in Non-Metastatic Patients. Scientific reports 6, 24517, doi:10.1038/srep24517 (2016).
Bessa, X. et al. Lack of prognostic influence of circulating tumor cells in peripheral blood of patients with colorectal cancer. Gastroenterology 120, 1084–1092, doi:10.1053/gast.2001.23245 (2001).
Ito, S. et al. Quantitative detection of CEA expressing free tumor cells in the peripheral blood of colorectal cancer patients during surgery with real-time RT-PCR on a LightCycler. Cancer letters 183, 195–203, doi:10.1016/S0304-3835(02)00157-X (2002).
Bessa, X. et al. Prognostic value of postoperative detection of blood circulating tumor cells in patients with colorectal cancer operated on for cure. Annals of surgery 237, 368–375, doi:10.1097/01.sla.0000055223.27623.f3 (2003).
Sadahiro, S. et al. Detection of carcinoembryonic antigen messenger RNA-expressing cells in portal and peripheral blood during surgery does not influence relapse in colorectal cancer. Annals of surgical oncology 12, 988–994, doi:10.1245/ASO.2005.03.565 (2005).
Douard, R. et al. Long-term prognostic value of detection of circulating colorectal cancer cells using CGM2 reverse transcriptase-polymerase chain reaction assay. Surgery 139, 556–562, doi:10.1016/j.surg.2005.09.025 (2006).
Deneve, E. et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clinical chemistry 59, 1384–1392, doi:10.1373/clinchem.2013.202846 (2013).
Sotelo, M. J. et al. Role of circulating tumor cells as prognostic marker in resected stage III colorectal cancer. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 26, 535–541, doi:10.1093/annonc/mdu568 (2015).
Kust, D. et al. Cytokeratin 20 positive cells in blood of colorectal cancer patients as an unfavorable prognostic marker. Acta Clinica Belgica 71, 235–243, doi:10.1080/17843286.2016.1177264 (2016).
van Dalum, G. et al. Importance of circulating tumor cells in newly diagnosed colorectal cancer. International journal of oncology 46, 1361–1368, doi:10.3892/ijo.2015.2824 (2015).
Sadahiro, S. et al. Detection of Carcinoembryonic Antigen Messenger RNA-Expressing Cells in Peripheral Blood 7 Days After Curative Surgery is a Novel Prognostic Factor in Colorectal Cancer. Annals of surgical oncology 14, 1092–1098, doi:10.1245/s10434-006-9289-0 (2007).
Lu, C. Y. et al. Circulating tumor cells as a surrogate marker for determining clinical outcome to mFOLFOX chemotherapy in patients with stage III colon cancer. British journal of cancer 108, 791–797, doi:10.1038/bjc.2012.595 (2013).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open medicine: a peer-reviewed, independent, open-access journal 3, e123–130 (2009).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology 25, 603–605, doi:10.1007/s10654-010-9491-z (2010).
Parmar, M. K., Torri, V. & Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in medicine 17, 2815–2834, doi:10.1002/(ISSN)1097-0258 (1998).
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S. & Sydes, M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16, doi:10.1186/1745-6215-8-16 (2007).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 21, 1539–1558, doi:10.1002/sim.1186 (2002).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 315, 629–634 (1997).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101, doi:10.2307/2533446 (1994).
Acknowledgements
This study was supported by the grants to Dr. Shen (81601839) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Contributions
N.S. conceived and designed this study. Y.L. and P.W. performed literature search, study selection and data extraction. J.P. and X.W. conducted statistical analysis. Y.L. and Y.Z. prepared tables and figures. Y.L., P.W. and N.S. wrote the manuscript. All these authors completely consented with all the data in the study and approved the final manuscript. N.S. had the primary responsibility for final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, Yj., Wang, P., Peng, J. et al. Meta-analysis Reveals the Prognostic Value of Circulating Tumour Cells Detected in the Peripheral Blood in Patients with Non-Metastatic Colorectal Cancer. Sci Rep 7, 905 (2017). https://doi.org/10.1038/s41598-017-01066-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01066-y
This article is cited by
-
Circulating tumour cells in gastrointestinal cancers: food for thought?
British Journal of Cancer (2023)
-
The clinical significance of CTC enrichment by GPC3-IML and its genetic analysis in hepatocellular carcinoma
Journal of Nanobiotechnology (2021)
-
CTCs as a prognostic and predictive biomarker for stage II/III Colon Cancer: a companion study to the PePiTA trial
BMC Cancer (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.