Abstract
The neutrophil-lymphocyte ratio (NLR) has been reported to be associated with prognosis in several cancers. The objective of our study was to evaluate the prognostic role of baseline NLR and change in NLR (ΔNLR) in advanced pancreatic cancer underwent chemotherapy. Between January 2010 and June 2015, 132 patients underwent chemotherapy were eligible for assessment. Based on our patients’ data, the cut-off value of NLR was 2.78 according to receiver operating characteristic curve. We observed that a high level of baseline NLR (NLR > 2.78) was a poor prognostic factor for overall survival (multivariable hazard ratio [HR] = 2.648, P < 0.001). Increased NLR (ΔNLR > 0) after 2 cycles of chemotherapy was associated with higher risk compared to ΔNLR ≤ 0 (multivariable HR = 1.894, P = 0.007). Combining both NLR and ΔNLR factors, multivariate analysis showed a significant higher risk (HR = 5.817, P < 0.001) for patients with high baseline NLR and increased NLR after 2 cycles of chemotherapy compared to patients with low baseline NLR and ΔNLR ≤ 0. In conclusion, both baseline NLR and ΔNLR are independent prognostic predictors for patients with advanced pancreatic cancer underwent chemotherapy.
Similar content being viewed by others
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in United States for both men and women1. Cases are usually diagnosed at an advanced stage with limited treatment options available. The 5-year survival rate is as low as <5%. The Karnofsky performance status (KPS) is usually worse in advanced stage pancreatic cancer, and precise patient selection for effective treatment is still unmet clinical need. There is lack of means to predict prognosis. Although pathological stage is known as a good predictor, it is difficult to obtain tumour tissue in most of the patients. Recently, blood neutrophil-to-lymphocyte ratio (NLR) was shown valuable to predict prognosis in cancer patients, as inflammatory responses play decisive roles at different stages of tumour development, including initiation, promotion, invasion, and metastasis2, 3. The tumour microenvironment contains innate immune cells (including macrophages, neutrophils, mast cells, myeloid-derived suppressor cells, dendritic cells, and natural killer cells) and adaptive immune cells (T and B lymphocytes) in addition to the cancer cells and their surrounding stroma. The net outcome of therapy-induced inflammation is controversial, as on the one hand it can have tumour-promoting functions just like the necrosis that accompanies rapid tumour growth4, but on the other hand it can enhance the cross-presentation of tumour antigens and subsequent induction of an anti-tumour immune response5.
Biomarkers of inflammation such as blood NLR and blood platelet-to-lymphocyte ratio (PLR) have been associated with clinical outcomes in a number of tumours including colorectal cancer, gastric cancer, non-small cell lung cancer, small cell lung cancer and pancreatic cancer6,7,8,9. However, neutrophil and lymphocyte count may be influenced by a host of clinical factors such as KPS, age, previous treatments, coexisting infection, and impaired renal or hepatic function. Chemotherapy could have a significant impact on patients’ inflammation environment. There were limited studies focused on the change in NLR (ΔNLR) when patients underwent chemotherapy. In this study, we investigated the prognostic role of both baseline NLR and ΔNLR in patients with advanced pancreatic adenocarcinoma underwent chemotherapy.
Results
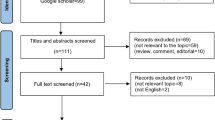
Total 132 patients (85 male and 47 female) with histologically confirmed advanced pancreatic cancer who received chemotherapy were eligible for assessment (Fig. 1). The median age at diagnosis was 57 (95% CI: 41.6–69.7). By July 30, 2016, 116 (87.9%) patients passed away, and the median survival was 7.66 months (95% CI: 6.13–9.23). Of the 31 (23.5%) patients were locally advanced and 101 (76.5%) patients had distant metastasis. We used 1-year survival as the time point to generate the receiver operating characteristic (ROC) curve and determined the optimal cut-off value of 2.78 for baseline NLR with the area under the curve (AUC) of 0.634. We used baseline NLR and NLR after 2 cycles to determine ΔNLR and categorized patients into ΔNLR ≤ 0 group and ΔNLR > 0 group (Table 1).
Univariable and multivariable analysis was performed to investigate the prognostic role of baseline NLR and ΔNLR in pancreatic cancer patients underwent chemotherapy. Univariate analysis indicated male (P = 0.017), a high white blood cell (WBC) count after 2 cycles (P < 0.001), a high baseline NLR (P < 0.001) and ΔNLR > 0 (P < 0.001) were poor prognostic factors for overall survival (OS) in this study cohort (Figs 2, 3). Age, location, stage at diagnosis, albumin, KPS, and WBC, platelets and hemoglobin at baseline, platelets and hemoglobin after 2 cycles were not significantly associated with prognosis (Table 2).
Overall survival curves in patients with advanced pancreatic cancer according to baseline NLR. Survival curves were estimated using Kaplan-Meier method. Total 132 patients, 116 passed away by July, 2016. The median OS of NLR > 2.78 and NLR ≤ 2.78 were 5.7 months (95% CI: 4.8–6.5) and 12.3 months (95% CI: 8.8–15.8), respectively.
Overall survival curves in patients with advanced pancreatic cancer according to ΔNLR. Survival curves were estimated using Kaplan-Meier method. Total 132 patients, 116 passed away by July, 2016. The median OS of ΔNLR > 0 and ΔNLR ≤ 0 were 4.8 months (95% CI: 3.4–6.2) and 9.1 months (95% CI: 7.6–10.6), respectively.
Multivariate analysis indicated that a high level of baseline NLR was a solid poor factor for OS (hazard ratio [HR] = 2.648, 95% CI: 1.631–4.300, P < 0.001) adjusted for gender, KPS, stage at diagnosis, and WBC, platelets, and hemoglobin at baseline and after 2 cycles. Whereas HR of death was 1.894 (1.160–3.091) for patients with ΔNLR > 0 compared to ΔNLR ≤ 0. Based on our analysis, baseline NLR and ΔNLR were independent prognostic factors for pancreatic cancer patients (Table 3).
Combining both baseline NLR and ΔNLR, we further categorized patients into 4 groups: group A (NLR ≤ 2.78 & ΔNLR ≤ 0), group B (NLR > 2.78 & ΔNLR ≤ 0), group C (NLR ≤ 2.78 & ΔNLR > 0) and group D (NLR > 2.78 & ΔNLR > 0). Patients in group B (HR = 2.189, 95% CI: 1.097–4.371, P = 0.026), group C (HR = 2.733, 95% CI: 1.510–4.947, P = 0.001), and group D (HR = 5.817, 95% CI: 2.862–11.821, P < 0.001) had poorer prognosis (OS) compared to group A by multivariate analysis adjusted for gender, KPS, stage at diagnosis, and WBC, platelets, and hemoglobin at baseline and after 2 cycles (Table 4) (Fig. 4).
Overall survival curves in patients with advanced pancreatic cancer according to 4 risk groups (combining baseline NLR and ΔNLR). Survival curves were estimated using Kaplan-Meier method. Total 132 patients, 116 passed away by July, 2016. The median OS of group A, B, C and D were 15.2 months (95% CI: 13.2–17.2), 7.6 months (95% CI: 4.9–10.2), 6.8 months (95% CI: 5.1–8.4) and 3.8 months (95% CI: 2.9–4.7), respectively.
Discussion
On the basis of our data, we found the independent prognostic value of baseline NLR and ΔNLR in advanced pancreatic cancer. Several studies indicated the poor survival was associated with high baseline NLR of the cancers in lung9, 10, colon11, gastric7, breast12, ovarian13, prostate14 and pancreas15,16,17. Our findings of baseline NLR was in consistent with previous studies. In addition, we found that HR of death were significantly higher in patients with ΔNLR > 0 compared to ΔNLR ≤ 0 (HR = 1.807, 95% CI: 1.202–2.714, P = 0.007). Baseline NLR and ΔNLR in patients underwent chemotherapy appear to be of significant clinical value. Combining both baseline NLR (≤2.78 or >2.78) and ΔNLR (≤0 or >0), group D (baseline NLR >2.78 & ΔNLR >0) had a significant higher risk (HR = 5.817, P < 0.001) compared to group A (NLR ≤ 2.78 & ΔNLR ≤ 0), which is indicative of patients’ potential resistance to treatment and predicts poor survival.
NLR and other similar indicators, such as C-reactive protein, albumin, the Glasgow prognostic score, thrombocytosis and PLR may reflect systemic inflammation18,19,20. Until now, several theories regarding these facts have been made. A high NLR goes along with either a larger neutrophil amount or a relatively lower number of lymphocytes.
First, literatures showed neutrophils can irritate angiogenesis and suppress the immune system for anti-tumour activities to induce tumour growth2, 21. Several studies indicated that neutrophils were involved in the release of various cytokines and chemokines including vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP)22, 23, which were important for tumour angiogenesis and metastasis24, 25. Fridlender et al.26 found that TGF-β within the tumour microenvironment induced a population of tumour-associated neutrophils (TAN) with a protumour phenotype. Lei Gong et al.27 confirmed that neutrophils played a very important role in the promotion of lung cancer which was strongly mediated through the IL-8/CXCR2 pathway and release of neutrophil elastase and development of a type 2 protumour microenvironment. KRAS mutation was the most common genetic alterations in pancreatic cancer28. Ji et al.29 indicated that activation of KRAS/RAF/MEK pathways could stimulate the accumulation of neutrophils, followed by intensive inflammatory response. Based on the above literatures, we think neutrophils could induce tumour growth and metastasis, and the increased number of tumour-associated neutrophils has been linked to poorer outcome in cancer patients. Similar findings in the literatures indicated that elevated neutrophils were significantly associated with larger tumour size and worse survival in patients with localized renal cell carcinoma30 and nasopharyngeal cancer31.
Second, in vitro studies showed that the cytolytic activity of lymphocytes and natural killer cells was suppressed when co-cultured with neutrophils, and the extent of suppression was proportionally enhanced to the addition of neutrophils32. Pillay et al.33 identified that neutrophil subpopulations could suppress T-cell proliferation by integrin Mac-1 and hydrogen peroxide. Lymphocytes were known to have a crucial role in tumour defense. Lymphocytes could induce cytotoxic cell death and inhibit tumour cell proliferation and migration21, 34. Therefore, a reduced lymphocyte count indicated a weaker immune reaction against tumour cells. Fogar et al.35 confirmed that lower total lymphocyte count in blood for patients with pancreatic cancer was associated with poor outcome. Therefore, the levels of baseline NLR and ΔNLR could reflect the inflammatory response and immune status of the patients undergoing chemotherapy, and theoretically both factors could be predictors for patients’ prognosis.
The limitations of this study included: (a) this was a single center retrospective study; (b) there were different chemotherapy regimens; (c) we had included limited sample size; and (d) the study was conducted only in the Chinese population.
In conclusion, we found both baseline NLR and ΔNLR were independent prognostic factors for patients with advanced pancreatic cancer underwent chemotherapy. Our results suggest that evaluation of baseline NLR and ΔNLR is helpful in prognosis prediction, drug dose adjustment and inflammatory status assessment. Further validation in a prospective study is warranted.
Methods
Patients
This was a retrospective study approved by the ethics committee of Chinese People’s Liberation Army (PLA) General Hospital. From January 1, 2010 to June 1, 2015, patients with advanced pancreatic cancer admitted for chemotherapy were included for analysis. Prior to chemotherapy initiation, written informed consent was reviewed and signed by the patients or their legal guardian. All relevant blood tests and treatments were performed based on institutional guidelines and regulations. Clinical data were retrieved and collected for retrospective analysis from the medical records of PLA General Hospital database electronically.
The inclusion criteria were: (1) patients were cytological or histologically confirmed pancreatic cancer and not eligible for operation; (2) patients received at least 2 cycles of chemotherapy (Please see Supplementary Table S1 for detailed information about chemotherapy regimens); (3) sufficient bone marrow function; (4) normal hepatic and renal function; (5) without targeted therapy or other biologics; (6) patients with a KPS score of 70 or more (on a scale from 0 to 100, with higher scores indicating better performance status); (7) no history of previous chemotherapy for advanced disease or adjuvant therapy within one year; (8) no radiotherapy. Exclusion criteria: (1) incomplete data of toxicities; (2) lost follow-up. Total 132 patients were eligible for analysis. Follow-up evaluations were performed every 3 months. Dates of death were obtained from the China disease prevention and control information system or telephone calls follow-up. Medical records were reviewed, and the cause of death was decided by investigator. Lost follow-up refers to the patient who was out of contact. We followed up until July 30, 2016 to obtain clinical and outcome information.
Data collection
All relevant clinic-pathological data were retrieved from patient medical records. Laboratory data, including neutrophil and lymphocyte, WBC, platelets, hemoglobin were obtained within 1 week before chemotherapy and after 2 cycles of chemotherapy. The absolute neutrophil count was calculated by the percentage of segmented neutrophils out of the WBC count. The NLR was determined by the absolute neutrophil count divided by the absolute lymphocyte count. ΔNLR was calculated by subtracting the baseline NLR from the NLR after 2 cycles of chemotherapy (cycle 2-cycle 0). Overall survival was defined as the time from date of treatment to death. The primary study endpoint was OS. Censoring occurred if patients were still alive at last follow-up.
Statistical analysis
Data were presented as median (interquartile) for continuous variables, and as frequency or percentage for categorical variables. Survival curves were estimated using the Kaplan–Meier method and compared by the log-rank test. Multivariate survival analyses were performed using Cox proportional hazards regression models. We used the ROC curve to determine the best cut-off values for OS with baseline NLR. All of the analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation). Statistical significance was defined as a two-sided P < 0.05.
Reference
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA: a cancer journal for clinicians 66, 7–30, doi:10.3322/caac.21332 (2016).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899, doi:10.1016/j.cell.2010.01.025 (2010).
Diakos, C. I., Charles, K. A., McMillan, D. C. & Clarke, S. J. Cancer-related inflammation and treatment effectiveness. The Lancet Oncology 15, e493–e503, doi:10.1016/s1470-2045(14)70263-3 (2014).
Ammirante, M., Luo, J. L., Grivennikov, S., Nedospasov, S. & Karin, M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464, 302–305, doi:10.1038/nature08782 (2010).
Zitvogel, L., Apetoh, L., Ghiringhelli, F. & Kroemer, G. Immunological aspects of cancer chemotherapy. Nature reviews. Immunology 8, 59–73, doi:10.1038/nri2216 (2008).
Zhou, X. et al. Prognostic value of PLR in various cancers: a meta-analysis. PloS one 9, e101119, doi:10.1371/journal.pone.0101119 (2014).
Zhang, X., Zhang, W. & Feng, L. J. Prognostic significance of neutrophil lymphocyte ratio in patients with gastric cancer: a meta-analysis. PloS one 9, e111906, doi:10.1371/journal.pone.0111906 (2014).
Takakura, K. et al. Comprehensive assessment of the prognosis of pancreatic cancer: peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site. Scandinavian journal of gastroenterology 51, 610–617, doi:10.3109/00365521.2015.1121515 (2016).
Wang, J. et al. Pretreatment Neutrophil to Lymphocyte Ratio Is Associated with Poor Survival in Patients with Stage I-III Non-Small Cell Lung Cancer. PloS one 11, e0163397, doi:10.1371/journal.pone.0163397 (2016).
Wang, X., Teng, F., Kong, L. & Yu, J. Pretreatment neutrophil-to-lymphocyte ratio as a survival predictor for small-cell lung cancer. OncoTargets and therapy 9, 5761–5770, doi:10.2147/OTT.S106296 (2016).
Stotz, M. et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. British journal of cancer 110, 435–440, doi:10.1038/bjc.2013.785 (2014).
Koh, C. H. et al. Utility of pre-treatment neutrophil–lymphocyte ratio and platelet–lymphocyte ratio as prognostic factors in breast cancer. British journal of cancer 113, 150–158, doi:10.1038/bjc.2015.183 (2015).
Williams, K. A. et al. Prognostic Significance and Predictors of the Neutrophil-to-Lymphocyte Ratio in Ovarian Cancer. Gynecologic oncology 132, 542–550, doi:10.1016/j.ygyno.2014.01.026 (2014).
Gu, X. et al. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: evidence from 16,266 patients. Scientific reports 6, 22089, doi:10.1038/srep22089 (2016).
Stotz, M. et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. British journal of cancer 109, 416–421, doi:10.1038/bjc.2013.332 (2013).
Shirai, Y. et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery 158, 360–365, doi:10.1016/j.surg.2015.03.043 (2015).
Luo, G. et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Annals of surgical oncology 22, 670–676, doi:10.1245/s10434-014-4021-y (2015).
Proctor, M. J. et al. Optimization of the systemic inflammation-based Glasgow prognostic score: a Glasgow Inflammation Outcome Study. Cancer 119, 2325–2332, doi:10.1002/cncr.28018 (2013).
Secchiero, P. et al. C-Reactive protein downregulates TRAIL expression in human peripheral monocytes via an Egr-1-dependent pathway. Clinical cancer research: an official journal of the American Association for Cancer Research 19, 1949–1959, doi:10.1158/1078-0432.ccr-12-3027 (2013).
van Rossum, A. P. et al. Granulocytosis and thrombocytosis in renal cell carcinoma: a pro-inflammatory cytokine response originating in the tumour. The Netherlands journal of medicine 67, 191–194 (2009).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867, doi:10.1038/nature01322 (2002).
Bausch, D. et al. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis 14, 235–243, doi:10.1007/s10456-011-9207-3 (2011).
Bekes, E. M. et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. The American journal of pathology 179, 1455–1470, doi:10.1016/j.ajpath.2011.05.031 (2011).
Zhao, X. et al. Lentivirus-mediated shRNA interference targeting vascular endothelial growth factor inhibits angiogenesis and progression of human pancreatic carcinoma. Oncology reports 29, 1019–1026, doi:10.3892/or.2012.2203 (2013).
Arnold, S. et al. Forced expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC. Experimental biology and medicine (Maywood, N.J.) 233, 860–873, doi:10.3181/0801-rm-12 (2008).
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell 16, 183–194, doi:10.1016/j.ccr.2009.06.017 (2009).
Gong, L. et al. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Molecular cancer 12, 154, doi:10.1186/1476-4598-12-154 (2013).
Waddell, N. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501, doi:10.1038/nature14169 (2015).
Ji, H. et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene 25, 2105–2112, doi:10.1038/sj.onc.1209237 (2006).
Jensen, H. K. et al. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 27, 4709–4717, doi:10.1200/jco.2008.18.9498 (2009).
He, J. R. et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head & neck 34, 1769–1776, doi:10.1002/hed.22008 (2012).
Shau, H. Y. & Kim, A. Suppression of lymphokine-activated killer induction by neutrophils. Journal of immunology (Baltimore, Md. : 1950) 141, 4395–4402 (1988).
Pillay, J. et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. The Journal of clinical investigation 122, 327–336, doi:10.1172/jci57990 (2012).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444, doi:10.1038/nature07205 (2008).
Fogar, P. et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas 32, 22–28, doi:10.1097/01.mpa.0000188305.90290.50 (2006).
Acknowledgements
We thank Xinglin Chen, PhD (Statistical consultant, X&Y Solution, lnc. Boston MA) and Xiaozhou Ma, MD (medical director, iCoreMed Technology and Service, LLC) for their helpful comments on the manuscript. Research was supported by the projects from National Natural Science Foundation of China (81372286), and National Youth Science Foundation of China (81402016).
Author information
Authors and Affiliations
Contributions
Conception and design: Yang Chen, Yan Shi and Guanghai Dai; Collection and assembly of data: Yang Chen, Yanrong Wang and Huan Yan; Data analysis and interpretation: Yang Chen, Huan Yan, Yanrong Wang and Guanghai Dai; Manuscript preparation: Yang Chen and Yan Shi; Revision of the manuscript: Yang Chen, Yan Shi and Guanghai Dai; Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Yan, H., Wang, Y. et al. Significance of baseline and change in neutrophil-to-lymphocyte ratio in predicting prognosis: a retrospective analysis in advanced pancreatic ductal adenocarcinoma. Sci Rep 7, 753 (2017). https://doi.org/10.1038/s41598-017-00859-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00859-5
This article is cited by
-
Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia
Nature Communications (2021)
-
Differences in Baseline Characteristics and White Blood Cell Ratios Between Racial Groups in Patients with Pancreatic Adenocarcinoma
Journal of Gastrointestinal Cancer (2021)
-
Changes and prognostic impact of inflammatory nutritional factors during neoadjuvant chemoradiotherapy for patients with resectable and borderline resectable pancreatic cancer
BMC Gastroenterology (2020)
-
Association of the prognostic model iSEND with PD-1/L1 monotherapy outcome in non-small-cell lung cancer
British Journal of Cancer (2020)
-
Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.