Abstract
The indirect interactions between insect vectors, such as whiteflies, and the viruses they transmit, such as begomoviruses, via host plants may produce a range of outcome depending on the species/strain of each of the three organisms involved, and the mechanisms underlying the variations are not well understood. Here, we observed the performance of whiteflies on three types of tomato, which vary in level of jasmonic acid (JA)-related resistance and were either uninfected or infected by a begomovirus, to investigate the role of JA-related resistance in mediating whitefly-begomovirus interactions. Compared to the performance of whiteflies on plants of the wild type, the performance was elevated on plants deficient in JA-related resistance but reduced on plants with a high level of JA-related resistance. Further, on plants with a high level of JA-related resistance, the whitefly performed better on virus-infected than on uninfected plants; however, on tomato plants deficient in JA-related resistance, whitefly performance was less affected by the virus-infection of plants. Additionally, the expression of the JA-regulated defense gene PI-II in tomato plants was repressed by virus infection. These findings suggest that JA-related resistance plays an important role in the tripartite interactions between whitefly, begomovirus and tomato plant.
Similar content being viewed by others
Introduction
In recent decades, increasing attention has been given to the research on the tripartite interactions between insect vectors, viruses and plants1, 2. Many case studies have shown that the effects of the tripartite interactions may constitute major determinants of the population dynamics of the organisms in the field3, 4. Both direct and indirect interactions between the three organisms may operate in a complex network resulting in various consequences. For example, the indirect interactions between insect vectors and the viruses they transmit via their host plants may produce positive, neutral to negative effects on the performance of the insect vectors and in turn on the transmission of the viruses and the performance of the plants2, 4,5,6.
The whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is a species complex composed of at least 35 morphologically indistinguishable cryptic species, some of which are invasive and important crop pests worldwide7,8,9,10,11. As the vectors of begomoviruses, which are the largest and economically the most important group of plant viruses in tropical and sub-tropical regions, the outbreaks of whiteflies in many regions are often accompanied by epidemics of plant diseases caused by begomoviruses, such as the virus diseases of tomato and other crops caused by Tomato yellow leaf curl virus (TYLCV) that have spread around the world in the past three decades12,13,14,15,16.
Like in other systems that have been investigated, plant-mediated whitefly-begomovirus interactions exert important influences on the population dynamics of the whiteflies and the epidemiology of the virus diseases17,18,19. These interactions are often complicated. For example, the effects of virus infection of the host plants on whitefly performance can be positive, negative or neutral depending on the combinations of whiteflies, begomoviruses and plants6, 17, 18. Thus, in some cases, a begomovirus may cause infected plants to promote the performance of its transmitting vector, which accelerates the spread of the pathogen, while in other cases the infection of the host plants by a begomovirus may have no or negative effects on the performance of the whiteflies6, 20, 21. The mechanisms underlying these variations remain largely unknown.
In this study, we firstly observed the performance of two cryptic species of whiteflies on three types of tomato plants that are known to vary in the level of jasmonic acid (JA)-related resistance. Next, we compared the performance of whiteflies on uninfected and begomovirus-infected plants for each type of the tomato. Finally, we examined the relative expression levels of genes related to JA signaling pathway between uninfected and virus-infected tomato plants. Our objectives were to investigate whether JA-related resistance participates in the tripartite interactions between whiteflies, begomoviruses and tomato plants.
Materials and Methods
Whitefly, virus and plant
The MEAM1 whitefly (mtCO1 GenBank accession no: KM821540) and MED whitefly (mtCO1 GenBank accession no: GQ371165) of the B. tabaci species complex were reared on cotton Gossypium hirsutum L. cv. Zhemian 1793 in insect-proof cages at 27 ± 1 °C, 70 ± 10% RH and a 14 L:10D light cycle in climate-controlled rooms. The purity of the cultures was monitored every three generations using PCR-RFLP (PCR-restriction fragment length polymorphism) and mtCO1 sequencing analysis as described before22.
Clones of TYLCV isolate SH2 (GenBank Acc. No.:AM282874) were provided by Professor Xueping Zhou, Institute of Biotechnology, Zhejiang University.
For plants of tomato Solanum lycopersicum, the cv. CastleMart was used as the wild type, and JA-deficient spr2 mutant plants and JA-overexpression 35S-prosystemin transgenic plants, both in the CastleMart background, were used. To obtain virus-infected tomato plants, each of the test plants at the 3–4 true-leaf stage was inoculated with 0.2 ml of the TYLCV culture by agroinoculation as previously described23. All virus-inoculated and un-inoculated plants were grown in a greenhouse under natural lighting supplemented with artificial illumination (light 05:00–18:00 hours) and controlled temperature at 25 ± 3 °C, and were used for whitefly bioassays when they reached the 6–7 true-leaf stage (approximately 25 days after agroinoculation of the virus). Virus infection of plants was determined by typical disease symptoms and further conformed by PCR24.
Performance of whiteflies on the three types of tomato plants
We designed six treatments, using two species of whiteflies MEAM1 and MED and three types of tomato CastleMart, spr2 and 35S-prosystemin, to investigate the effect of JA-regulated defence of the plants to whiteflies (Table 1). We observed the adult survival and fecundity of each species of whitefly feeding on each of the three types of tomato plants. For each treatment, 10–19 replicates were conducted, and for each replicate, newly emerged whiteflies (10 females and 10 males) were collected from the cultures on cotton and released onto the lower surface of a plant leaf (third to fourth leaf from the top) enclosed in a clip cage to feed, mate and oviposit. Seven days later, the live adult whiteflies in each replicate were recorded, and the eggs deposited on the leaf were counted under a microscope.
Performance of whiteflies on uninfected and TYLCV-infected tomato plants
Due to the workload and space required for the bioassays, we tested each of the three types of tomato plants at a time. For each of the three types of plants, i.e. CastleMart, spr2 and 35S-prosystemin, we designed four treatments involving two species of whiteflies MEAM1 and MED and two levels of status of the plants, i.e. uninfected and TYLCV-infected, to observe the effects of TYLCV infection of the plants on whitefly performance (Tables 2–4). For each treatment, we conducted 10–19 replicates. The protocols for observing whitefly adult survival and fecundity were the same as above.
RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
Following our observation that whitefly performance was better on TYLCV-infected 35S-prosystemin transgenic plants than that on uninfected plants, we compared the expression of JA biosynthesis and defense marker genes in the plants using qRT–PCR. Three replicate plants in each of the two treatments (uninfected and TYLCV-infected) were sampled. Total RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). RNA purity and quantity were determined using Nanodrop (Nanodrop Technologies), and 1000 ng of total RNA for each sample was reverse transcribed using the PrimeScript RT reagent Kit with gDNA Eraser (Takara, Japan) according to the manufacturer’s instructions. qRT-PCRs were performed on the Bio-Rad CFX96TM Real-Time System (Bio-Rad, CA, USA) using the SYBR Premix Ex TaqTM II (Takara, Japan). Three technical qRT-PCR replicates were analysed for each biological replicate. The relative expression levels were calculated with the 2−ΔΔCT method and further log2-transformed as described by Yuan et al.25 and Luan et al.26. The actin gene was used as reference. Primers for qRT-PCRs were shown in Table 5.
Data analysis
Statistical significance was evaluated using ANOVA at a 0.05 level followed by LSD tests for whitefly performance. Data of percentages of adult survival were transformed by arcsine square root before analysis. Differences in gene expression levels were analyzed by nonparametric tests. All the data analyses were performed using IBM Statistics SPSS 20.0.
Results
Whitefly performance on three types of tomato
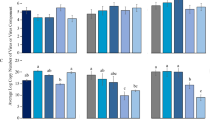
The survival of both females and males was not significantly affected by whitefly species or whitefly*plant interaction, but was significantly affected by the types of plants (Table 1). In both species of whiteflies, the percentages of survival were significantly reduced on 35S-prosystemin compared to those on either CastleMart or spr2, and in females of MEAM1 survival was significantly higher on spr2 than that on CastleMart (Table 1).
The fecundity was significantly affected by species of whiteflies, types of plants and the interaction between the two factors (Table 1). In MEAM1, fecundity was significantly higher on spr2 than that on either CastleMart or 35S-prosystemin; while in MED, fecundity was significantly higher on spr2 but lower on 35S-prosystemin than that on CastleMart (Table 1).
Whitefly performance on uninfected and TYLCV-infected CastleMart plants
On plants of CastleMart, the percentages of survival of both females and males were not significantly affected by species of whiteflies or virus-infection status of the plants, but were significantly affected by the interaction between the two factors (Table 2). The survival of MEAM1 females was significantly higher on TYLCV-infected plants than that on uninfected plants; however, the survival of MED females was not significantly affected by the virus-infection status of the plants. The survival of MEAM1 males was not significantly affected by the virus-infection status of the plants, while the survival of MED males was significantly lower on TYLCV-infected plants than that on uninfected plants (Table 2).
The fecundity was significantly affected by species of whiteflies, virus-infection status of the plants, and the interaction between the two factors (Table 2). While the fecundity of MEAM1 did not differ between uninfected and TYLCV-infected plants, the fecundity of MED was significantly higher on TYLCV-infected plants than that on uninfected plants (Table 2).
Whitefly performance on uninfected and TYLCV-infected spr2 plants
On plants of spr2, the percentages of survival of both females and males were significantly affected by the virus-infection status of the plants, but were not significantly affected by either the species of whiteflies or the interaction between the two factors (Table 3). The survival of females of both MEAM1 and MED was significantly higher on TYLCV-infected plants than that on uninfected plants; the percentages of survival of MEAM1 males did not differ between uninfected and TYLCV-infected plants, while survival of MED males was significantly higher on virus-infected plants than that on uninfected plants (Table 3).
The fecundity was significantly affected by species of whiteflies as well as the interaction between whitefly species and virus-infection status of the plants, but was not significantly affected by the virus-infection status of the plants (Table 3). For MEAM1, fecundity was not significantly affected by the virus-infection status of the plants, while the fecundity of MED was significantly lower on TYLCV-infected than that on uninfected plants (Table 3).
Whitefly performance on uninfected and TYLCV-infected 35S-prosystemin plants
On 35S-prosystemin transgenic plants, the percentages of survival of females were not significantly affected by species of whiteflies or the interaction between whitefly species and virus-infection status of the plants, but were significantly affected by the virus-infection status of the plants. In both whitefly species, survival of females was significantly higher on TYLCV-infected plants than that on uninfected plants (Table 4). The percentages of survival of males were significantly affected by species of whiteflies and the virus-infection status of the plants, but were not affected by the interaction between the two factors. In both whitefly species, survival of males was also significantly higher on TYLCV-infected than that on uninfected plants (Table 4).
The fecundity was significantly affected by the virus-infection status of the plants, but was not significantly affected by the species of whiteflies or the interaction between the two factors. While the fecundity of MEAM1 was significantly higher on TYLCV-infected plants than that on uninfected plants, the fecundity of MED did not differ between uninfected and TYLCV-infected plants (Table 4).
Expression of JA signaling pathway-related genes in uninfected and TYLCV-infected plants of 35S-prosystemin
The transcript levels of all four JA-biosynthesis-related genes we tested did not differ significantly between uninfected and TYLCV-infected 35S-prosystemin plants (Fig. 1a); however, the expression of the PI-II gene at the downstream of JA pathway was marginally repressed in TYLCV-infected plants compared to that in uninfected plants (P = 0.061; Fig. 1b).
Discussion
JA signaling pathway is involved extensively in plant resistance against herbivore insects27, 28. JA signaling pathway regulates the production of many plant secondary metabolites and defensive proteins that are harmful to herbivores, for example, terpenoids in tobacco plant play an important role in the resistance against whiteflies3, 29,30,31,32. Our data shows that, in tomato, the two species of whiteflies performed poorer on plants exhibiting a higher level of JA-related resistance (Table 1). TYLCV-infection of tomato plants repressed their JA-related resistance (Fig. 1), and TYLCV caused the infected plants to promote the performance of the whiteflies (Table 4). Further, this effect of promotion of whitefly performance by TYLCV infection was stronger on plants with a higher level of JA-related resistance than on plant with a lower level of JA-related resistance (Tables 2–4).
During the experiments, the plants of the CastleMart wild type, and JA-deficient spr2 mutant and JA-overexpression 35S-prosystemin transgenic plants grew to a similar size but did appear somewhat different. The differences among them are difficult to describe. In the experiments, test adults were caged on leaves of tomato plants, with the same number of adults enclosed in the same type of clip-cage. So the average area of leaf per insect was the same for all replicates in each of the treatments. We assumed that any differences in the phenotype of the plants had little effect on the performance of the whiteflies when we were comparing the performance of whiteflies in different treatments.
JA-induced defenses in Arabidopsis thaliana was shown to confer resistance to whiteflies33. Previous studies have demonstrated that the begomovirus Tomato Yellow leaf curl China virus (TYLCCNV) can promote the performance of whiteflies on infected tobacco plants through inhibiting JA-related defence32, 34. The pathogenicity factor ßC1 of TYLCCNV was found to be interacting with MYC2, and the interactions drive the down-regulation of JA signaling pathway and in turn suppression of terpenoid synthesis and release30. However, in this study, the begomovirus TYLCV infection did not inhibit the expression of the four JA-biosynthesis genes (AOC, AOS, OPR3, LOXD) in the 35S-prosystemin transgenic plants, but the transcript level of the JA-regulated defense gene (PI-II) was down-regulated (Fig. 1). It seemed likely that TYLCV caused the infected tomato plants to promote whitefly performance not via a direct repression of the JA synthesis pathway.
In this study, two species of whiteflies of the B. tabaci complex were included in the experiments. Statistical analyses of the data indicated that in some cases whitefly species had a significant role in the interactions (Tables 1–4). For example, on plants of the wild type, TYLCV-infection of the plants appeared to have some positive effects on the survival of MEAM1 females but negative effects on the survival of MED males. Further, TYLCV-infection of the plants did not have an effect on the fecundity of MEAM1 but a significant positive effect on the fecundity of MED. The mechanisms under these differences are not yet known. Similarly, in a previous study, we showed that both TYLCV and TYLCCNV caused the infected tomato plants of a widely grown cultivar Hezuo903 to promote the performance of the invasive MEAM1 but repressed the performance of an indigenous species of whitefly Asia II 321. In another study, we found that TYLCV infection of tomato plants had little effects on the performance of both the invasive MED and the indigenous Asia II 1 whiteflies20. These results are not surprising but do help to show the complexity that can occur in the tripartite interactions between whiteflies, begomoviruses and host plants. The data of this study indicate that the effects of virus infection of the plants on the performance of whiteflies vary with the intrinsic level of JA-related resistance (Tables 1–4), with plants that have higher intrinsic JA-related defence/resistance showing stronger positive effects on whitefly performance. Tomato plants are in general highly suitable to whitefly infestation possibly due to, in part, a low level of intrinsic JA-related defence/resistance. This inference may provide some clues to understand that in many cases viral infection of tomato plants does not have a major role in affecting whitefly performance on these highly suitable plants18,19,20, 35.
All in all, we show that begomovirus infection may suppresses JA-related resistance in tomato plants and in turn promote the performance of whiteflies. However, this benefit effect conferred to the whiteflies via infection of the host plants by the virus becomes significant or substantial only when JA-related resistance plays a major role in the plants against whiteflies. Our findings confirmed the general role of JA-related resistance against whiteflies, and further show that the effects of suppression of JA-related resistance in plants by begomoviruses on whitefly performance may vary depending on the intrinsic role of JA in that plant.
References
Biere, A. & Tack, A. J. M. Evolutionary adaptation in three-way interactions between plants, microbes and arthropods. Funct. Ecol. 27, 646–660 (2013).
Stout, M. J., Thaler, J. S. & Thomma, B. P. H. J. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu. Rev. Entomol. 51, 663–689 (2006).
Pieterse, C. M. & Dicke, M. Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends Plant Sci. 12, 564–569 (2007).
Hauser, T. P., Christensen, S., Heimes, C. & Kiaer, L. P. Combined effects of arthropod herbivores and phytopathogens on plant performance. Funct. Ecol. 27, 623–632 (2013).
Bennett, A. E. Can plant-microbe-insect interactions enhance or inhibit the spread of invasive species? Funct. Ecol. 27, 661–671 (2013).
Jiu, M. et al. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE. 2, e182, doi:10.1371/journal.pone.0000182 (2007).
De Barro, P. J., Liu, S. S., Boykin, L. M. & Dinsdale, A. B. Bemisia tabaci: A Statement of Species Status. Annu. Rev. Entomol. 56, 1–19 (2011).
Firdaus, S. et al. The Bemisia tabaci species complex: additions from different parts of the world. Insect Sci. 20, 723–733 (2013).
Hu, J. et al. Members of Bemisia tabaci (Hemiptera: Aleyrodidae) cryptic species and the status of two invasive alien species in the Yunnan Province (China). J. Insect Sci. 14, 281 (2014).
Liu, S. S., Colvin, J. & De Barro, P. J. Species concepts as applied to the whitefly Bemisia tabaci systematics: How many species are there? J. Integr. Agric. 11, 176–186 (2012).
Boykin, L. M., Armstrong, K. F., Kubatko, L. & De Barro, P. J. Species delimitation and global biosecurity. Evol. Bioinform. 8, 1–37 (2012).
Hogenhout, S. A., Ammar, E. D., Whitfield, A. E. & Redinbaugh, M. G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 46, 327–359 (2008).
Moffat, A. S. Geminiviruses emerge as serious crop threat. Science. 286, 1835–1835 (1999).
Mansoor, S., Briddon, R. W., Zafar, Y. & Stanley, J. Geminivirus disease complexes: an emerging threat. Trends Plant Sci. 8, 128–134 (2003).
Navas-Castillo, J., Fiallo-Olive, E. & Sanchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 49, 219–248 (2011).
Seal, S. E., van den Bosch, F. & Jeger, M. J. Factors influencing Begomovirus evolution and their increasing global significance: implications for sustainable control. Crit. Rev. Plant Sci. 25, 23–46 (2006).
Colvin, J. et al. Host-plant viral infection effects on arthropod-vector population growth, development and behaviour: management and epidemiological implications. Adv. Virus Res. 67, 419–452 (2006).
Luan, J. B., Wang, X. W., Colvin, J. & Liu, S. S. Plant-mediated whitefly-begomovirus interactions: research progress and future prospects. Bull. Entomol. Res. 104, 267–276 (2014).
Mauck, K. et al. Transmission mechanisms shape pathogen effects on host–vector interactions: evidence from plant viruses. Funct. Ecol. 26, 1162–1175 (2012).
Li, M., Liu, J. & Liu, S. S. Tomato yellow leaf curl virus infection of tomato does not affect the performance of the Q and ZHJ2 biotypes of the viral vector Bemisia tabaci. Insect Sci. 18, 40–49 (2011).
Liu, J., Zhao, H., Jiang, K., Zhou, X. P. & Liu, S. S. Differential indirect effects of two plant viruses on an invasive and an indigenous whitefly vector: implications for competitive displacement. Ann. Appl. Biol. 155, 439–448 (2009).
Qin, L., Wang, J., Bing, X.L. & Liu, S.S. Identifcation of nine cryptic species of Bemisia tabaci (Hemiptera: Aleyrodidae) from China by using the mtCOI PCR-RFLP technique. Acta Entomol. Sin. 56, 186–194, in Chinese with english abstract (2013).
Cui, X. F., Tao, X. R., Xie, Y., Fauquet, C. M. & Zhou, X. P. A DNAß associated with Tomato yellow leaf curl China virus is required for symptom induction. J. Virol. 78, 13966–13974 (2004).
Qian, Y. J. & Zhou, X. P. Pathogenicity and stability of a truncated DNAß associated with Tomato yellow leaf curl China virus. Virus Res. 109, 159–163 (2005).
Yuan, J. S., Reed, A., Chen, F. & Stewart, C. N. Statistical analysis of real-time PCR data. BMC Bioinformatics. 7, 85 (2006).
Luan, J. B. et al. Global analysis of the transcriptional response of whitefly to Tomato yellow leaf curl China virus reveals their relationship of coevolved adaptations. J. Virol. 85, 3330–3340 (2011).
Bari, R. & Jones, J. D. G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 69, 473–488 (2009).
Erb, M., Meldau, S. & Howe, G. A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17, 250–259 (2012).
Bingham, G., Alptekin, S., Delogu, G., Gurkan, O. & Moores, G. Synergistic manipulations of plant and insect defences. Pest Manag. Sci. 70, 566–571 (2013).
Li, R. et al. Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell. 26, 4991–5008 (2014).
Luan, J. B. et al. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 16, 390–398 (2013).
Zhang, T. et al. Begomovirus-whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Mol. Ecol. 21, 1294–1304 (2012).
Zarate, S. I., Kempema, L. A. & Walling, L. L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 143, 866–875 (2007).
Yang, J. Y. et al. βC1, the pathogenicity factor of TYLCCNV, interacts with AS1 to alter leaf development and suppress selective jasmonic acid responses. Genes Dev. 22, 2564–2577 (2008).
Matssuura, S. & Hoshino, S. Effect of tomato yellow leaf curl disease on reproduction of Bemisia tabaci Q biotype (Hemiptera: Aleyrodidae) on tomato plants. Appl. Entomol. Zool. 41,143–148 (2009).
Acknowledgements
We thank Professor Qiaomei Wang, Department of Horticulture, Zhejiang University for providing the seeds of all tomato plants used in this study. This research was supported by the National Natural Science Foundation of China (Project no.: 31390421).
Author information
Authors and Affiliations
Contributions
S.S.L. conceived the research, Y.C.S., L.L.P., F.Z.Y. and P.L. performed the experiments under the supervision of S.S.L. and X.W.W., Y.C.S., X.W.W. and S.S.L. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, YC., Pan, LL., Ying, FZ. et al. Jasmonic acid-related resistance in tomato mediates interactions between whitefly and whitefly-transmitted virus. Sci Rep 7, 566 (2017). https://doi.org/10.1038/s41598-017-00692-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00692-w
This article is cited by
-
Integrative transcriptomics reveals association of abscisic acid and lignin pathways with cassava whitefly resistance
BMC Plant Biology (2023)
-
Geminiviral betasatellites: critical viral ammunition to conquer plant immunity
Archives of Virology (2023)
-
The interplay of plant hormonal pathways and geminiviral proteins: partners in disease development
Virus Genes (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.