Abstract
A characterization of the thermal ecology of fishes is needed to better understand changes in ecosystems and species distributions arising from global warming. The movement of wild animals during changing environmental conditions provides essential information to help predict the future thermal response of large marine predators. We used acoustic telemetry to monitor the vertical movement activity of the common dentex (Dentex dentex), a Mediterranean coastal predator, in relation to the oscillations of the seasonal thermocline during two summer periods in the Medes Islands marine reserve (NW Mediterranean Sea). During the summer stratification period, the common dentex presented a clear preference for the warm suprathermoclinal layer, and adjusted their vertical movements following the depth changes of the thermocline. The same preference was also observed during the night, when fish were less active. Due to this behaviour, we hypothesize that inter-annual thermal oscillations and the predicted lengthening of summer conditions will have a significant positive impact on the metabolic efficiency, activity levels, and population dynamics of this species, particularly in its northern limit of distribution. These changes in the dynamics of an ecosystem’s keystone predator might cascade down to lower trophic levels, potentially re-defining the coastal fish communities of the future.
Similar content being viewed by others
Introduction
Temperature is a key environmental factor that, through profound physiological effects, influences the fitness and survival of ectothermic organisms1,2,3. Ectothermic organisms are usually adapted to live within a limited range of temperatures, which includes a thermal optimum that maximizes their physiological performance4. When conditions move away from this optimum, an organism experiences reduced growth, reproduction, foraging, or competitiveness5, 6. Temperature shifts from the preferred range will thus greatly affect the dynamics of a species, altering the relative abundances of a population and changing the horizontal and vertical distribution of individuals7,8,9,10. In the case of ecosystem keystone species such as apex predators, community-changing herbivores, or structure-forming species, temperature-driven changes in abundance may cause ripple-effects in other levels of the food web, changing the structure and functioning of the ecosystem11, 12. In the face of global climate change, a great deal of research effort is being expended to characterize the thermal ecology of marine organisms, as it is necessary to understand the trends in ecosystems arising from the warming of the global ocean6, 13.
The ability to move makes fishes much more resistant to environmental change than less mobile or sessile benthic species14. As with many other mobile animals, marine fishes can readily exploit thermal gradients to regulate their body temperature and increase their metabolic efficiency15, 16. Movement is thus a good behavioural response with which to infer the thermal ecology of a fish species. Controlled laboratory experiments have shown that fish move across thermal gradients to attain a preferred temperature17, 18, and have allowed the researchers to investigate the response of an individual’s internal temperature to a fluctuating environment16. However, laboratory experiments are not feasible for large marine predators, and hence studies in the wild using acoustic telemetry and bio-logging technologies are a much more practical approach to study the thermal preference of these animals10, 17, 19.
Within the complexity of oceanographic conditions, the thermocline is a prominent structure. Thermoclines are hydrographical structures caused by large temperature gradients and that generate a significant segregation of resources. Thermoclines set up a heterogeneous thermal environment wherein mobile organisms have developed behavioural responses according to the trade-off between their physiological requirements and energy demands. For instance, many oceanic predators need to maintain warm body temperatures in order to sustain their foraging activity, but often their preys concentrate in cold deep waters. In order to exploit those food resources, several tuna species15, 20, 21 as well as the ocean sunfish22 rewarm during relatively long periods in the suprathermoclinal layer before performing short excursions below the thermocline to forage. Less attention has been paid to this kind of behaviours in coastal predators17, despite their key roles in shaping coastal communities and their relevance as indicators of good ecosystem conservation status23,24,25.
In this study we focused on the common dentex, Dentex dentex (L. 1758), one of the main coastal apex predators in the Mediterranean Sea and an important fishery resource for both artisanal and recreational fisheries26,27,28. The common dentex is present along the Atlantic and Mediterranean coasts, but its populations are more abundant in central and southern Mediterranean and rare in the Northern Mediterranean Sea29, 30. A global decrease in fishery landings of common dentex has been reported by FAO during the last three decades30, reason why it is classified as ‘vulnerable’ by the International Union for the Conservation of Nature (IUCN) in the Red List of Threatened Species31. However, in several Mediterranean sectors its abundance seems to be increasing, as shown by an increase in fishery landings in several Spanish ports32, and the fast recovery of its populations in marine protected areas25. The common dentex inhabits infra- and circa-littoral rocky bottoms and seagrass meadows, and is more abundant at depths between 15–30 m33, 34. The usual depth distribution of adult individuals coincides with the depth range at which the seasonal thermocline establishes between May and October in the NW Mediterranean Sea35. Summer conditions in the Mediterranean Sea are characterized by high water column stability and high temperatures, resulting in a strong stratification of the water column. However, this thermocline is known to display strong vertical oscillations in short time periods, such as a few hours or days, which are mainly driven by the wind and movement of water masses, and are also dependent on local hydrographic conditions caused by coastal orientation and bathymetry35.
We monitored the vertical movements of the common dentex using acoustic telemetry and characterized the thermal environment using in situ temperature loggers during two consecutive summers in the Medes Island marine protected area (NW Mediterranean Sea, Fig. 1). Our objective was to describe the thermal preference of the common dentex by analysing its vertical movements and activity patterns during stratified and non-stratified hydrographical periods. Describing the thermal ecology of this iconic apex predator will help us to predict their population dynamics during future warm water periods and the probable cascade effects on coastal marine ecosystems.
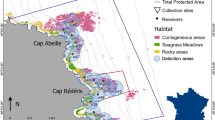
Bathymetric map of the study site and the location of acoustic receivers and temperature sensors. The approximate locations where the common dentex individuals were captured are also shown (see Table 1). In situ temperature sensors were placed every 5 m (between 5–40 m) in the marked location. Map was created using R60 version 3.3.1 (https://cran.r-project.org). The topographic base map (1:5.000), DEM and bathymetry are freely accessible through the Cartographic and Geologic Institute of Catalonia (www.icgc.cat) under Creative Commons Attribution License (CC BY 4.0).
Results
Thermal regime and depth of the thermocline
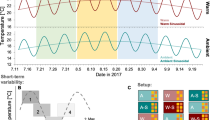
Surface temperatures ranged from a minimum of 12.4 °C in winter (March 2008) to maximum temperatures of 23.9 °C (August 2007) and 24.6 °C (August 2008) in summer (Fig. 2a,b). The stratification of the water column started in early May and became stronger as summer progressed, with the temperature gradient increasing in strength in July and August (Fig. 3). The temperature gradient relaxed at the end of summer (September), becoming weaker and deeper, until a complete breakdown at the end of October. Shortly after that, the water column was well mixed with a temperature of about 17 °C, before displaying a progressive cooling until the minimum winter temperature.
Daily temperature profiles (a,b) and vertical distributions of common dentex individuals (c,d). The panel in the left (c) corresponds to the set of individuals captured in May-June 2007 (n = 3), and the panel in right (d) to the set of individuals captured in December 2007-January 2008 (n = 9). Solid lines represent the daily mean depth of the thermocline.
Hourly thermocline depth (a) and temperature gradient (b) values, separated by month and year. The black line near the middle and the lower and upper box boundaries represent the median and the first and third quartiles of values, respectively. Ends of the whiskers represent values at 1.5 times the interquartile range of the box.
During the maximum stratification period, with surface temperatures above 19 °C (between mid-June and mid-September), significant oscillations of the thermocline were observed in both 2007 and 2008, but there were clear differences between years (Fig. 2). The summer of 2007 presented shallower thermocline positions than the summer of 2008 (Fig. 3a). The shallowest thermocline depths in 2007 were recorded in July, during which thermocline depths shallower than 15 m occurred on only two days. By contrast, in 2008 shallow thermoclines were registered during the entire summer (July, August and September), and observations of the thermocline shallower than 15 m occurred in 14 different days. Moreover, the maximum temperature gradients observed in July and August 2008 were much more elevated than the gradients observed in the same period in 2007 (Fig. 3b).
Vertical movement activity patterns
The total length of tagged individuals ranged between 42 and 65 cm (Table 1), with no differences in size between the first set of individuals (n = 3), tagged in May-June 2007, and the second set (n = 9), tagged in December 2007-January 2008 (Kruskal-Wallis test, χ2 = 2.203, df = 1, p = 0.138). However, the individuals from the first set utilized shallower depths (5–30 m) than the individuals from the second set (20–45 m) (Table 1 and Fig. 2a). The linear mixed-effects model used to test the differences between vertical movements (depth fluctuations for each day/night period) revealed a significant day-night and seasonal effect (Table 2). Overall, the vertical movement activity was greater during the day than during the night, and the seasonal pattern was marked by a higher vertical movement activity in spring compared to the rest of the seasons, which did not differ (Fig. 4).
Effects of the day/night cycle and the season on the vertical movement activity of common dentex. Vertical movement activity is quantified as the variance of depth (after a logarithmic transformation). Filled circles and error bars represent the mean values predicted by the linear mixed effects model and the 95% confidence intervals, respectively.
Effect of the thermocline on fish depth
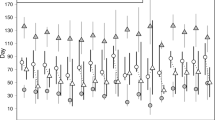
Tagged common dentex exhibited a characteristic pattern of vertical movements during the summer 2008, in which the depth of most of fish oscillated rapidly over periods of a few days (Fig. 2d). This pattern was not directly observable from tagged fish in the summer 2007 (Fig. 2c). A logistic mixed effects model explained the relationship between the observed depth patterns and the depth of the thermocline (Fig. 5 and Table 3). This model demonstrated a non-linear relationship between the average depths of tagged fish and the thermocline depth. When the thermocline was shallower than the depth-range used by each fish (20–45 m), the depth of the thermocline correlated positively and linearly with the mean fish depth. This relationship broke down and became asymptotic when the thermocline sank below the preferred depth range. Indeed, the individual asymptote values estimated by the model fell within the depth-range that each fish inhabited outside the summer season (Fig. 5). The effect of the day/night period was significant only in the estimation of one of the parameters of the model, the asymptote, but it had a relatively small effect (Table 3), thus indicating that individuals followed similar movement patterns above the thermocline indistinctively of the time of the day.
Relationship between mean fish depths and mean thermocline depths during the summer season, factorized by day/night periods, for each tagged fish (n = 12). Solid black lines represent the logistic models adjusted for each individual, and the magenta line is the mean prediction for the population. Black dotted lines highlight pure linear (1:1) relationships. Grey line and box in the bottom represent the median and the first and third quartiles, respectively, of the depths measured for each fish outside the summer period.
Despite this average relationship, several punctual excursions below the lower limit of the thermocline were observed during the summer period (Table 1). The amount of detections below the thermocline was low (<2%) for most of fishes, excepting for three individuals, #33, #42, and #45, which presented higher percentages. Overall, the percentage of detections below the thermocline was significantly higher during the daytime than during the night (paired samples t-test: t = 2.49; df = 11, p = 0.015).
Discussion
During the study period we observed the typical seasonal thermal cycle for the NW Mediterranean Sea, which is characterized by a mixed phase followed by a thermal stratification period35. Although this seasonal pattern repeats every year, there were inter-annual differences with respect to the maximum and minimum temperatures and the duration and magnitude of the stratification35, 36. During our study, the thermocline underwent several transient but recurring oscillations in the course of the stratification period. These oscillations included changes of up to 20 m in the depth of the thermocline and 10 °C in the temperature at certain depths. Our observations were similar to those described by Bensoussan et al.35, who indicated that thermocline oscillations represent an important fraction of the annual thermal variability despite their proportionately low duration (2.1 ± 0.8 days on average). Bensoussan et al.35 also reported that the relative variability of the temperature in summer in the study area increases with depth, reaching a maximum variability at depths between 25 and 40 m. At those depths, benthic communities are exposed to an average daily temperature variation of around 2 °C (with maximum values around 10 °C), and a weekly variation of around 7 °C (with maximum values of 11.5 °C). The swimming depth of the common dentex was strongly influenced by the temperature variations caused by the thermal structure of the water column, as shown by the relationship between the depth of the thermocline and the depths of tagged fish (Fig. 5). Transient thermocline rising events were related to upward displacements of individuals from the specific depths that they utilized outside the stratification period or during deeper thermocline events. Thus, common dentex demonstrated a clear preference for the suprathermoclinal warm water over the colder water below the thermocline.
Our main hypothesis is that a physiological optimization strategy was behind the observed behaviour of the common dentex. Temperature strongly affects the basal metabolic rate in fishes, restricting or enhancing physiological and behavioural processes2. Some studies on the effect of thermal gradients on fish have found that individuals spend most of their time (~75%) within ±2 °C of their preferred temperature, which coincides with the optimal physiological temperature that maximizes growth37. It has been also described that fish swimming speed or endurance is enhanced to a peak by an optimum temperature and is reduced when the temperature decreases or increases towards the tolerance limits38,39,40. The maximum swimming speed is a limiting factor for the foraging activity, and for this reason many oceanic predators must warm their body temperature in order to be able to hunt in cold deep waters15, 41. Thus, we hypothesize that the common dentex selects its thermal niche, presumably to approach the optimal temperature for growth and/or foraging in order to increase an individual’s net energy gain.
An alternative hypothesis would be that the observed movement behaviour of the common dentex was driven by indirect effects such as food distribution. The common dentex is a diurnal predator42, which primarily forages on other fishes (about 74% of prey items), and to a lesser degree on cephalopods and crustaceans26, 43. Consequently, foraging events were the most likely cause of the high vertical activity levels that we observed during the day (Fig. 4), while low activities during the night might be related to resting behaviour. Similar diel activity patterns have been observed in other coastal species42, 44 including other predators17. Regarding the relationship between the thermocline depth and the vertical distribution of individuals, our model indicated that the diel cycle had a negligible effect. This implies that a presumable concentration of prey items in the suprathermoclinal layer during the summer is not enough to explain the vertical movements of the common dentex. If that were the case, we would expect to observe a relaxation of the depth restriction imposed by the thermocline during the night, with individuals returning to their preferred depths to rest, similar to what has been described for the dogfish by Sims et al.17. On the contrary, most of the excursions below the thermocline were observed during the day, and were very probably related to punctual foraging events. Therefore, our results indicate that the observed vertical distributions of individuals during summer were more probably caused by a direct selection of the preferred temperature-range, rather than by an irregular distribution of preys items above and below the thermocline.
Our approach to estimate activity patterns could discern between day and night activity periods, but it was not adequate to resolve seasonal activity patterns. The common dentex resided within the study site throughout the year, facing two different thermal conditions (summer and winter) that we would expect to have a significant effect on fish physiology and activity. Cabled video observatories have already described a seasonal activity rhythm for the common dentex, which showed higher occurrences between August and October, indicating a change in its horizontal activity pattern coinciding with high water temperatures45. Similarly, Abdelkader and Ktari46 described an increase in the food intake of common dentex between April and May, coinciding with the timing of the spawning, and a decrease in consumption from September to February. These movement and feeding patterns are thought to be related to seasonal variations on the abundance of their prey items, mainly coastal fishes such as sparids, labrids and picarels (Spicara maena)26, which tipically are more abundant in summer than in winter. Interestingly, the common dentex has shown an unsual ability to withstand long periods without food, which seems to be an adaptation to cope with unfavourable periods47, such as the low prey availability and low temperatures during winter. However, our analysis did not show significant differences between the vertical activities of summer and winter. Nevertheless, it was able to detect an increase of the activity during spring, very probably related to the spawning, which has been described to occur between April and May26. During this period, the common dentex is thought to perform excursions to deep rocky outcrops, where it aggregates to spawn26, 30. Characterizing spawning movements and periods is key for designing effective conservation measures for emblematic fish species48, 49, and thus they should be studied more carefully in the future.
Changes in the distribution of individuals associated with seasonal and inter-annual environmental fluctuations provide insights into how populations may shift under global climate change. The current global change is driving not only a steady increase of global water temperatures, but also a lengthening of the summer period50. For instance, the thermal stratification has increased in the NW Mediterranean Sea during the last three decades, which has already lengthened the duration of yearly summer conditions by a ~40%50. Consequently, warm water fish species are being positively affected, leveraging the higher presence of favourable conditions that enhance both their metabolic and foraging efficiency, thus improving their survival rate and reproductive success51, 52. These kinds of environmental fluctuations are proposed as the cause of the inter-annual fluctuations in the capture rates of many important demersal fishery resources53, including the common dentex30, 34. The redistribution of preferred temperature ranges is generating changes in both the horizontal and vertical distributions of coastal fish assemblages7, 8. For instance, recent local data suggest that the common dentex might be expanding in the northern Mediterranean25, 32. Our results, by confirming the preference of this species for warm water, provides valuable information on the ecology of the species and a mechanistic understanding to the mentioned population fluctuations and expansion.
In this study, we used acoustic telemetry to provide some valuable insights on the thermal ecology of the common dentex and the possible future trends of its populations. The common dentex and other apex predators are keystone species in marine food webs, and are often used as indicators of the structure and functioning of ecosystems23, 24. Also, the abundances of species occupying high trophic levels shape biological communities through top-down trophic effects54, 55, although the extent of the impact is still under debate56. Climate change will differentially affect different species, depending on their physiological and behavioural traits6, 12, and as a result generate unpredictable effects that will interact with other perturbations such as overfishing. To make reliable predictions of future biological assemblages and main ecological trends, it is necessary to understand the mechanisms underpinning the responses of different species to climate change.
Methods
Acoustic telemetry study
Information about movements from 12 D. dentex individuals was collected from an acoustic telemetry study carried out in the Medes Islands MPA (Catalonia, NW Mediterranean Sea) between 2007 and 2008. Fish were captured by jigging hook-and-line fishing gear from a boat and tagged with V13P-1H acoustic transmitters (VEMCO, Nova Scotia; dimensions: 48 × 13 mm; power output: 153 dB; weight in water: 6.5 g), which were implanted in the peritoneal cavity using a standard surgical procedure57, 58. The tagging protocol followed the guidelines provided by the Ministry of Agriculture, Livestock, Fisheries and Food of the Catalan Government (decree 214/1997), and was approved by the Committee on the Ethics of Animal Experimentation of the University of Barcelona. The Department of Environment of the Catalan Government granted permissions for fishing, operating and releasing the animals in the Medes Islands Marine Reserve. All surgery was performed under 2-phenoxyethanol anaesthesia, and all efforts were made to minimize suffering. The sex of individuals could not be determined due to the lack of sexual dimorphism in this species. Transmitters were equipped with a pressure sensor and were programmed to emit signals with a random delay between 80 and 180 s. Movements of tagged individuals were monitored by a network of 17 acoustic receivers placed around the study area (Fig. 1). Signal range-tests were performed in the area and revealed an average detection range of 150 m around the receivers (see Aspillaga et al.44 for more details). Individuals were divided into two sets depending on their capture date (May-June 2007: n = 3; December 2007-January 2008: n = 9). All the analyses were restricted to a different time period for each set (Jun/4 2007 to May/21 2008, and Jan/1 2008 to Nov/11 2008, respectively), corresponding to the period in which all the individuals in the set were simultaneously tracked.
Temperature data
Hourly measures of in situ temperature were provided by the T-MedNet network (http://www.t-mednet.org). Temperature was registered by autonomous sensors (HOBO Water Temp Pro v2) placed in rocky ledges at depth intervals of 5 m (from 5 to 40 m depth) at one location of the study site (Fig. 1). Additional temperature data, corresponding to manually-operated sensors for one sampling station situated at 2.5 nautical miles offshore the eastern side of the islands, was provided by J. Pascual (http://meteolestartit.cat). At that station, temperature profiles were generated every 2–3 days using a CTD that recorded measures every meter, from the surface to a depth of 90 m.
Data analysis
The thermocline depth was calculated from hourly temperature profiles. A four parameter logistic regression was fitted to each profile, and the mean depth of the thermocline was then determined as the depth at which the first derivate of the model presented its maximum value (as in McKinzie et al.59). The upper and lower limits of the thermocline were also determined from the peaks in the second derivate of the model. The strength of the thermocline was then calculated as the temperature gradient (°C · m−1) between its upper and lower limits. In order to calculate the depth of the thermocline when it was below the depth-range of the autonomous sensors (40 m), the in situ temperature profiles were complemented with an interpolation of the manual CTD casts taken between 40 and 80 m depth. Thermocline depth was only calculated for the profiles where the total temperature difference between the surface and the deepest measures was higher than 3 °C.
Acoustic telemetry data was pooled in 5 min intervals and the mean depth was calculated for each common dentex individual, in order to remove duplicated detections in different receivers and to homogenize the data distribution along time. These intervals were then classified into consecutive day/night periods, defined by local sunset and sunrise time provided by the NOAA Solar Calculator (www.esrl.noaa.gov/gmd/grad/solcalc/) for the coordinates of the Medes Islands (42°03′N 3°13′E, WGS84), and the mean depths and variances were calculated for each period. Variance of the depth was used as an approximation to fish vertical activity, assuming that the range of vertical movements is bigger when fish are active (e.g. when foraging) than when they are resting. In order to detect diel and seasonal patterns of fish activity, a linear mixed-effects model was applied to the variance data after applying a logarithmic transformation, considering the day/night period and the season as fixed factors and the fish tag number as a random factor. To test the effect of the thermocline depth on the fish depth, only data corresponding to the summer was used, as it was the only season in which a well-developed thermocline was present. A non-linear mixed-effects model was applied to test this relationship, where the mean depth of the thermocline and day/night period were considered the primary and secondary covariates, respectively, and the fish tag number as a random factor. All the data was managed and analysed in R60, and mixed effects models were fitted using the ‘nlme’ package61. Performances of the models were visually inspected in residual distribution and residual vs. fitted values plots.
References
Crawshaw, L. I. Physiological and Behavioral Reactions of Fishes to Temperature Change. J. Fish. Res. Board Can. 34, 730–734 (1977).
Magnuson, J. J., Crowder, L. B. & Medvick, P. A. Temperature as an Ecological Resource. Am. Zool. 19, 331–343 (1979).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a methabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Martin, T. L. & Huey, R. B. Why ‘Suboptimal’ Is Optimal: Jensen’s Inequality and Ectotherm Thermal Preferences. Am. Nat. 171, E102–E118 (2008).
Huey, R. B. & Kingsolver, J. G. Evolution of Thermal Sensitivity of Ectotherm Performance. Trends Ecol. Evol. 4, 131–135 (1989).
Pörtner, H. O. & Farrell, A. P. Physiology and Climate Change. Science 322, 690–692 (2008).
Dulvy, N. K. et al. Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. J. Appl. Ecol. 45, 1029–1039 (2008).
Last, P. R. et al. Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices. Glob. Ecol. Biogeogr. 20, 58–72 (2011).
Montero-Serra, I., Edwards, M. & Genner, M. J. Warming shelf seas drive the subtropicalization of European pelagic fish communities. Glob. Chang. Biol. 21, 144–153 (2015).
Freitas, C., Olsen, E. M., Moland, E., Ciannelli, L. & Knutsen, H. Behavioral responses of Atlantic cod to sea temperature changes. Ecol. Evol. 5, 2070–2083 (2015).
Vergés, A. et al. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 281, 20140846 (2014).
Kortsch, S., Primicerio, R., Fossheim, M., Dolgov, A. V. & Aschan, M. Climate change alters the structure of arctic marine food webs due to poleward shifts of boreal generalists. Proc. R. Soc. B 282, 20151546 (2015).
Harley, C. D. G. et al. The impacts of climate change in coastal marine systems. Ecol. Lett. 9, 228–241 (2006).
Pagès, J. F. et al. The Mediterranean Benthic Herbivores Show Diverse Responses to Extreme Storm Disturbances. PLoS ONE 8, e62719 (2013).
Holland, K. N., Brill, R. W., Chang, R. K. C., Sibert, J. R. & Fournier, D. A. Physiological and behavioural thermoregulation in bigeye tuna (Thunnus obesus). Nature 358, 410–412 (1992).
Pépino, M., Goyer, K. & Magnan, P. Heat transfer in fish: are short excursions between habitats a thermoregulatory behaviour to exploit resources in an unfavourable thermal environment? J. Exp. Biol. 218, 3461–3467 (2015).
Sims, D. W. et al. Hunt warm, rest cool: bioenergetic strategy underlying diel vertical migration of a benthic shark. J. Anim. Ecol. 75, 176–190 (2006).
Cerqueira, M. et al. Thermal preference predicts animal personality in Nile tilapia Oreochromis niloticus. J. Anim. Ecol. 85, 1389–1400 (2016).
Hussey, N. E. et al. Aquatic animal telemetry: A panoramic window into the underwater world. Science 348, 1255642-1–10 (2015).
Block, B. A. et al. Environmental preferences of yellowfin tuna (Thunnus albacares) at the northern extent of its range. Mar. Biol. 130, 119–132 (1997).
Kitagawa, T. et al. Effect of ambient temperature on the vertical distribution and movement of Pacific bluefin tuna Thunnus thynnus orientalis. Mar. Ecol. Prog. Ser. 206, 251–260 (2000).
Nakamura, I., Goto, Y. & Sato, K. Ocean sunfish rewarm at the surface after deep excursions to forage for siphonophores. J. Anim. Ecol. 84, 590–603 (2015).
Stevenson, C. et al. High apex predator biomass on remote Pacific islands. Coral Reefs 26, 47–51 (2007).
Sala, E. et al. The Structure of Mediterranean Rocky Reef Ecosystems across Environmental and Human Gradients, and Conservation Implications. PLoS ONE 7, e32742 (2012).
García-Rubies, A., Hereu, B. & Zabala, M. Long-Term Recovery Patterns and Limited Spillover of Large Predatory Fish in a Mediterranean MPA. PLoS ONE 8, e73922 (2013).
Morales-Nin, B. & Moranta, J. Life history and fishery of the common dentex (Dentex dentex) in Mallorca (Balearic Islands, western Mediterranean). Fish. Res. 30, 67–76 (1997).
Morales-Nin, B. et al. The recreational fishery off Majorca Island (western Mediterranean): some implications for coastal resource management. ICES J. Mar. Sci. 62, 727–739 (2005).
Font, T. & Lloret, J. Biological implications of recreational shore angling and harvest in a marine reserve: the case of Cape Creus. Aquatic Conserv: Mar. Freshw. Ecosyst. 21, 210–217 (2011).
Bayle-Sempere, J. T., Ramos-Espla, A. A. & Mas Hernandez, J. Observations on Dentex dentex (L., 1758) in the Spanish Mediterranean in Les Espèces Marines à Protéger en Méditerranée (eds Boudouresque, C. F., Avon, M. & Gravez, V.) 245–253 (GIS Posidonie Publ., 1991).
Marengo, M., Durieux, E. D. H., Marchand, B. & Francour, P. A review of biology, fisheries and population structure of Dentex dentex (Sparidae). Rev. Fish Biol. Fisheries 24, 1065–1088 (2014).
Carpenter, K. E. & Russel, B. Dentex dentex. The IUCN Red List of Threatened Species 2014. http://dx.doi.org/10.2305/IUCN.UK.2014-3.RLTS.T170245A1300534.en (2014).
Orozco, M. J., Sánchez-Lizaso, J. L. & Fernández, A. M. Capturas del dentón (Dentex dentex) en dos puertos del Mediterráneo ibérico. Mediterránea. Ser. Estud. Biológicos. Epoca II. Número Espec. (2011).
Bauchot, M. L. & Hureau, J. C. Sparidae in Fishes of the North-Eastern Atlantic and the Mediterranean vol. 2 (eds Whitehead, P. J. P., Bauchot, M. L., Hureau, J. C., Nielsen, J. & Tortonese, E.) 883–907 (UNESCO, Paris, 1986).
Ramos-Esplá, A. A. & Bayle-Sempere, J. Estatuto del Dentex dentex (Linnaeus, 1758) en el Mediterráneo in Les Espèces Marines à Protéger en Méditerranée (eds Boudouresque, C. F., Avon, M. & Gravez, V.) 237–244 (GIS Posidonie Publ., 1991).
Bensoussan, N., Romano, J. C., Harmelin, J. G. & Garrabou, J. High resolution characterization of northwest Mediterranean coastal waters thermal regimes: To better understand responses of benthic communities to climate change. Estuar. Coast. Shelf Sci. 87, 431–441 (2010).
Pascual, J., Bensoussan, N., Salat, J., Garrabou, Q. & Garrabou, J. Clima i règim tèrmic de les aigües de les illes Medes i el Montgrí in El fons marí de les illes Medes i el Montgrí: quatre dècades de recerca per a la conservació (eds Hereu, B. & Quintana, X.) 65–77 (Càtedra d’ecosistemes litorals mediterranis. Museu de la Mediterrània, 2012).
Magnuson, J. J. & Destasio, B. T. Thermal niche of fishes and global warming in Global Warming: Implications for Freshwater and Marine Fish (eds Wood, C. M. & McDonald, D. G.) 377–408 (Cambridge University Press, 1997).
Randall, D. & Brauner, C. Effects of Environmental Factors on Exercise in Fish. J. Exp. Biol. 160, 113–126 (1991).
Lee, C. G. et al. The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J. Exp. Biol. 206, 3239–3251 (2003).
Claireaux, G., Couturier, C. & Groison, A.-L. Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J. Exp. Biol. 209, 3420–3428 (2006).
Furukawa, S. et al. Vertical movements of Pacific bluefin tuna (Thunnus orientalis) and dolphinfish (Coryphaena hippurus) relative to the thermocline in the northern East China Sea. Fish. Res. 149, 86–91 (2014).
Aguzzi, J. et al. Daily activity rhythms in temperate coastal fishes: insights from cabled observatory video monitoring. Mar. Ecol. Prog. Ser. 486, 223–236 (2013).
Chemmam-Abdelkader, B., Kraïem, M. M. & El Abed, A. Etude de l’âge et de la croissance de deux espèces de dentés Dentex dentex et de Dentex maroccanus des côtes Tunisiennes. Bull. Inst. Natn Scien. Tech. Mer de Salammbô 31, 43–51 (2004).
Aspillaga, E. et al. Ordinary and Extraordinary Movement Behaviour of Small Resident Fish within a Mediterranean Marine Protected Area. PLoS ONE 11, 1–19 (2016).
Aguzzi, J. et al. Coastal observatories for monitoring of fish behaviour and their responses to environmental changes. Rev. Fish Biol. Fisheries 25, 463–483 (2015).
Abdelkader, B. & Ktari, M. H. Régime alimentaire des dentés (Genre Dentex), poissons sparidés de Tunisie. Bull. Soc. Nat., Tunisie 17, 19–25 (1985).
Pérez-Jiménez, A. et al. Metabolic adjustments of Dentex dentex to prolonged starvation and refeeding. Fish Physiol. Biochem. 38, 1145–1157 (2012).
Sala, E., Ballesteros, E. & Starr, R. M. Rapid Decline of Nassau Grouper Spawning Aggregations in Belize: Fishery Management and Conservation Needs. Fisheries 26, 23–30 (2001).
Sadovy de Mitcheson, Y. & Colin, P. L. Reef Fish Spawning Aggregations: Biology, Research and Management. Fish and Fisheries Series. Vol. 35 (Springer Netherlands, 2012).
Coma, R. et al. Global warming-enhanced stratification and mass mortality events in the Mediterranean. Proc. Natl. Acad. Sci. USA 106, 6176–6181 (2009).
Walther, G.-R. et al. Ecological responses to recent climate change. Nature 416, 389–395 (2002).
Rijnsdorp, A. D., Peck, M. A., Engelhard, G. H., Möllmann, C. & Pinnegar, J. K. Resolving the effect of climate change on fish populations. ICES J. Mar. Sci. 66, 1570–1583 (2009).
Massutí, E. et al. The influence of oceanographic scenarios on the population dynamics of demersal resources in the western Mediterranean: Hypothesis for hake and red shrimp off Balearic Islands. J. Mar. Syst. 71, 421–438 (2008).
Myers, R. A., Baum, J. K., Shepherd, T. D., Powers, S. P. & Peterson, C. H. Cascading Effects of the Loss of Apex Predatory Sharks from a Coastal Ocean. Science 315, 1846–1850 (2007).
Heithaus, M. R., Frid, A., Wirsing, A. J. & Worm, B. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210 (2008).
Roff, G. et al. The Ecological Role of Sharks on Coral Reefs. Trends Ecol. Evol. 31, 395–407 (2016).
Lino, P. G., Bentes, L., Abecasis, D., Neves dos Santos, M. & Erzini, K. Comparative behavior of wild and hatchery reared white sea bream (Diplodus sargus) released on artificial reefs off the Algarve (Southern Portugal) in Tagging and Tracking of Marine Animals with Electronic Devices (eds Nielsen, J. L. et al.) 23–34 (Springer Netherlands, 2009).
Koeck, B., Gudefin, A., Romans, P., Loubet, J. & Lenfant, P. Effects of intracoelomic tagging procedure on white seabream (Diplodus sargus) behavior and survival. J. Exp. Mar. Bio. Ecol. 440, 1–7 (2013).
McKinzie, M. K., Jarvis, E. T. & Lowe, C. G. Fine-scale horizontal and vertical movement of barred sand bass, Paralabrax nebulifer, during spawning and non-spawning seasons. Fish. Res. 150, 66–75 (2014).
R Core Team R: A language and evironment for statistical computing, version 3.3.1. http://www.r-project.org/ (2016).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-128 (2016).
Acknowledgements
This research was funded by the Spanish Ministry of Science (CGL2005-05238/BOS) and the Generalitat de Catalunya (AG-2014-654). EA was supported by the Spanish Ministry of Education (FPU scholarship No. AP2012-0141). We would like to thank J. Pascual, observer from the meteorological station of l’Estartit, for providing us his valuable oceanographic data. We are also thankful to I. Montero-Serra, P. Capdevila, A. Medrano, A. Pagès, and D. Gomez for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
B.H., R.M.S., C.L., M.Z., D.D. and A.L. conceived the acoustic telemetry study and conducted the fieldwork. J.G. obtained and processed the in situ temperature data. E.A., F.B., J.G. and B.H. analysed the data. E.A. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Aspillaga, E., Bartumeus, F., Starr, R.M. et al. Thermal stratification drives movement of a coastal apex predator. Sci Rep 7, 526 (2017). https://doi.org/10.1038/s41598-017-00576-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00576-z
This article is cited by
-
Movements and spatial distribution of an endangered fish (Sciaena umbra) within a marine protected area
Scientific Reports (2024)
-
Residency and space use estimation methods based on passive acoustic telemetry data
Movement Ecology (2023)
-
Diel patterns of depth use and swimming activity of post-release greater amberjack (Seriola dumerili) in the northern Gulf of Mexico
Environmental Biology of Fishes (2023)
-
Water column structure influences long-distance latitudinal migration patterns and habitat use of bumphead sunfish Mola alexandrini in the Pacific Ocean
Scientific Reports (2021)
-
Seasonal influence on the bathymetric distribution of an endangered fish within a marine protected area
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.