Abstract
Drought stress conditions in soil or air hinder plant growth and development. Here, we report that the hot pepper (C apsicum a nnuum) RING type E3 Ligase 1 gene (CaREL1) is essential to the drought stress response. CaREL1 encodes a cytoplasmic- and nuclear-localized protein with E3 ligase activity. CaREL1 expression was induced by abscisic acid (ABA) and drought. CaREL1 contains a C3H2C3-type RING finger motif, which functions in ubiquitination of the target protein. We used CaREL1-silenced pepper plants and CaREL1-overexpressing (OX) transgenic Arabidopsis plants to evaluate the in vivo function of CaREL1 in response to drought stress and ABA treatment. CaREL1-silenced pepper plants displayed a drought-tolerant phenotype characterized by ABA hypersensitivity. In contrast, CaREL1-OX plants exhibited ABA hyposensitivity during the germination, seedling, and adult stages. In addition, plant growth was severely impaired under drought stress conditions, via a high level of transpirational water loss and decreased stomatal closure. Quantitative RT-PCR analyses revealed that ABA-related drought stress responsive genes were more weakly expressed in CaREL1-OX plants than in wild-type plants, indicating that CaREL1 functions in the drought stress response via the ABA-signalling pathway. Taken together, our results indicate that CaREL1 functions as a negative regulator of ABA-mediated drought stress tolerance.
Similar content being viewed by others
Introduction
Plants are sessile organisms, and hence they frequently encounter various stress conditions such as high light, extreme temperature, and water stress. Among these stress conditions, water stress in soil dramatically affects crop yield and agricultural quality worldwide. Plants alleviate the adverse effects of water stress via alteration of ion transport and regulation of stomatal aperture to reduce transpirational water loss. Abscisic acid (ABA) plays a key role in plant defence responses, especially the drought stress response. When exposed to drought stress, plants increase ABA biosynthesis and accumulation in the leaves and initiate signal transduction leading to plant defence responses1,2,3. A large number of stress-related genes involved in these defence mechanisms are regulated by ABA4. The ABA- and drought-signalling pathways have been extensively investigated; however, the specific mechanisms underlying the functional modifications remain unclear.
Ubiquitination with the 26S proteasome system is a common protein modification mechanism in eukaryotic cells5, 6. In plants, ubiquitination is necessary for various cellular signalling processes with a diverse range of targets such as transcription factors, hormone receptors, and damaged proteins7,8,9,10. The process of ubiquitin attachment to the target protein is conserved and involves three proteins—E1 (ubiquitin-activating enzyme), E2 (ubiquitin-conjugating enzyme), and E3 (ubiquitin ligase)11. Ubiquitin is composed of 76 amino acids and contains seven lysines that serve as the site of modification12. Ubiquitination via these enzymes conjugates single or multiple ubiquitins to the target proteins, which are recognized by the 26S proteasome for degradation. In this process, E3 ubiquitin ligase plays a major role as the target recognition component responsible for recruiting the target protein and transferring ubiquitin to the substrate. E3 ubiquitin ligase has a large number of multiple isoforms and determines the specificity of the target protein8, 10. E3 ubiquitin ligases are divided into two distinct superfamilies according to whether the structural domain contains a single or multi-subunit. One superfamily comprises the Really Interesting New Gene (RING), Homology to E6-AP Carboxyl Terminus (HECT), and U-box E3 ligases13,14,15,16. The other superfamily is composed of CULLIN4-Damaged-specific DNA binding protein1 (CUL4-DDB1), Anaphase Promoting Complex (APC), and Skp1-Cullin-F-box (SCF) E3 ligases17,18,19. The Arabidopsis genome contains more than 1,400 E3 ubiquitin ligases10. These include approximately 477 RING finger domain-containing E3 U-box ligases, which are subdivided into eight subgroups according to the type of RING domain20. To date, however, the precise function of only a few RING type E3 ligases has been elucidated.
In the present study, we identified the C apsicum a nnuum RING type E3 Ligase 1 gene (CaREL1), which encodes a RING type E3 ligase. We used CaREL1-silenced pepper plants and CaREL1-overexpressing (OX) transgenic Arabidopsis plants to examine the potential roles of CaREL1 in the abiotic stress response. CaREL1-silenced pepper plants exhibited an ABA-hypersensitive phenotype with significantly enhanced tolerance to drought stress. On the other hand, CaREL1-OX Arabidopsis plants exhibited an ABA-insensitive and drought-sensitive phenotype characterized by a high transpiration rate and altered expression levels of stress-related genes. Our findings indicate that the CaREL1 RING type E3 ligase negatively regulates drought stress tolerance via the ABA-signalling pathway.
Results
Isolation and sequence analysis of the CaREL1 gene
The CaREL1 gene was isolated from a cDNA library constructed from ABA-treated pepper leaves by using differential hybridization21. The putative CaREL1 consists of a 663-bp open reading frame encoding 220 amino acid residues with an isoelectric point (pI) of 4.52 and a calculated molecular mass of 24.3 kDa (Fig. 1a). Multiple sequence alignment analysis revealed high amino acid sequence identity between CaREL1 (accession no. KU557246) and RING finger proteins of Solanum lycopersicum (accession no. XP_004245586.1, 89.6% identity), Solanum tuberosum (accession no. XP_006343956.1, 89.2% identity), Nicotiana tomentosiformis (accession no. XP_009591218.1, 84.2% identity), Vitis vinifera (accession no. XP_002280000.1, 52.8% identity), Malus domestica (accession no. XP_008392592.1, 49.3% identity), and Arabidopsis thaliana (accession no. NP_568590.1, 43.4% identity) (Fig. 1a and b). All of these proteins have a highly conserved C3H2C3 type RING finger domain (Fig. 1c). The RING domain of CaREL1 contains eight conserved cysteine and histidine residues, which are necessary for E3 ubiquitin ligase activity9, 22 (Fig. 1c).
Amino acid sequence analysis of the pepper CaREL1 (C apsicum a nnuum RING type E3 Ligase 1) protein. (a) Comparisons of the deduced amino acid sequence of the CaREL1 protein (accession no. KU557246) with those of the Solanum lycopersicum (accession no. XP_004245586.1), Solanum tuberosum (accession no. XP_006343956.1), Nicotiana tomentosiformis (accession no. XP_009591218.1), Vitis vinifera (accession no. XP_002280000.1), Malus domestica (accession no. XP_008392592.1), and Arabidopsis thaliana (accession no. NP_568590.1) proteins. Identical amino acid residues are highlighted in black, and the box reveals the Really Interesting New Gene (RING) zinc finger domain. (b) Phylogenetic tree analysis of the CaREL1 protein. Blast search was performed using the deduced amino acid sequences of the CaREL1 gene, and the top-ranked sequences from each plant species were collected. (c) Alignment of the RING zinc finger C3H2C3-type domain. Asterisks indicate conserved cysteine (C) and histidine (H) residues.
Subcellular localization and in vitro ubiquitination of CaREL1
To analyse the subcellular localization of the CaREL1 protein in plant cells, we fused the reporter gene of the green fluorescent protein (GFP) to the CaREL1 coding region under the control of 35S promoter, to produce 35S:CaREL1-GFP fusion. The 35S:CaREL1-GFP fusion protein generated GFP signals in the cytoplasm and nucleus of Nicotiana benthamiana epidermal cells (Fig. 2a). We used DAPI staining as a control and observed blue signals in the nucleus. Our results suggest that the CaREL1 protein functions in the cytoplasm and nucleus.
CaREL1 is localized at nucleus and has E3 ligase activity. (a) Subcellular localization of the 35S:CaREL1-GFP fusion protein in Nicotiana benthamiana epidermal cells. The 35S:CaREL1-GFP construct was expressed via agroinfiltration of N. benthamiana leaves and observed using a confocal laser-scanning microscope. DAPI staining was used as a marker for the nucleus. The scale bar represents 20 μm. (b) Auto-ubiquitination of CaREL1. In the presence of ubiquitin, E1 (UbE1), and E2 (UBHPC H5b), maltose-binding protein (MBP)–CaREL1 fusion proteins displayed E3 ubiquitin ligase activity. Detection of MBP–CaREL1 auto-ubiquitination. MBP–CaREL1C205S/C208S was loaded as negative control. MBP–CaREL1 fusion proteins were detected using MBP and ubiquitin antibodies; shifted bands indicate the attachment of ubiquitin molecules.
The CaREL1 protein contains a C3H2C3-type RING domain, which has E3 ligase activity7, 23, 24. We investigated whether CaREL1 has E3 ligase activity by conducting an in vitro self-ubiquitination assay with maltose-binding protein tagged CaREL1 (MBP::CaREL1). The purified MBP::CaREL1 fusion protein was incubated with or without ubiquitin, E1, and E2 and separated using SDS-PAGE gel. The ubiquitinated protein was detected using western blot analysis with anti-MBP and anti-ubiquitin antibodies (Fig. 2b). In the presence of ubiquitin, E1, and E2 enzymes, we detected additional higher molecular-weight bands. However, a catalytic dead mutant of CaREL1, CaREL1C205S/C208S, did not show self-ubiquitination activity. These data indicate that CaREL1 has E3 ligase activity.
Induction of the CaREL1 gene in pepper tissues
We performed RT-PCR analysis to determine whether the CaREL1 gene is constitutively expressed in different tissues of pepper plants (Supplementary Fig. S1). CaREL1 was expressed in root, stem, leaf, and flower tissues; moreover, high transcript levels were found in root and flower tissues. CaREL1 was isolated from drought-treated leaves; hence, we examined whether the expression of this gene is induced by abiotic stresses such as ABA and drought (Fig. 2c). In ABA-treated leaf tissues, CaREL1 expression was not upregulated at 0–12 h; however, high transcript levels were detected at 24 h. Moreover, the steady-state levels of CaREL1 transcripts were slightly upregulated by drought treatment. Our results indicate that CaREL1 functions in the ABA-signalling and drought stress responses.
ABA hypersensitivity and enhanced drought tolerance of CaREL1-silenced pepper plants
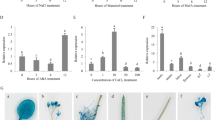
To investigate the response of CaREL1 to abiotic stress, we used a virus-induced gene silencing (VIGS) system with the tobacco rattle virus (TRV) vector to analyse loss-of-function CaREL1 mutants25. CaREL1 transcript accumulation was approximately 50% lower in CaREL1-silenced pepper plants than in control plants (Supplementary Fig. S2). Abiotic stress and ABA signals share common signal transduction components; however, drought stress signalling has ABA-dependent and ABA-independent pathways26. CaREL1 transcripts were accumulated after ABA and drought stress treatments, suggesting that CaREL1 functions in stress-induced signalling. Thus, we compared the ABA sensitivity of CaREL1-silenced pepper plants with that of control plants (Fig. 3a–e). First, we measured the leaf temperature—as an indication of stomatal aperture—in fully expanded leaves of control and CaREL1-silenced pepper plants after exposure to 50 μM ABA (Fig. 3a and c). CaREL1-silenced pepper plants had higher leaf temperatures than control plants. Stomatal opening leads to an increase in evaporative cooling and therefore to decrease in leaf temperatures27, 28; thus, we predicted that ABA-treated CaREL1-silenced pepper plants would show increased stomatal closure relative to control plants. We exposed control and CaREL1-silenced pepper plants to 20 μM ABA for 3 h and measured the stomatal pore sizes. Consistent with the leaf temperatures, the stomatal apertures were smaller in CaREL1-silenced pepper plants than in control plants (Fig. 3d and e).
Enhanced tolerance of CaREL1-silenced pepper plants to drought stress. (a) qRT-PCR analysis of CaREL1 gene expression in pepper leaves 0–24 h after treatment with 50 μM abscisic acid (ABA) or drought. (b and c) Increased leaf temperatures of CaREL1-silenced pepper plants in response to ABA treatment. Leaf temperatures after treatment with 50 μM ABA were measured using thermal imaging and representative images were taken (b). Data represent the mean ± standard error of three independent experiments (c). (d and e) Stomatal apertures in control and CaREL1-silenced pepper plants treated with ABA. Leaf peels were harvested from 4-week-old plants of each line and incubated in SOS buffer containing 0 μM or 20 μM ABA. Stomatal apertures were measured under the microscope and representative images were taken (d). Data represent the mean ± standard error of three independent experiments (e). (f) The drought-tolerant phenotype of CaREL1-silenced pepper plants. Control and CaREL1-silenced pepper plants were grown in pots for 6 weeks under well-watered conditions. Thereafter, watering was withheld for 11 days, followed by re-watering for 2 days. (g) Survival rates of control and CaREL1-silenced pepper plants after 2 days of re-watering. Data represent the mean ± standard error of three independent experiments, each evaluating 20 plants. (h) Transpirational water loss from the leaves of empty vector control and CaREL1-silenced pepper plants at various times after detachment of leaves.
Under well-watered conditions, we observed no phenotypic differences between CaREL1-silenced pepper plants and control plants (Fig. 3f, left panel). However, when plants were subjected to drought stress by withholding watering for 11 days, followed by re-watering for 2 days, the CaREL1-silenced pepper plants exhibited a drought-tolerant phenotype (Fig. 3f, middle and right panels). Moreover, after re-watering, 87.5% of the CaREL1-silenced pepper plants resumed their growth, whereas the survival rate of control plants was only 37.5% (Fig. 3f). Hence, CaREL1 expression led to enhanced drought tolerance. To ascertain whether the enhanced drought tolerance of CaREL1-silenced pepper plants was caused by increased water retention in leaf tissues, we monitored the transpirational water loss by measuring the fresh weight of leaves 8 h after detachment (Fig. 3g). Consistent with the drought-tolerant phenotype, the leaf water content was higher in CaREL1-silenced pepper than in control plants. Our results indicate that the enhanced drought tolerance of CaREL1-silenced pepper was derived from an increase in water retention capacity owing to ABA hypersensitivity.
ABA hyposensitivity of CaREL1-OX plants
To further examine the in vivo function of CaREL1, we generated Arabidopsis transgenic plants constitutively expressing CaREL1 (CaMV 35S:CaREL1). We performed RT-PCR analysis to examine the expression levels of two independent T3 homozygous transgenic progeny (CaREL1-OX) (Fig. 4a). Under normal growth conditions, we observed no phenotypic differences between CaREL1-OX plants and wild-type plants, suggesting that conferred expression of CaREL1 does not influence normal growth and development (Figs 4 and 5). To investigate whether conferred expression of CaREL1 affects the ABA response, we analysed the ABA sensitivity of wild-type and CaREL1-OX plants during the germination and seedling stages (Fig. 4). We germinated seeds on growth media supplemented with various ABA concentrations (0.0 μM, 0.5 μM, 0.75 μM, or 1.0 μM). In the absence of ABA, we determined no significant differences in germination rates between wild-type and CaREL1-OX seeds (Fig. 4b). In the presence of 0.5 μM ABA, wild-type and CaREL1-OX seeds showed germination rates of almost 100% after 5 days; however, CaREL1-OX seeds germinated faster than wild-type seeds. These differences were more distinguishable in the presence of higher ABA concentrations (Fig. 4b). To analyse the ABA response at the seedling stage, we measured seedling establishment and root growth in the presence of various ABA concentrations (Fig. 4c–f). After 5 days, seedling establishment was higher in CaREL1-OX plants than in wild-type plants (Fig. 4c and d). In addition, primary root growth of CaREL1-OX plants was less impaired than that of wild-type plants (Fig. 4e and f). In the absence of ABA, we determined no significant differences in the primary root lengths between wild-type and CaREL1-OX plants. However, in the presence of various ABA concentrations, the primary roots of CaREL1-OX plants were significantly longer than those if wild-type plants. Our data indicate that altered expression of CaREL1 affects ABA sensitivity in Arabidopsis.
Reduced sensitivity of CaREL1-OX transgenic Arabidopsis lines to ABA. (a) Reverse transcription-polymerase chain reaction (RT-PCR) analysis of CaREL1 expression in wild-type and CaREL1-OX plants. Actin8 was used as an internal control gene. (b) Seed germination of wild-type and CaREL1-OX plants in response to ABA for 0–5 days after treatment. Seeds were germinated on 0.5× MS agar plates containing 0.0 μM, 0.5 μM, 0.75 μM, or 1.0 μM ABA. (c) Growth of wild-type and CaREL1-OX seedlings on 0.5× MS agar plates containing 0.0 μM, 0.5 μM, 0.75 μM, or 1.0 μM ABA. Representative photographs were taken 10 days after plating. (d) Quantification of green cotyledons in the wild-type and each mutant line was performed 10 days after plating on 0.5× MS agar plates containing 0.0 μM, 0.5 μM, 0.75 μM, or 1.0 μM ABA. Data represent the mean ± standard error of three independent experiments, each evaluating 30 seeds. (e) Root elongation of wild-type and transgenic plants in response to ABA. (f) The root length of each plant was measured 10 days after plating. Data represent the mean ± standard error of three independent experiments. Different letters indicate significant differences between wild-type and transgenic lines (P < 0.05; ANOVA followed by Fisher’s LSD test).
Reduced tolerance of CaREL1-OX plants to drought stress. (a) Drought tolerance of CaREL1-OX transgenic plants. Three-week-old wild-type and transgenic plants were subjected to drought stress by withholding water for 9 days and then re-watering for 2 days. Survival rates of plants were measured after re-watering. (b) Reduced biomass of CaREL1-OX plants. The plant biomass was measured after re-watering. (c) Transpirational water loss from the leaves of wild-type and transgenic plants at various times after detachment of leaves. (d and e) Decreased leaf temperatures of CaREL1-OX plants in response to ABA treatment. Leaf temperatures of plants treated with 50 μM ABA were measured using thermal imaging and representative images were taken (d). Data represent the mean ± standard error of three independent experiments (e). (f and g) Stomatal apertures in wild-type and CaREL1-OX plants treated with ABA. Leaf peels were harvested from 3-week-old plants of each line and incubated in SOS buffer containing 0 μM or 20 μM ABA. Stomatal apertures were measured under the microscope and representative images were taken (e). Data represent the mean ± standard error of three independent experiments (f), each evaluating 20 plants. Different letters indicate significant differences between wild-type and transgenic lines (P < 0.05; ANOVA followed by Fisher’s LSD test).
Reduced drought tolerance of CaREL1-OX plants
CaREL1-silenced pepper plants and CaREL1-OX Arabidopsis plants showed drought-tolerant and ABA-hyposensitive phenotypes, respectively (Figs 3 and 4); hence, we predicted that CaREL1-OX plants would show an altered drought stress response. We compared the phenotypes of CaREL1-OX plants in response to drought and ABA stresses. Under favourable growth conditions, we observed no phenotypic differences between CaREL1-OX and wild-type plants (Fig. 5a, left panel). However, when watering was withheld for 9 days, followed by re-watering for 2 days, CaREL1-OX plants displayed a more wilted phenotype than wild-type plants (Fig. 5a, middle and right panels). We measured the survival rates of CaREL1-OX and wild-type plants after re-watering and found that 85% of wild-type plants survived, whereas the survival rates of CaREL1-OX #2 and CaREL1-OX #4 plants were only 20% and 35%, respectively (Fig. 5a). Moreover, the reduction in biomass was higher in transgenic plants than in wild-type plants (Fig. 5b). Hence, conferred expression of CaREL1 contributes to the impaired drought tolerance of CaREL1-OX plants. To examine whether the altered phenotype exhibited by CaREL1-OX plants in response to drought stress is derived from reduced capacity for water retention, we monitored the transpirational water loss by measuring the fresh weight of detached leaves (Fig. 5c). Consistent with the drought-sensitive phenotype, the leaf water content was lower in the leaf tissues of CaREL1-OX plants than in those of wild-type plants.
Several studies have suggested that increased stomatal closure in response to ABA leads to a reduction in water loss via decreased transpiration. Therefore, we examined whether conferred expression of CaREL1 influences ABA-mediated stomatal closure. First, we exposed wild-type and transgenic plants to 50 μM ABA and measured the leaf temperatures. After 3 h, the leaf temperatures of CaREL1-OX plants were lower than those of wild-type plants (Fig. 5d and e). Next, we measured the stomatal apertures in the presence and absence of ABA treatment (Fig. 5f and g). In the absence of ABA treatment, the stomatal apertures did not differ significantly between wild-type and CaREL1-OX plants. However, after 4 h of exposure to 20 μM ABA, the stomatal apertures were larger in the leaves of transgenic plants than in the leaves of wild-type plants. Our results indicate that hyposensitivity to ABA in the guard cells of CaREL1-OX plants may lead to decreased water retention and therefore reduced drought tolerance.
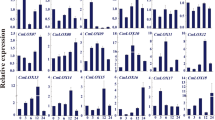
The expression of stress-responsive genes is associated with stress tolerance. Hence, we examined whether several drought-inducible genes are directly or indirectly regulated by CaREL1. We subjected wild-type and CaREL1-OX plants to dehydration stress and performed qRT-PCR analysis of leaves 3 h after detachment (Fig. 6). Induction of NCED3, which is associated with ABA synthesis, was significantly higher in the leaves of CaREL1-OX plants than in the leaves of wild-type plants. In addition, we observed lower accumulation of various stress-responsive genes, including DREBA, RAB18, RD20, RD29B, RD29A, and KIN2, in the leaves of CaREL1-OX plants than in the leaves of wild-type plants. Our data indicate that the expression level of CaREL1 not only influences ABA biosynthesis, but is also involved in modulating the response to drought stress. CaREL1 may function as a negative regulator of the drought stress response via ABA-dependent signalling. Thus, we examined whether the drought stress-responsive function of CaREL1 is ABA-dependent or ABA-independent, by measuring the expression levels of ABI1 and ERD1 in the leaves of wild-type and CaREL1-OX plants. Previous studies showed that ABI1 and ERD1 function in the drought stress response via ABA-dependent and ABA-independent pathways, respectively2, 29. Three hours after detachment, the levels of ABI1 transcripts were significantly lower in the leaves of transgenic plants than in the leaves of wild-type plants; however, the levels of ERD1 transcripts did not differ significantly between the leaves of transgenic and wild-type plants (Fig. 6). Our data indicate that CaREL1 expression negatively influences the expression levels of drought-inducible marker genes via the ABA-dependent pathway, and this contributes to the reduced drought tolerance of CaREL1-OX plants.
Expression of drought stress-responsive genes in CaREL1-OX plants. CaREL1-OX plants were exposed to drought stress and the expression levels of ABA-dependent stress-related genes were measured 3 h after detachment using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis. The relative expression levels (∆∆CT) of each gene were normalized to the geometric mean of Actin8 as an internal control gene. Data represent the mean ± standard error of three independent experiments. Different letters indicate significant differences between wild-type and transgenic lines (P < 0.05; ANOVA followed by Fisher’s LSD test).
The expression level of NCED3, which is associated with ABA biosynthesis, was significantly higher in the leaves of CaREL1-OX plants than in the leaves of wild-type plants. To ascertain whether overexpression of CaREL1 is associated with ABA biosynthesis, we measured the ABA contents in the leaves of wild-type and CaREL1-OX plants after exposure to drought stress (Fig. 7). Consistent with the NCED3 expression level, the ABA content was higher in the leaves of CaREL1-OX plants than in the leaves of wild-type plants.
Discussion
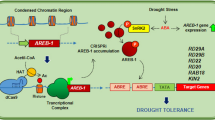
Recently, several studies have reported that ABA-signalling components, which function as positive or negative regulators of abiotic stress responses, are ubiquitinated and degraded by E3 ubiquitin ligase and 26S proteasome, respectively30, 31. In the present study, we have demonstrated that CaREL1 functions as a negative regulator of ABA sensitivity and drought tolerance. CaREL1 contains a RING-zinc finger domain in the C-terminal region; this domain is highly homologous with other C3H2C3 type RING E3 ubiquitin ligases found in diverse species (Fig. 1), including pepper (Supplementary Fig. S3). The presence of the RING finger domain in CaREL1 implies that this protein ubiquitinates target proteins. Ubiquitination is a post-translational modification process in eukaryotes, and it plays a key role in the recognition of and response to various hormone signals by controlling the protein stability8, 32, 33. To date, RING type E3 ligases have been associated with transferring ABA signals24, 30, 34, 35.
The expression of CaREL1 was induced by ABA and drought stress (Fig. 2c), suggesting that this protein functions in the response to ABA-mediated drought stress. To determine the biological role of CaREL1 in the ABA and drought stress responses, we generated CaREL1-silenced pepper plants and CaREL1-overexpressing Arabidopsis plants, because the transformation efficiency was very low in pepper plants. Detailed genetic analyses revealed that CaREL1-silenced pepper plants and CaREL1-OX Arabidopsis plants displayed drought-tolerant and drought-sensitive phenotypes, respectively. Moreover, these plants exhibited altered phenotypes in response to ABA. Generally, ABA sensitivity and drought tolerance in plants are determined by at least one cellular and molecular parameters, such as marker gene expression and stomatal aperture. Several studies have measured stomatal movement and established that high ABA sensitivity leads to increased drought tolerance21, 25. Consistent with the results of our phenotypic analysis in response to drought stress, CaREL1-silenced pepper plants and CaREL1-overexpressing Arabidopsis plants exposed to various ABA concentrations showed small and large stomatal pore sizes, respectively. Our data imply that the in vivo function of CaREL1 is related to the drought stress response, and that this is mediated by ABA-dependent signalling.
In the present study, we were unable to identify the substrate of CaREL1; however, the expression levels of ABA-dependent stress-related genes suggested that CaREL1 is involved in the ABA-dependent drought stress response. The results of a biochemical assay revealed that under drought stress conditions, ABA is more strongly accumulated in CaREL1-OX plants than in wild-type plants. Previous genetic studies have revealed that aba1, aba2, and aba3 (ABA-deficient mutants) exhibited drought- and dehydration-sensitive phenotypes36, 37 characterized by reduced water potential and proline accumulation under water-deficit conditions38. Under these conditions, NCED3 functions in ABA biosynthesis, which is crucial for the drought stress response in plants39. The up-regulation of ABA biosynthesis and NCED3 expression in CaREL1-OX plants might be expected to amplify ABA-dependent defence signalling and therefore contribute to a drought-tolerant phenotype. In addition, NCED3 expression positively modulates the transcription levels of stress-related genes, as well as the ABA level40. However, our genetic analyses revealed that CaREL1-OX plants exhibited a drought-sensitive phenotype characterized by ABA hyposensitivity (Figs 4 and 5) and decreased expression levels of ABA-dependent stress-related genes (Fig. 6). These findings cannot fully be explained by higher expression of NCED3 and increased accumulation of ABA in CaREL1-OX plants. However, if CaREL1 is located upstream of ABA-dependent stress-related genes and inhibits defence response to drought stress mediated by these genes, the drought-sensitive phenotype of CaREL1-OX plants can be explained. Moreover, CaREL1-OX plants are unable to alleviate drought stress; hence, CaREL1-OX plants presumably recognize being in a stressful condition, leading to NCED3 gene expression and ABA biosynthesis.
In conclusion, our results demonstrate that CaREL1 functions as a negative regulator of drought stress via altered sensitivity to ABA, as evidenced by changes in leaf temperature and stomatal apertures. We have demonstrated the involvement of CaREL1 in the defence responses to several abiotic stresses; however, the mechanism by which CaREL1 functions as a negative regulator of abiotic stress responses remains unclear. To elucidate the precise in vivo function of CaREL1 in response to abiotic stress, further studies to identify the substrates of this E3 ligase are required.
Methods
Plant materials
Seeds of pepper (Capsicum annuum L., cv. Hanbyul) and tobacco (Nicotiana benthamiana) were sown in a steam-sterilized compost soil mix (peat moss, perlite, and vermiculite, 5:3:2, v/v/v), sand, and loam soil (1:1:1, v/v/v). The pepper plants were raised in a growth room at 27 ± 1 °C under white fluorescent light (80 μmol photons·m−2·s−1; 16 h per day) as described previously41. The tobacco plants were maintained in a growth chamber at 25 ± 1 °C under a 16-h light/8-h dark cycle. Arabidopsis thaliana (ecotype Col-0) seeds were germinated on Murashige and Skoog (MS) salt (Duchefa Biochemie, Haarlem, Netherlands) supplemented with 1% sucrose and Microagar (Duchefa Biochemie); the seeded plates were incubated in a growth chamber at 24 °C under a 16-h light/8-h dark cycle. The Arabidopsis seedlings were maintained in a steam-sterilized compost soil mix (peat moss, perlite, and vermiculite, 9:1:1, v/v/v) under controlled environmental conditions as follows: 24 °C and 60% relative humidity under fluorescent light (130 μmol photons·m−2·s−1) with a 16-h light/8-h dark cycle. All seeds were vernalized at 4 °C for 2 days before being placed in the growth chamber.
Sequence alignment and phylogenetic tree analysis
The encoded amino acid sequences for CaREL1 and its homologs were obtained using BLAST searches (http://www.ncbi.nlm.nih.gov/BLAST). The amino acid alignment was performed using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2), and the results were edited using Genedoc software (http://www.nrbsc.org/gfx/genedoc). The amino acid alignments were manually regulated to compare the cDNA clones of CaREL1 with those of other organisms. Based on the multiple sequence alignment data, a phylogenetic tree was drawn with MEGA software (version 5.2). To investigate sequence identity and similarity between two proteins, pairwise sequence alignment was performed using EMBOSS Needle webtool (http://www.ebi.ac.uk/Tools/psa/emboss_needle) with default parameters.
Virus-induced gene silencing and overexpression of CaREL1
We used the tobacco rattle virus (TRV)-based virus-induced gene silencing (VIGS) system to generate CaREL1 gene knockdown in pepper plants. We used the full-length CaREL1 cDNA to generate CaREL1-overexpressing (OX) transgenic Arabidopsis plants, according to the protocol described previously42.
ABA and drought treatments in pepper leaves
To examine the expression level of CaREL1 in pepper leaves after ABA treatment, six-leaf-stage pepper plants were sprayed with 50 μM ABA. For the drought treatment, pepper plants were carefully removed from the soil to avoid injury. The plants were placed onto 3-mm filter paper (Whatman, Clifton, UK). Leaves were harvested 0–24 h after treatment and were subjected to RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR) analysis.
To measure the rate of germination, root elongation, and seedling establishment, 36 seeds each of wild-type and CaREL1-OX transgenic Arabidopsis plants were stratified at 4 °C for 3 days and were then plated on 0.5× MS agar medium supplemented with various concentrations of ABA. The plates were incubated at 24 °C under white fluorescent light (130 μmol photons·m−2·s−1) with a 16-h light/8 h-dark cycle.
Three-week-old wild-type and CaREL1-OX seedlings were randomly planted and were then subjected to drought stress by withholding watering for 9 days and re-watering for 2 days. Survival rates were measured in each individual sample, and each experiment was performed three times with 30 plants. For pepper plants, drought stress was imposed on four-leaf-stage plants by withholding watering for 11 days. Plants were re-watered for 2 days to allow recovery, and the survival rate of the plants was then calculated. Survival rates were measured in each individual sample, and each experiment was performed three times with 20 plants. The drought resistance was determined in a quantitative manner by measuring the transpirational water loss. Fifty leaves were detached from four-leaf-stage pepper plants and 3-week-old Arabidopsis plants and placed in Petri dishes. The dishes were maintained in a growth chamber at 40% relative humidity, and the loss of fresh weight was determined at the indicated time points. All the experiments were performed at least in triplicate.
Thermal imaging
For thermal imaging analysis, 4-week-old pepper plants having full expanded first and second leaves and 3-week-old Arabidopsis plants were treated with 50 μM ABA. Thermal images were obtained using an infrared camera (FLIR systems; T420) and the leaf temperature was measured with FLIR Tools+ ver 5.2 software.
Stomatal aperture
To measure the stomatal aperture, epidermal peels were stripped from rosette leaves of 3-week-old plants and floated in a stomatal opening solution (SOS: 50 mM KCl and 10 mM MES-KOH, pH 6.15, 10 µM CaCl2) in the light. After incubation for 3 h, the buffer was replaced with fresh SOS containing 10 μM or 20 μM ABA. After an additional 2 h of incubation, stomatal apertures were measured in each individual sample, and each experiment was performed three times with 20 leaves.
RNA isolation and semi-quantitative and quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated from the Arabidopsis leaf tissues, which were dehydrated or infected with the bacterial pathogen using an RNeasy Mini kit (Qiagen, Valencia, CA, USA). To remove genomic DNA, all RNA samples were digested with RNA-free DNase. After quantification using a spectrophotometer, 1 μg of total RNA was used to synthesize cDNA using a Transcript First Strand cDNA Synthesis kit (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions. Concomitantly, cDNAs were synthesized without reverse transcriptase and were subjected to semi-quantitative RT-PCR to rule out the possibility of contamination by genomic DNA in the cDNA samples. For quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis, the synthesized cDNA was amplified in a CFX96 TouchTM Real-Time PCR detection system (Bio-Rad) with iQTM SYBR Green Supermix and specific primers (Supplementary Table S1). Every reaction was performed in triplicate. The PCR was programmed as follows: 95 °C for 5 min; 45 cycles each at 95 °C for 20 s and 60 °C for 20 s; and 72 °C for 20 s. The relative expression of each gene was calculated using the ∆∆Ct method, as described previously43. The Arabidopsis actin8 gene (AtACT8) was used for normalization.
In vitro ubiquitination
The procedure for the expression and purification of the maltose-binding protein (MBP)–CaREL1 recombinant protein is described in Park et al.42. For the in vitro ubiquitination assay, the purified MBP–CaREL1 (500 ng) was mixed with ubiquitination reaction buffer [50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 0.05 mM ZnCl2, 1 mM Mg-ATP, 0.2 mM DTT, 10 mM phosphocreatine, and 0.1 unit of creatine kinase (Sigma-Aldrich)] containing 250 ng of recombinant human UBE1 (Boston Biochemicals, Cambridge, MA, USA), 250 ng of recombinant human H5b (Enzo Life Sciences, Farmingdale, NY), and 10 μg of bovine ubiquitin (Sigma-Aldrich). After incubation at 30 °C for 3 h, the reacted proteins were separated using SDS-PAGE and analysed using immunoblotting with anti-ubiquitin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-MBP antibody (New England Biolabs, Ipswich, MA).
Measurement of ABA content
For determination of ABA content, leaves were harvested from pepper and Arabidopsis plants treated with dehydration for 2 and 4 h and immediately frozen in liquid nitrogen. Approximately 50 mg of ground tissue were extracted overnight in 1 ml of ABA extraction buffer (methanol, containing 100 mg butylated hydroxyl toluene, 0.5 g citric acid monohydrate) at 4 °C on a rotary shaker. After centrifuged at 1500 g, the supernatant was transferred to new tube and dried using a speed vac. ABA content of each sample was quantified by using the Phytodetek-ABA kit (Agdia Inc., Elkhart, IN, USA) according to manufacturer’s instruction. ABA contents were expressed as pmol mg−1 fresh weight of the tissue.
Statistical analyses
To determine significant differences between different plant lines in response to treatments, statistical analyses were performed using one way analysis of variance (ANOVA) or student’s t-test. A P value of <0.05 was considered significant.
References
Lim, C. W., Luan, S. & Lee, S. C. A prominent role for RCAR3-mediated ABA signaling in response to Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis. Plant Cell Physiol 55, 1691–1703 (2014).
Lee, S. C. & Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ 35, 53–60 (2012).
Golldack, D., Li, C., Mohan, H. & Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front Plant Sci 5, 151 (2014).
Kilian, J. et al. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50, 347–363 (2007).
Moon, J., Parry, G. & Estelle, M. The ubiquitin-proteasome pathway and plant development. Plant Cell 16, 3181–3195 (2004).
Dreher, K. & Callis, J. Ubiquitin, hormones and biotic stress in plants. Ann Bot 99, 787–822 (2007).
Lee, D. H., Choi, H. W. & Hwang, B. K. The pepper E3 ubiquitin ligase RING1 gene, CaRING1, is required for cell death and the salicylic acid-dependent defense response. Plant Physiol 156, 2011–2025 (2011).
Sadanandom, A., Bailey, M., Ewan, R., Lee, J. & Nelis, S. The ubiquitin-proteasome system: central modifier of plant signalling. New Phytol 196, 13–28 (2012).
Kim, J. H. & Kim, W. T. The Arabidopsis RING E3 ubiquitin ligase AtAIRP3/LOG2 participates in positive regulation of high-salt and drought stress responses. Plant Physiol 162, 1733–1749 (2013).
Vierstra, R. D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol 10, 385–397 (2009).
Ciechanover, A. & Schwartz, A. L. The ubiquitin-proteasome pathway: the complexity and myriad functions of proteins death. Proc Natl Acad Sci USA 95, 2727–2730 (1998).
Yin, X. J. et al. Ubiquitin lysine 63 chain forming ligases regulate apical dominance in Arabidopsis. Plant Cell 19, 1898–1911 (2007).
Hatakeyama, S., Yada, M., Matsumoto, M., Ishida, N. & Nakayama, K. I. U box proteins as a new family of ubiquitin-protein ligases. J Biol Chem 276, 33111–33120 (2001).
Marin, I. Evolution of plant HECT ubiquitin ligases. PLoS One 8, e68536 (2013).
Kim, S. J. & Kim, W. T. Suppression of Arabidopsis RING E3 ubiquitin ligase AtATL78 increases tolerance to cold stress and decreases tolerance to drought stress. FEBS Lett 587, 2584–2590 (2013).
Miao, Y. & Zentgraf, U. A HECT E3 ubiquitin ligase negatively regulates Arabidopsis leaf senescence through degradation of the transcription factor WRKY53. Plant J 63, 179–188 (2010).
Zheng, N. et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 416, 703–709 (2002).
Chang, L., Zhang, Z., Yang, J., McLaughlin, S. H. & Barford, D. Molecular architecture and mechanism of the anaphase-promoting complex. Nature 513, 388–393 (2014).
Pazhouhandeh, M., Molinier, J., Berr, A. & Genschik, P. MSI4/FVE interacts with CUL4-DDB1 and a PRC2-like complex to control epigenetic regulation of flowering time in Arabidopsis. Proc Natl Acad Sci USA 108, 3430–3435 (2011).
Stone, S. L. et al. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol 137, 13–30 (2005).
Lim, S., Baek, W. & Lee, S. C. Identification and functional roles of CaDIN1 in abscisic acid signaling and drought sensitivity. Plant Mol Biol 86, 513–525 (2014).
Ding, S., Zhang, B. & Qin, F. Arabidopsis RZFP34/CHYR1, a ubiquitin E3 ligase, regulates stomatal movement and drought tolerance via SnRK2.6-mediated phosphorylation. Plant Cell 27, 3228–3244 (2015).
Ryu, M. Y., Cho, S. K. & Kim, W. T. The Arabidopsis C3H2C3-type RING E3 ubiquitin ligase AtAIRP1 is a positive regulator of an abscisic acid-dependent response to drought stress. Plant Physiol 154, 1983–1997 (2010).
Zhang, H. et al. The RING finger ubiquitin E3 ligase SDIR1 targets SDIR1-INTERACTING PROTEIN1 for degradation to modulate the salt stress response and ABA signaling in Arabidopsis. Plant Cell (2015).
Lim, C. W. & Lee, S. C. Functional roles of the pepper MLO protein gene, CaMLO2, in abscisic acid signaling and drought sensitivity. Plant Mol Biol 85, 1–10 (2014).
Jakab, G. et al. Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol 139, 267–274 (2005).
Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M. & Waner, D. Guard cell signal transduction. Annu Rev Plant Phys 52, 627–658 (2001).
Park, S. Y. et al. Agrochemical control of plant water use using engineered abscisic acid receptors. Nature 520, 545–548 (2015).
Babula-Skowronska, D. et al. Involvement of genes encoding ABI1 protein phosphatases in the response of Brassica napus L. to drought stress. Plant Mol Biol 88, 445–457 (2015).
Bueso, E. et al. The single-subunit RING-type E3 ubiquitin ligase RSL1 targets PYL4 and PYR1 ABA receptors in plasma membrane to modulate abscisic acid signaling. Plant J 80, 1057–1071 (2014).
Kong, L. et al. Degradation of the ABA co-receptor ABI1 by PUB12/13 U-box E3 ligases. Nat Commun 6, 8630 (2015).
Lyzenga, W. J., Booth, J. K. & Stone, S. L. The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1-carboxylate synthase 7. Plant J 71, 23–34 (2012).
Cho, S. K., Chaabane, S. B., Shah, P., Poulsen, C. P. & Yang, S. W. COP1 E3 ligase protects HYL1 to retain microRNA biogenesis. Nat Commun 5, 5867 (2014).
Gao, W., Liu, W., Zhao, M. & Li, W. X. NERF encodes a RING E3 ligase important for drought resistance and enhances the expression of its antisense gene NFYA5 in Arabidopsis. Nucleic Acids Res 43, 607–617 (2015).
Irigoyen, M. L. et al. Targeted degradation of abscisic acid receptors is mediated by the ubiquitin ligase substrate adaptor DDA1 in Arabidopsis. Plant Cell 26, 712–728 (2014).
Tuteja, N. Abscisic Acid and abiotic stress signaling. Plant Signal Behav 2, 135–138 (2007).
Maia, J., Dekkers, B. J., Dolle, M. J., Ligterink, W. & Hilhorst, H. W. Abscisic acid (ABA) sensitivity regulates desiccation tolerance in germinated Arabidopsis seeds. New Phytol 203, 81–93 (2014).
Verslues, P. E. & Bray, E. A. Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J Exp Bot 57, 201–212 (2006).
Urano, K. et al. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant Journal 57, 1065–1078 (2009).
Iuchi, S. et al. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27, 325–333 (2001).
Lee, S. C., Hwang, I. S., Choi, H. W. & Hwang, B. K. Identification and functional expression of the novel pepper antimicrobial protein, CaAMP1 enhances broad-spectrum disease resistance in transgenic Arabidopsis. Plant Physiol 148, 1004–1020 (2008).
Park, C., Lim, C. W., Baek, W. & Lee, S. C. RING type E3 ligase CaAIR1 in pepper acts in the regulation of ABA signaling and drought stress response. Plant Cell Physiol 56, 1808–1819 (2015).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Acknowledgements
This work was supported by a grant from the “Next-Generation BioGreen 21 Program for Agriculture & Technology Development (Project No. PJ01101001),” by the Rural Development Administration, and by the Research Foundation of Korea funded by the government of the Republic of Korea (NRF-2015R1A2A2A01002674) and this research was supported by the Chung-Ang University Graduate Research Scholarship in 2016.
Author information
Authors and Affiliations
Contributions
C.W.L., C.P., J.-H.K., H.J. and E.H. performed the experiments and analysed the results. S.C.L. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lim, C.W., Park, C., Kim, JH. et al. Pepper CaREL1, a ubiquitin E3 ligase, regulates drought tolerance via the ABA-signalling pathway. Sci Rep 7, 477 (2017). https://doi.org/10.1038/s41598-017-00490-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00490-4
This article is cited by
-
MfbHLH38, a Myrothamnus flabellifolia bHLH transcription factor, confers tolerance to drought and salinity stresses in Arabidopsis
BMC Plant Biology (2020)
-
Characterization of two novel drought responsive genes in wheat
Journal of Plant Biochemistry and Biotechnology (2020)
-
Characterization on the water deprivation-associated physiological traits as well as the related differential genes during seed filling stage in wheat (T. aestivum L.)
Plant Cell, Tissue and Organ Culture (PCTOC) (2020)
-
Association mapping identifies loci for canopy temperature under drought in diverse soybean genotypes
Euphytica (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.