Abstract
Transplantation of cultured oral mucosal epithelial cells (OMECs) is a promising treatment strategy for limbal stem cell deficiency. In order to improve the culture method, we investigated the effects of four culture media and tissue harvesting sites on explant attachment, growth, and phenotype of OMECs cultured from Sprague-Dawley rats. Neither choice of media or harvesting site impacted the ability of the explants to attach to the culture well. Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM) and Roswell Park Memorial Institute 1640 medium (RPMI) supported the largest cellular outgrowth. Fold outgrowth was superior from LL explants compared to explants from the buccal mucosa (BM), HP, and transition zone of the lower lip (TZ) after six-day culture. Putative stem cell markers were detected in cultures grown in DMEM and RPMI. In DMEM, cells from TZ showed higher colony-forming efficiency than LL, BM, and HP. In contrast to RPMI, DMEM both expressed the putative stem cell marker Bmi-1 and yielded cell colonies. Our data suggest that OMECs from LL and TZ cultured in DMEM give rise to undifferentiated cells with high growth capacity, and hence are the most promising for treatment of limbal stem cell deficiency.
Similar content being viewed by others
Introduction
The integrity of the outermost layer of the cornea, the epithelium, is dependent on stem cells located in the corneal periphery, the limbus. These stem cells can be damaged by a number of diseases, but also external factors, such as those causing chemical and thermal burns. In limbal stem cell deficiency (LSCD), the cornea can become opaque and painful. Since 1997, LSCD has been treated successfully by transplanting cultured limbal epithelial stem cells from donors1,2,3. In bilateral LSCD, limbal tissue can be provided from a relative or a deceased individual, however, any non-autologous source requires prolonged immunosuppressive treatment.
To avoid the risks associated with prolonged use of immunosuppressants, numerous non-limbal autologous cell sources have been investigated for the treatment of bilateral LSCD in animal models over the past 13 years4. However, only cultured conjunctival epithelial cells5 and cultured oral mucosal epithelial cells (OMECs)6 have been evaluated in humans. Of these cell sources, OMECs are by far the most extensively studied7. However, the effects of the harvesting site and culture medium for generating an undifferentiated epithelium and sufficient cell growth have not yet been compared. Since 2010, following a study by Rama et al., the degree of differentiation of cultured epithelia for treating LSCD has been a major issue in corneal regenerative medicine3. Rama and colleagues demonstrated that transplantation of undifferentiated limbal epithelial sheets yields significantly better clinical results compared to the use of more differentiated equivalents. Thus, it may be clinically important to determine how the phenotype of cultured OMECs is influenced by the choice of tissue harvesting site and culture medium.
The culture media investigated in this study were chosen for the following reasons: EpiLife is serum-free and has a low calcium-content, which is known to promote an undifferentiated phenotype8, 9; oral keratinocyte medium (OKM) with oral keratinocyte growth supplement, which includes pituitary extract, protects cells from H2O2-induced cell death, DNA fragmentation, and has a positive effect on cell viability10; Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM) and Roswell Park Memorial Institute 1640 medium (RPMI) are among the most commonly used cell culture media. Furthermore, cholera toxin and epidermal growth factors, which are typically added to DMEM, increase the proliferation capacity of oral epithelial cells11.
Although the harvesting site for culture of OMECs has not been described in all studies12,13,14,15,16,17, the most commonly reported harvesting site is the inferior buccal mucosa6, 18,19,20,21,22. However, no studies have compared various harvesting sites for ex vivo expanded OMECs. As the phenotype, degree of keratinization, and morphology of the oral mucosa vary throughout the oral cavity23, 24, we hypothesized that the harvesting site could affect the growth capacity and phenotype of ex vivo expanded OMECs.
In the current study, the effects of harvesting site and culture medium on attachment, growth, and phenotype of cultured OMECs were investigated. We found that OMECs from the lower lip and transition zone of the lower lip cultured in DMEM give rise to undifferentiated cells with high growth capacity, and hence are the most promising for treatment of LSCD.
Methods
EpiLife medium, EpiLife defined growth supplement (EDGS), and trypsin-EDTA were purchased from Life Technologies (Grand Island, NY). Oral keratinocyte medium, oral keratinocyte growth supplement, and penicillin/streptomycin solution (P/S) were obtained from ScienCell Research Laboratory (Carlsbad, CA). Dulbecco’s modified Eagle’s medium/Ham’s F12, insulin, cholera toxin from vibro cholera, and human recombinant epidermal growth factor (EGF) were delivered by Sigma-Aldrich (St. Louis, MO). Roswell Park Memorial Institute medium 1640, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), L-glutamine, non-essential amino acids (NEAA), and sodium pyruvate were obtained from Lonza (Walkersville, MD). Fetal bovine serum (FBS) was purchased from Hyclone Laboratories (Logan, UT). All cell culture and plastic wares were purchased from Thermo Fisher Scientific (Waltham, MA).
Animal
Sprague-Dawley rats were used for the experiments. The Schepens Eye Research Institute (SERI) Animal Care and Use Committee approved the study employing rat oral mucosal tissue. All experiments using the animal were carried out in accordance with the approved guidelines.
Explant Culture
Oral mucosal epithelial cells were obtained from four harvesting sites: hard palate (HP), buccal mucosa (BM), lower lip (LL), and transition zone of the lower lip (TZ) of Sprague-Dawley rats (Fig. 1). The harvested tissue was rinsed three times with phosphate-buffered saline (PBS). The submucosal connective tissue was removed by dissection using forceps, scalpel, and a dissection microscope (Leica ZOOM 200, Leica Microsystems Inc., Buffalo, IL). The tissue samples were cut into 1–3 mm2 explants and immersed in the various media containing antibiotics (50 IU/ml P/S). The explants were transferred to 24-well tissue culture dishes, in which they were seeded with 180 μl of culture medium to allow adhesion whilst still supplying nutrition. The explants attached to the surface within 24 to 48 hours. 500 μl of culture medium was then added to each well to submerge the explants. The culture media used were: (1) EpiLife with EDGS, 50 IU/mL P/S (termed EpiLife in the present study); (2) OKM with oral keratinocyte growth supplement and 50 IU/mL P/S (termed OKM in the present study); (3) DMEM supplemented with 10% FBS, 5 μg/mL insulin, 0.1 nM cholera toxin, 10 ng/mL human recombinant EGF, and 50 IU/mL P/S (termed DMEM in the present study); and (4) RPMI with 10% FBS, 2 mM L-glutamine, 87 μM NEAA solution, 870 μM sodium pyruvate, 8.7 mM HEPES, and 50 IU/mL P/S (termed RPMI in the present study). Explants were grown for either six or 13 days. Cultures were incubated at 37 °C with 5% CO2, and the medium was changed every other day.
Cell Attachment and Outgrowth
For attachment analysis, explants from the four harvesting sites were cultured for 48 hours in culture wells containing 180 μl of EpiLife, OKM, DMEM or RPMI. Explants were considered to be attached if after 48 hours, they were adherent to the culture well surface and did not float in the medium. The percentage of attached explants was calculated by dividing the total number of attached explants by the total number of seeded explants ×100%.
After six and 13 days in culture, the tissue outgrowth was measured for TZ and LL explants. To quantify the outgrowth, cultures were rinsed with PBS, fixed with 100% methanol for 30 minutes, and then rinsed again in PBS. Serial photographs were captured at 40x magnification using a phase contrast light microscope to visualize the entire outgrowth area of the culture. The outgrowth area was then quantified using ImageJ (National Institutes of Health, Bethesda, MD)25. Fold outgrowth was calculated using the following equation:
Immunocytochemistry
Explants cultured for six days were fixed with methanol and incubated for one hour at room temperature in blocking buffer that consisted of 1% bovine serum albumin and 0.2% Triton X-100 dissolved in PBS. After the incubation, the cells were washed with fresh PBS and then incubated with primary antibodies overnight at 4 °C. Antibodies used in the study are listed in Table 1. Following incubation, the cells were washed three times with fresh PBS for 5 minutes on a shaker. Secondary antibodies conjugated to either Cy2 or Cy3 were used for the detection of protein-bound primary antibodies. The cells were incubated for 1 hour with secondary antibodies and then rinsed with PBS before mounting. A drop of photo-bleach protecting mounting media containing a 1:1000 dilution of 4′,6-diamidino-2-phenylindole (DAPI) was applied directly to the culture. A round glass coverslip was then placed on top of the culture before image acquisition. The cultures were visualized with an epifluorescence microscope (Olympus IX51; Center Valley, PA). Negative control consisted of replacing the primary antibodies with PBS. Expression of the markers was assessed by manual counting at 400x magnification. Cells were counted in photomicrographs captured near the explant and at the leading edge to assess if the degree of cell differentiation varied according to the distance from the explant26. Expression of proliferating cell nuclear antigen (PCNA) and nerve growth factor (NGF) p75 were calculated according to the following formula:
The remaining markers Bmi-1 (B lymphoma Mo-MLV insertion region 1 homolog), the transcription factor p63α, pan-cytokeratin (CK), and CK-4 were assessed using a semi-quantitative scoring system: cultures with less than 25% positive cells for the given marker were scored as ‘+’, between 25% and 50% were ‘++’, between 50% and 75% were ‘+++’ and more than 75% were ‘++++’. Cultures without positive cells were scored as ‘0’. Expression of all immunocytochemical markers was assessed by an experienced investigator blinded to the origin of the samples.
Colony-Forming Efficiency Assay
Colony-forming efficiency (CFE) assay was performed to measure the growth capacity of cells from the LL, BM, HP, and TZ. Harvested tissue was exposed to 1.2 U/ml dispase diluted in the respective culture media for 1 hour to separate the epithelium from the basal membrane. The epithelial tissues were then incubated for five minutes in trypsin-EDTA (0.25%) at 37 °C to obtain a single cell suspension. The cells were seeded in six-well plates at a density of 2.5 × 103/cm2. After 12 days of culture in DMEM or RPMI, the colonies were stained with crystal violet solution27. A colony was defined as a group of at least eight contiguous cells28. Colony-forming efficiency was calculated as follows:
The colonies stained with crystal violet solution were captured using a regular paper scanner. To assess the morphology of the cells light microscope was used.
Statistical Analysis
The appropriate statistical test was determined with consideration of sample size and whether the data fitted a normal distribution or not. For comparisons of continuous variables of two groups, Mann-Whitney test was used, as the data was not always normally distributed. To compare three or more groups while adjusting for multiple testing, one-way analysis of variance (ANOVA) was used followed by a Tukey’s post-hoc test. P < 0.05 was considered significant. Values were presented as mean ± standard error of the mean (SEM).
Results
Effect of Culture Medium and Harvesting Site on Cell Attachment
Explant attachment to the culture substrate is a pre-requisite for epithelial cell outgrowth. The percentage of attached explants was compared between each culture medium and harvesting site used. Location wise, the percentages of attached explants in EpiLife were 55%, 51% 41%, and 27% for LL, BM, HP and TZ, respectively. For OKM the attachment percentages were 81%, 66%, 43%, and 83% for LL, BM, HP and TZ, respectively. In DMEM the percentages were 90%, 62%, 60% and 70% for LL, BM, HP and TZ, respectively. In RPMI the percentage values were 71%, 55%, 75% and 90% for explants harvested from LL, BM, HP and TZ respectively (Fig. 2). Neither choice of media or harvesting site revealed any statistically significant difference in the ability of the explants to attach to the culture well.
The percentage of attached explants following 48 hours of culture in four different media was compared using explants from four different rat oral mucosa harvesting sites. Each set of bar chart showing percentage of attached explants in EpiLife, oral keratinocyte medium (OKM), Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM), and Roswell Park Memorial Institute 1640 medium (RPMI). The ability of the explants to attach to the culture wells was independent of both harvesting site and type of media.
Effect of Culture Medium and Harvesting Site on Cell Outgrowth
Fold outgrowth in relation to explant size was calculated following six-day culture using the four culture media (Fig. 3A). EpiLife (1.4 ± 0.2) and OKM (0.04 ± 0.03) gave rise to a low fold outgrowth, which was lower than that promoted by DMEM (18 ± 6; P = 0.044 and P = 0.027, respectively) and RPMI (20 ± 5; P = 0.03 and P = 0.018, respectively) (Fig. 3B). Therefore, all subsequent experiments were performed using only DMEM or RPMI.
(A) Light microscopy images showing representative outgrowth of epithelial cells from rat oral mucosa explants from lower lip region cultured for six days in EpiLife, oral keratinocyte medium (OKM), Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM), and Roswell Park Memorial Institute 1640 medium (RPMI). Red circles indicate the explant area. Green circles indicate the leading edge of the cellular outgrowth. The area between the red and the green circle represents the outgrowth area. (B) Bar chart showing fold outgrowth in EpiLife, OKM, DMEM, and RPMI. *P < 0.05. Fold outgrowth was calculated by dividing the outgrowth area by the explant area. Images are representative of four experiments.
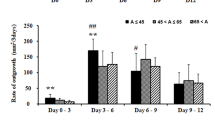
We then assessed fold outgrowth following six-day culture using explants from the four harvesting sites. When cultured in DMEM, explants from LL (36 ± 7) generated a higher fold outgrowth than explants from BM (11 ± 3; P < 0.001), HP (7 ± 0.7; P < 0.001), and TZ (19 ± 3; P = 0.046) (Fig. 4A). Similarly, explants from LL cultured for six days in RPMI (32 ± 12) generated significantly higher fold outgrowth than explants from BM (22 ± 8; P = 0.044), HP (10 ± 1; P < 0.001), and TZ (15 ± 4; P = 0.005) (Fig. 4B).
Bar charts showing fold outgrowth from rat oral mucosa explants harvested from the lower lip (LL), buccal mucosa (BM), hard palate (HP), and transition zone of the lower lip (TZ). (A) Explants were cultured in Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM) or (B) Roswell Park Memorial Institute 1640 medium (RPMI) for six days. (C) Fold outgrowth from LL and TZ explants was also measured after 13 days of culture in DMEM. *P < 0.05 and **P < 0.005.
Fold outgrowth was also measured after long-term culture in DMEM. After 13 days of culture, the fold outgrowth from LL explants did not differ significantly from that of TZ explants (Fig. 4C). Collectively, our results show that DMEM and RPMI yielded the highest fold outgrowth, whereas explants from LL gave the biggest fold outgrowth following six-day culture and comparable outgrowth to TZ following 13-day culture.
Effect of Culture Medium and Harvesting Site on Cell Phenotype
To determine the effect of the choice of culture medium, as well as tissue harvesting site, on phenotype of cells in primary culture, the expression of immunocytochemical markers related to differentiated and undifferentiated cells (Table 1) was compared among the groups.
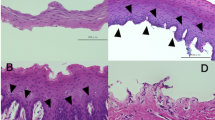
We first investigated the expression of undifferentiated cell markers Bmi-129 and the transcription factor p63α30. Bmi-1, a protein required for maintenance of adult stem cells31, was detected in the cell nucleus of only a few cells (Fig. 5A). p63α was detected in a minority of cell nuclei (Fig. 5B). Most of the cultured cells demonstrated cytoplasmic staining for the epithelial cell marker pan-CK (Fig. 5C) and the stratified squamous epithelial cell marker CK-4 (Fig. 5D).
Photomicrographs showing immunostaining of the putative stem cell markers Bmi-1 (green) (A) and p63α (red) (B), as well as the epithelial cell marker anti-pan-cytokeratin (CK; green) (C) and the stratified squamous epithelial cell marker anti-CK-4 (red) (D), in rat oral mucosal epithelial cells from lower lip cultured for six days in Dulbecco’s modified Eagle’s medium/Ham’s F12. Images are representative of three samples. Nuclei were counterstained with DAPI (blue). The arrow in image A indicates Bmi-1 staining (green). The arrow in image B indicates p63α staining (red). Magnification: 400x.
Bmi-1 positive cells were detected in fewer than 25% of the cells from all harvesting sites cultured in DMEM. However, in cultures from HP and TZ, Bmi-1 was undetectable at the leading edge (Table 2). In DMEM, no p63α positive cells were observed at the leading edge of the cultures originating from LL and HP, whereas p63α positive cells were detectable in fewer than 25% of the cells at the leading edge of BM and TZ cultures. Near the explant, fewer than 25% of the cells were positive for p63α in cultures from LL, BM, and HP, whereas in cultures from TZ 25% to 50% of the cells near the explant were p63α positive. With the exception of BM cultures, cultures from all other harvesting sites showed a lower percentage of p63 positive cells at the leading edge than near the explant (Table 2). In DMEM, the percentages of both pan-CK and CK-4 positive cells were similar (75–100%) irrespective of harvesting site and irrespective of the location of the expanded cells (i.e., near the explant or at the leading edge), indicating that the absolute majority of the cultured cells were epithelial (Table 2).
In contrast to DMEM, none of the cultures grown in RPMI displayed Bmi-1 positive cells (Table 3). In RPMI, up to 50% of the cells were positive for p63α irrespective of both the location in culture and of the harvesting site. Only cultures derived from LL and TZ showed a lower percentage of p63α positive cells at the leading edge (<25%) compared to near the explant (25–50%) (Table 3). As for cultures grown in DMEM, a similar percentage of pan-CK and CK-4 positive cells were obtained from all harvesting sites when growing cells in RPMI (Table 3).
The percentage of proliferative cells was assessed by counting PCNA positive cells following six-day culture in DMEM or RPMI. Positive staining for PCNA was seen in cell nuclei (Fig. 6A). PCNA positive cells cultured in DMEM were significantly lower in culture from HP compared to the culture from LL (P = 0.012), BM (P = 0.014) and TZ (P = 0.009) (Fig. 6B). Explants cultured in RPMI harvested from LL, BM, HP, and TZ, showed comparable percentages of PCNA positive cells in all cultures (Fig. 6C).
Rat oral mucosal epithelial cells were cultured for six days and then immunostained with anti-proliferating cell nuclear antigen (PCNA) to assess the percentage of proliferating cells. (A) Corresponding photomicrograph of cultured cell nuclei stained with DAPI (blue; left and right image) and immunostained with anti-PCNA (green; middle and right image). The cells were harvested from the lower lip (LL) oral mucosa and cultured in Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM). Images are representative of three samples. Magnification: 400x. (B) PCNA expression of cultured cells in DMEM from explants harvested from LL, buccal mucosa (BM), hard palate (HP), and transition zone of the lower lip (TZ) (C) PCNA expression of cultured cells in RPMI from explants harvested from LL, BM, HP, and TZ. *P < 0.05.
The undifferentiated cell marker NGF p75 was detected in the cytoplasm of the cultured cells (Fig. 7A). The percentage of cells positive for NGF p75 in DMEM cultures derived from LL, BM, HP, and TZ were 50% ± 4%, 34 ± 8%, 38 ± 9%, and 61% ± 5%, respectively (Fig. 7B). Culture derived from LL, BM, HP, and TZ in RPMI, showed 47% ± 5%, 12% ± 2%, 34% ± 7%, and 49% ± 4% percentage of NGF p75 positive cells, respectively. BM cultures had a significantly lower percentage of NGF p75 positive cells compared to the cultures derived from LL (P < 0.001) and TZ (P < 0.001) (Fig. 7C). Together, these results suggest that an undifferentiated phenotype is supported in both DMEM and RPMI. However, expression of NGF p75 varies depending on the harvesting location in DMEM. Furthermore, explants from LL and TZ tended to support a higher percentage of proliferative cells and a more undifferentiated phenotype than explants from HP and BM, respectively.
Rat oral mucosal epithelial cells were cultured for six days and then immunostained with anti-nerve growth factor (NGF) p75 to assess the percentage of undifferentiated cells. (A) Corresponding photomicrograph of cultured cell nuclei stained with DAPI (blue; left and right image) and immunostained with anti-NGF p75 (red; middle and right image). The cells were harvested from the lower lip (LL) oral mucosa and cultured in Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM). Images are representative of three samples. Magnification: 400x. (B) NGF p75 expression of cultured cells in DMEM from explants harvested from LL, buccal mucosa (BM), hard palate (HP), and transition zone of the lower lip (TZ) (C) NGF p75 expression of cultured cells in RPMI from explants harvested from LL, BM, HP, and TZ. *P < 0.001.
Effect of Culture Medium and Harvesting Site on Colony-Forming Efficiency
A colony-forming efficiency assay was performed to assess clonal growth capacity. In DMEM, LL (0.003% ± 0.002%), BM (0.003% ± 0.001%), and HP (0.016% ± 0.002%) cells yielded lower CFE than TZ (0.593% ± 0.004%; P < 0.001) (Fig. 8A). Cells from LL and BM gave rise to the smallest colonies (presumed paraclones), cells from HP yielded medium-sized colonies (presumed meroclones), whereas cells from TZ gave rise to the largest colonies (presumed holoclones) (Fig. 8B). In contrast to DMEM, RPMI did not give rise to any colonies after 12 days of culture (Fig. 8C).
Rat oral mucosal epithelial cells harvested from four sites in the oral cavity (lower lip (LL), buccal mucosa (BM), hard palate (HP), and transition zone of the lower lip (TZ)) were assayed for colony-forming efficiency (CFE) for 12 days in Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM) or Roswell Park Memorial Institute 1640 medium (RPMI). (A) Bar chart showing the percentage of colonies formed when using DMEM as culture medium. *P < 0.001. (B) Photographs (top row) show representative colonies grown in DMEM and stained with crystal violet. Photomicrographs (bottom row; magnification: 200x) show corresponding bright-field images of the clonal cells stained with crystal violet. Images are representative of at least five samples. (C) Photographs showing no colonies when cells were cultured in RPMI. Images are representative of three samples.
Discussion
In the present study, OMECs were harvested from four sites in the oral cavity of rats and cultured in four different media (EpiLife, OKM, DMEM, and RPMI) and the explant attachment, cell outgrowth, proliferation, and phenotype of the cells were compared. Our results indicate that OMECs cultured in EpiLife, OKM, DMEM, and RPMI have similar attachment ability. Epithelial cells are anchorage dependent, and therefore cell adhesion is necessary for their proliferation32.
DMEM and RPMI yielded a higher fold outgrowth of cells than EpiLife and OKM. The presence of growth promoting supplements in the media plays an important role in ex vivo cell proliferation33,34,35. Formanek et al. found that among numerous tested additives, DMEM supplemented with fetal calf serum, EGF, insulin, and hydrocortisone exhibited the greatest capacity for promoting oral keratinocyte growth36. Our finding that DMEM generated a higher fold outgrowth than OKM and EpiLife is consistent with this study36. However, RPMI yielded similar fold outgrowth despite not being supplemented with EGF, insulin, and hydrocortisone.
In the current study, we were able to produce undifferentiated epithelial sheets in several of our cultures. An undifferentiated phenotype in cultured limbal epithelial stem cell transplants has been found to be a strong predictor of clinical success following transplantation in patients with LSCD3. Further studies are warranted to explore the effects of the phenotype of OMCEs on clinical results following transplantation.
Kolli et al. reported a loss of p63 positive cells with increasing distance from limbal explants37. In agreement with this study, we found a lower percentage of p63α positive cells at the leading edge than near the explant in cultures from LL and TZ grown in DMEM or RPMI26. Cells harvested from HP cultured in DMEM, but not in RPMI, also showed a lower percentage of p63α positive cells at the leading edge than near the explant. Bmi-1, another putative stem cell marker applied in our study, demonstrated a similar tendency, strengthening the argument that increasing distance from the explant results in a higher degree of differentiation of cultured epithelial cells. As an undifferentiated phenotype is considered highly advantageous in corneal regenerative medicine3, our study also lends support to the idea of using shredded explants for treating LSCD38, especially if the explants are spread apart in the culture wells. Considering our observations together with the reports from Kolli et al., two cell types appear to change phenotypically with respect to the distance from the explant. Further studies are warranted to explore whether this tendency appears to be irrespective of cell types and, thus, represents a general principle in ex vivo expansion of cells from explants for use in regenerative medicine4.
Pan-CK and CK-4 expression were detected in more than 75% of the cells across both DMEM and RPMI. This finding indicates that the majority of the cultured cells were epithelial cells.
Cultures from HP had a significantly lower expression of the proliferation marker PCNA than cultures from LL, BM and TZ, which agrees with our finding that explants from HP demonstrated the least outgrowth.
Nerve growth factor p75 has been suggested as a stem/progenitor cell marker for OMECs and cells positive for this marker typically cluster in specific regions of the oral mucosal epithelium39. It has been reported that NGF p75 positive cells have a high in vitro proliferative capacity and clonal growth potential39. In RPMI, explants from BM gave rise to the lowest percentage of NGF p75 positive cells.
Buccal mucosa has been employed as harvesting site for OMEC transplants in several studies6, 12, 13, 16,17,18,19,20,21, 40,41,42,43,44,45,46,47,48. However, our study showed that OMECs from TZ yielded higher CFE than BM. RPMI did not yield any colonies nor did the cells cultured in this medium express Bmi-1. The ability of OMECs to form colonies may be related to the maintenance of Bmi-1 expression as Bmi-1 was found to be related to the self-renewal capacity of human limbal epithelial stem cells49. We did not use a feeder layer when performing the CFE assay in our study. In the absence of a feeder layer, a cyclic adenosine monophosphate inducer such as cholera toxin, has been shown to be necessary for rat OMECs to form colonies50. This is in agreement with our results in which we only obtained colonies when culturing the cells in a cholera toxin-supplemented medium (DMEM).
Explants from LL and TZ exhibited a higher proliferative capacity than explants from BM and HP, as demonstrated by measurement of fold outgrowth and CFE. Stem cells reside in a particular microenvironment known as a niche51. One benefit of using explants for ex vivo expansion of OMECs is that the stem cells remain in, and are supported by, their niche, which is known to control stem cell function52. Asaka et al. showed that label-retaining cells in rat oral mucosal epithelium, which may represent stem cells, were localized to the basal layer. Where the epithelium had rete ridges the label-retaining cells were seen at the bottom of these ridges, however, where the epithelium had less pronounced rete ridges the label-retaining cells were randomly located in the basal layer53. In our study, we harvested tissues from the BM and LL (representing lining mucosa), where epithelial rete ridges are shallow and gentle39, 54, and HP (representing masticatory mucosa), where they are steep and deep39, 54. No study has directly compared the rete ridges of rats in LL, BM, HP, and TZ. However, TZ in rodents has been shown to have more prominent rete ridges than the adjacent epidermis55. The higher percentage of colonies formed from TZ cells compared to the other three harvesting sites tested in our study infers a relatively larger stem cell content in TZ. As TZ has prominent rete ridges this increases the surface area of the basement membrane and therefore the relative number of basal cells (where stem cells are likely to be located) compared to supra-basal cells. Analogous to this, thin epithelia have been shown to have higher CFE than thick epithelia, possibly due to a relatively larger content of basal stem cells50. Compared to cells from the other three tested harvesting sites, TZ cells could also be less dependent on their niche for colony-formation. However, as LL explants yielded higher fold outgrowth than TZ, we speculate that: (1) LL has a relatively higher percentage of transient amplifying cells with high proliferative capacity; (2) outgrowth of LL cells is more dependent on the stem cell niche (which is included with explant culture) than TZ cells; or (3) outgrowth of LL cells is dependent on a higher cell density (which is achieved when using explants rather than cell suspension) than TZ cells. Cell density has been shown to greatly affect proliferation of rat OMECs50. An in-depth analysis of differences in the stem cell niches of the four harvesting sites is needed to provide a more definite answer.
In conclusion, the choice of tissue harvesting site and culture medium influence the attachment, growth, and phenotype of cultured OMECs. Explants from LL and TZ cultured in DMEM are more favorable than BM. This is particularly interesting as BM has been the by far most commonly reported harvesting site to generate OMEC sheets for transplantation. Comparative studies are warranted to assess whether optimization of the choices of harvesting site and culture medium can improve the clinical success of transplantation of cultured OMEC to patients with LSCD.
References
Pellegrini, G. et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349, 990–993, doi:10.1016/s0140-6736(96)11188-0 (1997).
Utheim, T. P. Limbal epithelial cell therapy: past, present, and future. Methods Mol Biol 1014, 3–43, doi:10.1007/978-1-62703-432-6_1 (2013).
Rama, P. et al. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J Med 363, 147–155, doi:NEJMoa0905955 [pii]; doi:10.1056/NEJMoa0905955 (2010).
Sehic, A., Utheim, O. A., Ommundsen, K. & Utheim, T. P. Pre-Clinical Cell-Based Therapy for Limbal Stem Cell Deficiency. Journal of functional biomaterials 6, 863–888, doi:10.3390/jfb6030863 (2015).
Di Girolamo, N. et al. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation 87, 1571–1578, doi:10.1097/TP.0b013e3181a4bbf2 (2009).
Nishida, K. et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. The New England journal of medicine 351, 1187–1196, doi:10.1056/NEJMoa040455 (2004).
Utheim, T. P. Concise review: transplantation of cultured oral mucosal epithelial cells for treating limbal stem cell deficiency-current status and future perspectives. Stem Cells 33, 1685–1695, doi:10.1002/stem.1999 (2015).
Litvinov, I. V. et al. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res. 66, 8598–8607, doi:10.1158/0008-5472.can-06-1228 (2006).
Deyrieux, A. F. & Wilson, V. G. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology 54, 77–83, doi:10.1007/s10616-007-9076-1 (2007).
Kent, K. D. & Bomser, J. A. Bovine pituitary extract provides remarkable protection against oxidative stress in human prostate epithelial cells. In vitro cellular & developmental biology. Animal 39, 388–394, doi:10.1290/1543-706x(2003)039<0388:bpeprp>2.0.co;2 (2003).
Muller-Glauser, W. & Preisig, E. The effect of cholera toxin and epidermal growth factor on the in-vitro growth of human oral epithelial cells. Archives of oral biology 28, 765–771 (1983).
Gaddipati, S. et al. Oral epithelial cells transplanted on to corneal surface tend to adapt to the ocular phenotype. Indian J. Ophthalmol. 62, 644–648, doi:10.4103/0301-4738.109517 (2014).
Takeda, K. et al. Ocular surface reconstruction using the combination of autologous cultivated oral mucosal epithelial transplantation and eyelid surgery for severe ocular surface disease. American journal of ophthalmology 152, 195–201 e191, doi:10.1016/j.ajo.2011.01.046 (2011).
Nakamura, T., Takeda, K., Inatomi, T., Sotozono, C. & Kinoshita, S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br. J Ophthalmol 95, 942–946, doi:bjo.2010.188714 [pii]; doi:10.1136/bjo.2010.188714 (2011).
Ang, L. P. et al. Autologous serum-derived cultivated oral epithelial transplants for severe ocular surface disease. Arch. Ophthalmol. 124, 1543 (2006).
Inatomi, T. et al. Ocular surface reconstruction with combination of cultivated autologous oral mucosal epithelial transplantation and penetrating keratoplasty. American journal of ophthalmology 142, 757–764, doi:10.1016/j.ajo.2006.06.004 (2006).
Nakamura, T. et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. The British journal of ophthalmology 88, 1280–1284, doi:10.1136/bjo.2003.038497 (2004).
Priya, C. G. et al. Adult human buccal epithelial stem cells: identification, ex-vivo expansion, and transplantation for corneal surface reconstruction. Eye (London, England) 25, 1641–1649, doi:10.1038/eye.2011.230 (2011).
Satake, Y. et al. Barrier function and cytologic features of the ocular surface epithelium after autologous cultivated oral mucosal epithelial transplantation. Arch. Ophthalmol. 126, 23–28, doi:10.1001/archopht.126.1.23 (2008).
Sotozono, C. et al. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology 120, 193–200, doi:10.1016/j.ophtha.2012.07.053 (2013).
Satake, Y., Higa, K., Tsubota, K. & Shimazaki, J. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology 118, 1524–1530, doi:10.1016/j.ophtha.2011.01.039 (2011).
Kolli, S. et al. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells 32, 2135–2146, doi:10.1002/stem.1694 (2014).
Rao, R. S., Patil, S. & Ganavi, B. S. Oral cytokeratins in health and disease. J Contemp Dent Pract 15, 127–136 (2014).
Hamilton, A. I. & Blackwood, H. J. Cell renewal of oral mucosal epithelium of the rat. Journal of anatomy 117, 313–327 (1974).
Eidet, J. R. et al. Effect of biopsy location and size on proliferative capacity of ex vivo expanded conjunctival tissue. Investigative ophthalmology & visual science 53, 2897–2903, doi:10.1167/iovs.11-8269 (2012).
Fostad, I. G. et al. Biopsy harvesting site and distance from the explant affect conjunctival epithelial phenotype ex vivo. Experimental eye research 104, 15–25, doi:10.1016/j.exer.2012.09.007 (2012).
Barrandon, Y. & Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proceedings of the National Academy of Sciences of the United States of America 84, 2302–2306 (1987).
Nakamura, T. et al. The use of autologous serum in the development of corneal and oral epithelial equivalents in patients with Stevens-Johnson syndrome. Invest. Ophthalmol. Vis. Sci. 47, 909–916 (2006).
Jones, K. B. & Klein, O. D. Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. International journal of oral science 5, 121–129, doi:10.1038/ijos.2013.46 (2013).
Tao, Q. et al. p63 and its isoforms as markers of rat oral mucosa epidermal stem cells in vitro. Cell Biochem. Funct. 27, 535–541, doi:10.1002/cbf.1612 (2009).
Park, I. K. et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423, 302–305, doi:10.1038/nature01587 (2003).
Stoker, M., O’Neill, C., Berryman, S. & Waxman, V. Anchorage and growth regulation in normal and virus-transformed cells. Int. J. Cancer 3, 683–693 (1968).
Cohen, S. The stimulation of epidermal proliferation by a specific protein (EGF). Dev. Biol. 12, 394–407 (1965).
Cohen, S. Epidermal growth factor. In Vitro Cell. Dev. Biol. 23, 239–246, doi:10.1007/bf02623704 (1987).
Brunner, D. et al. Serum-free cell culture: the serum-free media interactive online database. Altex 27, 53–62 (2010).
Formanek, M., Millesi, W., Willheim, M., Scheiner, O. & Kornfehl, J. Optimized growth medium for primary culture of human oral keratinocytes. Int. J. Oral Maxillofac. Surg. 25, 157–160 (1996).
Kolli, S., Lako, M., Figueiredo, F., Mudhar, H. & Ahmad, S. Loss of corneal epithelial stem cell properties in outgrowths from human limbal explants cultured on intact amniotic membrane. Regen. Med. 3, 329–342, doi:10.2217/17460751.3.3.329 (2008).
Sangwan, V. S., Vemuganti, G. K., Iftekhar, G., Bansal, A. K. & Rao, G. N. Use of autologous cultured limbal and conjunctival epithelium in a patient with severe bilateral ocular surface disease induced by acid injury: a case report of unique application. Cornea 22, 478–481 (2003).
Nakamura, T., Endo, K.-i. & Kinoshita, S. Identification of Human Oral Keratinocyte Stem/Progenitor Cells by Neurotrophin Receptor p75 and the Role of Neurotrophin/p75 Signaling. Stem Cells 25, 628–638, doi:10.1634/stemcells.2006-0494 (2007).
Sotozono, C. et al. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta ophthalmologica 92, e447–e453, doi:10.1111/aos.12397 (2014).
Chen, H. C. et al. Persistence of transplanted oral mucosal epithelial cells in human cornea. Investigative ophthalmology & visual science 50, 4660–4668, doi:10.1167/iovs.09-3377 (2009).
Chen, H. C. et al. Expression of angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Investigative ophthalmology & visual science 53, 5615–5623, doi:10.1167/iovs.11-9293 (2012).
Ma, D. H. et al. Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye (London, England) 23, 1442–1450, doi:10.1038/eye.2009.60 (2009).
Schwab, I. R., Reyes, M. & Isseroff, R. R. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea 19, 421–426 (2000).
Inatomi, T. et al. Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. American journal of ophthalmology 141, 267–275, doi:10.1016/j.ajo.2005.09.003 (2006).
Nakamura, T. et al. Phenotypic investigation of human eyes with transplanted autologous cultivated oral mucosal epithelial sheets for severe ocular surface diseases. Ophthalmology 114, 1080–1088, doi:10.1016/j.ophtha.2006.09.034 (2007).
Burillon, C. et al. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Investigative ophthalmology & visual science 53, 1325–1331, doi:10.1167/iovs.11-7744 (2012).
Hirayama, M., Satake, Y., Higa, K., Yamaguchi, T. & Shimazaki, J. Transplantation of cultivated oral mucosal epithelium prepared in fibrin-coated culture dishes. Investigative ophthalmology & visual science 53, 1602–1609, doi:10.1167/iovs.11-7847 (2012).
Barbaro, V. et al. C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J. Cell Biol. 177, 1037–1049, doi:10.1083/jcb.200703003 (2007).
Kondo, M. et al. Significantly different proliferative potential of oral mucosal epithelial cells between six animal species. Journal of biomedical materials research. Part A 102, 1829–1837, doi:10.1002/jbm.a.34849 (2014).
Schofield, R. The stem cell system. Biomed. Pharmacother. 37, 375–380 (1983).
Watt, F. M. & Hogan, B. L. Out of Eden: stem cells and their niches. Science 287, 1427–1430 (2000).
Asaka, T., Akiyama, M., Kitagawa, Y. & Shimizu, H. Higher density of label-retaining cells in gingival epithelium. J. Dermatol. Sci. 55, 132–134, doi:10.1016/j.jdermsci.2009.03.006 (2009).
Wu, T. et al. Morphogenesis of rete ridges in human oral mucosa: a pioneering morphological and immunohistochemical study. Cells, tissues, organs 197, 239–248, doi:10.1159/000342926 (2013).
Riau, A. K., Barathi, V. A. & Beuerman, R. W. Mucocutaneous junction of eyelid and lip: a study of the transition zone using epithelial cell markers. Current eye research 33, 912–922, doi:10.1080/02713680802485147 (2008).
Acknowledgements
The authors thank Robin R. Hodges, Dayu Li, Marie Shatos, Ashkon Seyed-Safi and and Lalana Songra at the Schepens Eye Research Institute/Massachusetts Eye and Ear, Harvard Medical School, Boston, MA for their excellent assistance.
Author information
Authors and Affiliations
Contributions
R.I., T.P.U., J.R.E., D.A.R., E.M. and M.G. conceived the experiments; R.I. conducted the experiments, R.I., R.A.B., and M.L. analyzed the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Islam, R., Eidet, J.R., Badian, R.A. et al. Tissue Harvesting Site and Culture Medium Affect Attachment, Growth, and Phenotype of Ex Vivo Expanded Oral Mucosal Epithelial Cells. Sci Rep 7, 674 (2017). https://doi.org/10.1038/s41598-017-00417-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00417-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.