Abstract
Intracerebral hemorrhage (ICH) is common in China. However, the sex differences in clinical features, risk factors, and outcomes of ICH remain controversial. Between 2005 and 2014, we recruited patients with primary ICH in Tianjin, China, and evaluated sex differences in clinical features, risk factors, and outcomes at 3, 12, and 36 months after ICH. The 1,325 patients included 897 men (67.7%) and 428 women (32.3%). The mean age at ICH onset was younger among men (59.14 years) than among women (63.12 years, P < 0.001). Men were more likely to have a hematoma in the basal ganglia, while women were more likely to have one in the thalamus. Women had higher frequencies of urinary tract infections, diabetes mellitus, cardiovascular diseases, and obesity. Men had a greater risk of death at 3 months after ICH. However, no sex differences were observed for mortality at 12 and 36 months after ICH or for recurrence and dependency at 3, 12, and 36 months after ICH. These findings suggested that it crucial to strengthen management of AF and complications in patients with ICH, especially management of blood pressure in men for reducing the mortality rates and the burden of ICH in China.
Similar content being viewed by others
Introduction
Several studies have evaluated sex-related differences in functional outcomes among patients with intracerebral hemorrhage (ICH)1,2,3; however, mortality rates and outcomes following ICH remain controversial. For example, some studies have reported a higher mortality rate among women4,5,6, while others have reported a higher mortality rate among men7, 8. Other studies have reported no sex-related differences in mortality after ICH3, 9, 10. Moreover, there are limited data regarding sex-related differences in long-term outcomes (particularly outcomes at >1 year), including recurrence and dependency rates after ICH. Therefore, the present study aimed to evaluate sex-related differences in functional outcomes (mortality, dependency, and recurrence rates) in the short-term (3 months), medium-term (12 months), and long-term (36 months) after ICH.
Results
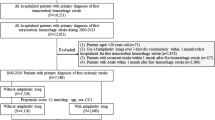
During the study period, 1,533 consecutive patients diagnosed with first-ever hemorrhagic stroke were registered in our database. Among these patients, 1,330 patients fulfilled our inclusion criteria, and we analyzed the records of 1,325 patients with complete data. The patient selection flow chart is shown in Fig. 1. Of the 1,325 patients who had experienced at least 3 months after stroke onset, 1,287 patients (97.1%) completed the 3-month follow-up; among 1,170 patients who had experienced at least 12 months after stroke onset, 1,092 patients (93.3%) completed the 12-month follow-up; and among 893 patients who had experienced at least 36 months after stroke onset, 770 patients (86.2%) completed the 36-month follow-up.
The present study included 897 men (67.7%) and 428 women (32.3%). The mean age at ICH onset was younger in men than in women (59.14 years vs. 63.12 years, respectively), and men were more likely to be <45 years of age at ICH onset (11.5% vs. 3.0%; P < 0.001 for all). Men were also more likely to have a basal ganglia hematoma (47.4% vs. 36.2%; P < 0.001), although women were more likely to have a thalamus hematoma (21.7% vs. 12.4%; P < 0.001). Moreover, the frequency of number for multi-hematoma was similar between men and women (8.7% vs. 11.7%, P = 0.085). Regarding in-hospital complications, urinary tract infections were more prevalent in women than in men (3.7% vs. 1.6%; P = 0.013), although there were no other statistically significant sex-related differences in complication rates. Women had significantly greater neurological function deficits, with lower Barthel indices (BIs) and higher modified Rankin scale (mRS) scores at admission; men and women had similar National Institutes of Health Stroke Scale (NIHSS) scores.
There were higher prevalence rates of diabetes mellitus (DM) (22.9% vs. 17.7%; P < 0.001), cardiovascular disease (26.6% vs. 17.7%; P < 0.001), and obesity (15.7% vs. 10.8%; P = 0.012) in women than in men; however, there were no other significant sex-related differences in medical history factors (P > 0.05 for all). The rates of current smoking status (47.2% vs. 11.9%; P < 0.001) and alcohol consumption (30.5% vs. 1.6%; P < 0.001) were higher in men than in women. Women had significantly higher levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), fasting glucose (FG), and glycosylated hemoglobin (HbA1c) (P < 0.05 for all), and men had significantly higher diastolic blood pressure (DBP). We did not observe any significant sex-related differences in levels of triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), or systolic blood pressure (SBP) (Table 1).
In the univariate analysis, we observed significant sex-related differences in mortality rates at each follow-up period. The mortality rates at 3 (13.5% vs. 9.0%, P = 0.021), 12 (17.2% vs. 13.4%, P = 0.111), and 36 months (25.3% vs. 21.9%, P = 0.307) were higher for men than for women. The unadjusted OR (95%CI) was 1.58 (1.07–2.33) at 3 months after ICH. However, there were no significant sex-related differences in recurrence or dependency rates (Table 2).
Furthermore, the results stratified by age groups showed that mortality at 3 months after ICH was significantly higher in men than in women among those aged 45–59 years, and the dependency rate was greater in men than in women at 3 and 12 months among patients aged <45 years (Table 2).
Table 3 presents the adjusted ORs for men at the 3-, 12-, and 36-month follow-ups. Men had a higher risk of death at 3 months after ICH (OR, 2.32; 95% confidence interval [CI], 1.45–3.72; P < 0.001) than did women after adjustment for those covariates found to be significant in the univariate analysis, including age, stroke severity, hematoma number, hypertension, atrial fibrillation (AF), dyslipidemia, complications, current smoking, and alcohol consumption. Moreover, severe stroke, AF, and complications were independent risk factors for mortality at 3 months after ICH. However, there were no significant sex differences for recurrence and dependency rates at 3, 12, and 36 months after ICH. Severity, AF, complications, and multiple hematomas were associated with high risk of mortality at 12 months after ICH; severity, complications, and multiple hematomas were associated with a high risk of mortality at 36 months after ICH. Furthermore, older age, greater severity, and complications were determinants of dependency at 3 and 12 months after ICH, but severity and AF were determinants of dependency at 36 months after ICH.
Discussion
To our knowledge, this is the first study to examine sex-related differences in long-term outcomes after ICH. Using a large hospital-based registry, we evaluated mortality, recurrence, and dependency rates at 3, 12, and 36 months after ICH. Our findings revealed various sex-related differences in patients’ demographic and clinical characteristics. Women were more likely than men to be older; have a greater frequency of urinary tract infections, DM, cardiovascular disease, obesity; and have higher levels of TC, HDL-C, LDL-C, FG, and HbA1c. However, men were more likely than women to be younger and have higher DBP. Men aged 45–59 years had significantly higher mortality at 3 months after ICH; male sex was an independent risk factor for mortality after adjusting for covariates. Moreover, severity and complications were determinants of mortality and dependency after ICH. Recurrence was associated with older age only at 36 months after ICH.
Over the past few decades, the incidence of ICH in developed countries has remained unchanged or decreased2, 11,12,13,14. However, the trends in China are inconsistent, as the incidence of ICH has decreased in urban areas but increased in rural areas (for both sexes)15, 16. Interestingly, studies have also found that men are more likely to experience their first stroke at a younger age than women are. A study with exclusion criteria similar to those utilized in the present study reported that women were on average 8 years older than men at ICH onset11. Another study found that women were on average 6 years older than men at the time of stroke17.
Similarly, we found that men were approximately 4 years younger at ICH onset than women were. However, conflicting trends have been reported in other studies; an American study reported that women in North Carolina experienced ICH 4 years before men did18, and other studies have reported no significant differences between sexes in the age of onset19, 20. In the present study, men aged 45–59 years had a significantly higher mortality at 3 months after ICH. It is possible that the neuroprotective effects of female gonadal hormones delay the onset of ICH among women, as these hormones play roles in decreasing lipid levels and altering rapid vasomotor responses in vessel walls21,22,23. There is a lower rate of hormone replacement therapies in Chinese women; the rate of regularly using hormone replacement therapies (1 year and over) was 1.1% in mainland China24 and 13.5% in Taiwan25 among postmenopausal women. In this study, none of the women were taking hormone replacement therapies; therefore, the neuroprotective effects of estrogen may play an important role in delaying the presence of ICH among women.
Previous studies have demonstrated that stroke burden is higher among women than among men due to higher rates of pre- and post-stroke disability17, 18, 26, 27. However, sex-related differences in stroke outcomes may be due to the study design, which include setting (population-based or hospital-based), population (Asian or Western), inclusion criteria, analytical methods, and duration of follow-up19. For example, some studies have reported higher mortality rates among women after ICH4,5,6, some have reported similar mortality rates between men and women after ICH3, 9, 10, and others have reported higher mortality rates among men after ICH7, 8. In the present study, we observed higher mortality rates among men at 3 months after ICH. This higher mortality rate may be explained by the higher prevalence of smoking and alcohol consumption and the higher DBP levels among men; in addition, lower levels of TC and HDL-C, which have been reported to be associated with a higher risk of death after ICH, may contribute to the higher mortality rate in men after ICH28, 29.
Several studies have reported that hypercholesterolemia is a protective factor for ICH. For example, the Ludwigshafen Stroke Study in Germany found that the absence of hypercholesterolemia before ICH was associated with a 22% higher mortality rate after ICH30. Another study identified an association between a history of hypercholesterolemia and a decreased risk of ICH31, and TC has been reported to be negatively associated with hemorrhagic and total stroke mortality32.
Recently, two studies reported the long-term mortality rates and functional outcomes among stroke patients31, 32. A study from the Swedish Stroke Register indicated there were higher mortality rates among women than among men at 3 and 12 months after stroke, and elderly women (aged 75 years and over) were most susceptible to deterioration, with dependency rates increasing from 23.2% to 45.5%33. Another Collaborative Evaluation of Rehabilitation in Stroke Across Europe Study showed that functional and motor outcomes at 5 years were equal to those 2 months after stroke. Increasing age and increasing stroke severity negatively affected outcomes34. However, only a few studies have reported sex-related differences in long-term (>1 year) functional outcomes after ICH. For example, one Chinese study found that women had a higher risk of dependency at 3 and 6 months after ICH19. In contrast, we found no sex-related differences in recurrence and dependency rates at 3, 12, and 36 months after ICH.
Although this study included a large sample of patients diagnosed with ICH and evaluated long-term outcomes, there are also several limitations. First, all patients were from a single hospital in northern China, and it cannot be assumed that our findings are representative of the general Chinese population. However, given the high incidence of ICH in northern China, our large sample provides reliable information regarding local sex-related differences in ICH outcomes. Second, we did not collect information regarding pre-stroke medications. This omission may have confounded our analysis of sex-related differences for various factors. Third, our registry did not contain information regarding hematoma volume, which could affect outcomes after ICH. However, we replaced hematoma volume with the number of hematomas, which could roughly estimate the hematoma volume. Finally, information regarding rehabilitation therapy after the acute phase was not provided in this study, but it might have an effect on the evaluation of prognosis after ICH. Finally, a follow-up rate of <90% at 36 months after ICH could impact the evaluation of outcomes at 36 months after ICH.
Conclusions
This is the first study to evaluate sex-related differences in the clinical features, risk factors, and short- to long-term outcomes among Chinese patients diagnosed with ICH. Our findings revealed that ICH onset occurred approximately 4 years earlier in men than in women. Women were more likely than men to be older; have a greater frequency of urinary tract infections, DM, cardiovascular disease, obesity; and have higher levels of TC, HDL-C, LDL-C, FG, and HbA1c. However, men were more likely than women to be younger and have higher DBP. Men aged 45–59 years had significantly higher mortality at 3 months after ICH; male sex was an independent risk factor for mortality after adjusting for covariates. Moreover, severity and complications were determinants of mortality and dependency after ICH. Recurrence was associated with older age only at 36 months after ICH. These findings suggest that it is crucial to strengthen the management of AF and complications in patients with ICH, especially the management of blood pressure in men, to reduce mortality rates and the burden of ICH in China.
Materials and Methods
This study evaluated data from a prospectively maintained database of patients diagnosed with ICH who were admitted to the stroke unit of Tianjin Huanhu Hospital, China, between January 2005 and September 2014. We assessed the outcomes at 3, 12 and 36 months after ICH in December 31, 2014. Of these, those patients who registered before September 30, 2014 were qualified to assess the outcomes at 3 months after ICH; those patients who registered before December 31, 2013 were qualified to assess the outcomes at 12 months after ICH; and those patients who registered before December 31, 2011 were qualified to assess the outcomes at 36 months after ICH.
A diagnosis of ICH was made according to the World Health Organization's criteria, and all diagnoses were confirmed using brain computed tomography findings35. All patients with ICH were admitted to the hospital within 72 h of stroke onset and were ≥18 years of age at the time of database inclusion.
We excluded patients diagnosed with subarachnoid hemorrhage, traumatic hemorrhage, and brain hemorrhage caused by vascular malformations, as well as cases of coagulopathy, aneurysmal rupture, and recurrent ICH. Furthermore, patients with premorbid dependency (defined as mRS score >2) and those who died after completing the neuroimaging diagnosis but before admission to the stroke unit were excluded from this study. For the included patients, we collected data regarding their baseline characteristics (including demographic information), clinical features, medical history, risk factors, routine laboratory test results, and outcomes 3, 12, and 36 months after ICH. Patients with ICH were treated with medications that included diuretics (mannitol, glycerol fructose, furosemide, torsemide, and albumin), antihypertensives, and medications to treat complications occurring during hospitalization.
All investigative protocols were approved by the ethics committee of Tianjin Huanhu Hospital. The procedures were performed according to approved guidelines, and a written informed consent was obtained from each patient.
The clinical features of ICH included in this analysis were hematoma location, neurological function deficits, severity, and in-hospital complications. Hematoma location was categorized as: basal ganglia, lobar, thalamus, brain stem, cerebellum, or ≥2 locations, as determined using brain computed tomography findings. Neurological function deficits were evaluated using the NIHSS score, BI, and mRS score at admission. Stroke severity was categorized into 3 groups using the NIHSS score: mild (NIHSS score ≤ 7), moderate (NIHSS score 8–16), or severe (NIHSS score ≥ 17)36. We also identified cases that experienced pulmonary infection, urinary tract infection, gastrointestinal hemorrhage, seizure, electrolyte disturbance, and liver/renal toxicity in the hospital. Furthermore, we collected data on patients’ levels of TC, TG, HDL-C, LDL-C, FG, and HbA1c at admission.
Regarding patient medical history, we collected data on the presence of hypertension (defined as a history of hypertension or antihypertensive drug use), DM (defined as a history of DM or hypoglycemic drug use), atrial fibrillation (AF, defined as a history of AF confirmed by at least one electrocardiogram, or the presence of arrhythmia during hospitalization), and cardiovascular disease (including coronary heart disease or myocardial infarction). We also evaluated patients’ modifiable lifestyle factors, which included current smoking status (≥1 cigarette per day for ≥1 year), alcohol consumption (≥1 drink per week for 1 year), and obesity (body mass index ≥ 30 kg/m2).
Patient outcomes included mortality, recurrence, and dependency rates at 3, 12, and 36 months after ICH. All outcome data were collected via in-person examinations or telephone follow-ups. Death was defined as all-cause mortality during the corresponding periods after ICH. Recurrence was defined as a new-onset vascular event, which included ICH, ischemic stroke, myocardial infarction, and venous thrombosis within 30 days after stroke. We included patients who died as a result of these vascular events, although we excluded patients with a confirmed non-vascular cause of death. Dependency was defined as an mRS score ≥3 at the time of follow-up; patients who died were excluded from the analysis of dependency rates37.
Follow-ups were performed according to a predetermined procedure, with trained neurologists re-examining the patients in the outpatient department at 3, 12, and 36 months after ICH. All patients completed follow-up with face-to-face interviews or with telephone interviews for patients who could not attend an in-person follow-up.
Descriptive statistics were used to evaluate sex-related differences. Age and levels of TG, TC, HDL-C, LDL-C, FG, and HbA1c are reported as means ± standard deviations, while NIHSS scores, BI, and mRS scores are reported as medians (ranges). Continuous variables were compared using the Student t-test or Mann-Whitney U test as appropriate. Dichotomous variables, including stroke subtypes, stroke severity, medical history, stroke risk factors, and outcomes at 3, 12, and 36 months after ICH, are reported as numbers (percentages). The chi-squared test was used to compare dichotomous variables. All patients missing from each follow-up period were excluded from calculations of mortality, dependency, and recurrence rates. We also excluded patients from the dependency rate calculation who completed follow-up via telephone. Sex-related differences in outcomes were assessed using logistic regression models, and the risk is reported as unadjusted ORs with 95% CIs.
A multivariate analysis of sex differences in outcomes was performed with a logistic regression model that was adjusted by those variables found to be significantly associated with outcomes at 3, 12, and 36 months after stroke in the univariate analysis, such as age, stroke severity, hematoma location, medical history, risk factors, and in-hospital complications (i.e., pulmonary infection and gastrointestinal hemorrhage). The results of the multivariate analysis are presented as adjusted ORs and 95% CIs. All statistical analyses were performed using SPSS software (version 15.0; SPSS Inc., Chicago, IL), and all tests were two-tailed. Statistical significance was defined as a P-value of <0.05.
References
Wagner, I. et al. Sex differences in perihemorrhagic edema evolution after spontaneous intracerebral hemorrhage. Eur J Neurol. 19, 1477–1481 (2012).
van Asch, C. J. et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 9, 167–176 (2010).
Togha, M. & Bakhtavar, K. Factors associated with in-hospital mortality following intracerebral hemorrhage: a three-year study in Tehran, Iran. BMC Neurol. 4, 9 (2003).
Zhang, L. F. et al. Proportion of different subtypes of stroke in China. Stroke. 34, 2091–2096 (2003).
Wu, S. H. et al. Sex differences in stroke incidence and survival in Hong Kong, 2000–2007. Neuroepidemiology. 38, 69–75 (2012).
Ganti, L. et al. Female gender remains an independent risk factor for poor outcome after acute nontraumatic intracerebral hemorrhage. Neurol Res Int. 2013, 219097 (2013).
Ayala, C. et al. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995–1998. Stroke. 33, 1197–1201 (2002).
Wang, X. et al. Surveillance of trend and distribution of stroke mortality by subtype, age, gender, and geographic areas in Tianjin, China, 1999–2006. Int J Stroke. 4, 169–174 (2009).
Yesilot, N. F., Koyuncu, B. A., Coban, O., Tuncay, R. & Bahar, S. Z. Gender differences in acute stroke: Istanbul medical school stroke registry. Neurol India. 9, 174–179 (2011).
Sheikh, K. & Bullock, C. M. Effect of measurement on sex difference in stroke mortality. Stroke. 38, 1085–1087 (2007).
Sivenius, J. et al. FINSTROKE study. Continuous 15-year decrease in incidence and mortality of stroke in Finland: the FINSTROKE study. Stroke. 35, 420–425 (2004).
Lovelock, C. E., Molyneux, A. J. & Rothwell, P. M. Oxford Vascular Study. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol. 6, 487–493 (2007).
Islam, M. S. et al. Trends in incidence and outcome of stroke in Perth, Western Australia during 1989 to 2001: the Perth Community Stroke Study. Stroke. 39, 776–782 (2008).
Benatru, I. et al. Stable stroke incidence rates but improved case-fatality in Dijon, France, from 1985 to 2004. Stroke. 37, 1674–1679 (2006).
Zhao, D. et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke. 39, 1668–1674 (2008).
Wang, J. et al. Increasing stroke incidence and prevalence of risk factors in a low-income Chinese population. Neurology. 84, 374–381 (2015).
Roquer, J., Campello, A. R. & Gomis, M. Sex differences in first-ever acute stroke. Stroke. 34, 1581–1585 (2003).
Umeano, O. et al. Gender and Age Interact to Affect Early Outcome after Intracerebral Hemorrhage. PLoS One. 8, e81664 (2013).
Zhou, J. et al. Sex differences in clinical characteristics and outcomes after intracerebral haemorrhage: results from a 12-month prospective stroke registry in Nanjing, China. BMC Neurol. 14, 172 (2014).
Wagner, I. et al. Sex differences in perihemorrhagic edema evolution after spontaneous intracerebral hemorrhage. Eur J Neurol. 12, 1477–1481 (2012).
Roof, R. L. & Hall, E. D. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 17, 367–388 (2000).
Nakamura, T. et al. Estrogen therapy for experimental intracerebral hemorrhage. J Neurosurg. 103, 97–103 (2005).
Nakamura, T. et al. Effects of endogenous and exogenous estrogen on intracerebral hemorrhageinduced brain damage in rats. Acta Neurochir Suppl. 96, 218–221 (2006).
Zhang, S. W. et al. The investigation and analysis of the hormone replacement therapy of perimenopausal women in Beijing. Chinese journal of women and children health. 19, 45–47 (2004).
Chang, C. & Chang, C. H. Menopause and hormone using experiences of Chinese women in Taiwan. Health Care Women Int. 17, 307–318 (1996).
Glader, E. L. et al. Sex differences in management and outcome after stroke a Swedish national perspective. Stroke. 34, 1970–1975 (2003).
Di Carlo, A. et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 34, 1114–1119 (2003).
Suzuki, K., Izumi, M., Sakamoto, T. & Hayashi, M. Blood pressure and total cholesterol level are critical risks especially for hemorrhagic stroke in Akita, Japan. Cerebrovasc Dis. 31, 100–106 (2011).
Iso, H., Jacobs, D. R. Jr., Wentworth, D., Neaton, J. D. & Cohen, J. D. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the Multiple Risk Factor Intervention Trial. N Engl J Med. 320, 904–910 (1989).
Palm, F. et al. Intracerebral haemorrhage in a population-based stroke registry (LuSSt): incidence, aetiology, functional outcome and mortality. J Neurol. 260, 2541–2550 (2013).
Woo, D. et al. Hypercholesterolemia, HMG-CoA reductase inhibitors, and risk of intracerebral hemorrhage: a case-control study. Stroke. 35, 1360–1364 (2004).
Prospective Studies Collaboration et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 370, 1829–1839 (2007).
Ullberg, T., Zia, E., Petersson, J. & Norrving, B. Changes in functional outcome over the first year after stroke: an observational study from the Swedish stroke register. Stroke. 46, 389–394 (2015).
Meyer, S. et al. Functional and motor outcome 5 years after stroke is equivalent to outcome at 2 months: follow-up of the collaborative evaluation of rehabilitation in stroke across Europe. Stroke. 46, 1613–1619 (2015).
World Health Organization Task Force on Stroke and Other Cerebrovascular Disorders: Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. 20, 1407–1431 (1989).
Kim, J. S., Lee, K. B., Roh, H., Ahn, M. Y. & Hwang, H. W. Gender Differences in the Functional Recovery after Acute Stroke. J Clin Neurol. 6, 183–188 (2010).
Banks, J. L. & Marotta, C. A. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 38, 1091–1096 (2007).
Acknowledgements
We thank all participants in this study and the local physicians for their enthusiasm, tireless work, and sustained support, Mr. yueqian Ning for advice on revision of the manuscript. This study was funded by the Tianjin Health Bureau of Science and Technology Fund Key Projects (contract: KY12, and 2013KG120).
Author information
Authors and Affiliations
Contributions
Y.X. was involved in data interpretation and drafting this manuscript. Y.X., Z.A., X.N., and J.W. were involved in conception and design, data collection, data interpretation, and critical review. Y.X., X.Z., N.Y., and W.Z. were involved in data collection, case diagnosis, and confirmation. J.W. and X.N. were involved in data analysis.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xing, Y., An, Z., Zhang, X. et al. Sex Differences in the Clinical Features, Risk Factors, and Outcomes of Intracerebral Hemorrhage: a Large Hospital-based Stroke Registry in China. Sci Rep 7, 286 (2017). https://doi.org/10.1038/s41598-017-00383-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00383-6
This article is cited by
-
A comparison of the disease occurrence of cerebrovascular diseases, diabetes mellitus, hypertensive diseases, and ischaemic heart diseases among hospitalized older adults in Thailand
Scientific Reports (2024)
-
Impact of Increased Hemoglobin on Spontaneous Intracerebral Hemorrhage
Neurocritical Care (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.