Abstract
Forty-nine patients with stage IIb cervical cancer were included to investigate the changes in bladder volume in response to different approaches to maintaining consistent bladder filling. The impacts of age (P age), water consumption (P wat ), and body mass index (BMI, P bmi ) on the mean urinary inflow rate (v tot ) were analysed. The bladder volume (BV) increased linearly over time. A large variation in v tot among individuals was observed, ranging from 0.19 to 5.13 ml/min. The v tot was correlated with P age (R = −0.53, p = 0.01) and P wat (R = 0.84, p = 0.00), and no correlation between v tot and P bmi was found (p > 0.05). Therefore, v tot could be parameterized using two methods: multivariable linear regression and iterative fitting. There was no statistically significant difference between the two methods. The model accuracy was successfully assessed with several validation tests for patients with good compliance (79.2% of all patients), and the proportion of radiotherapy (RT) fractions with zero wait time (one ultrasound (US) scan) increased from 6.5% to 41.2%. The optimal US scanning number and RT time could be provided using this model. This adaptive RT approach could reduce patient discomfort caused by holding onto urine and reduce technician labour as well as cost.
Similar content being viewed by others
Highly conformal radiotherapy (RT) techniques for cervical cancer enable adequate dose coverage of the clinical target volume (CTV) and the sparing of normal tissue1, 2. However, large inter-fraction organ motion of the cervix and uterus increases the risk of under-dosing the CTV or exposing the small bowel to a high dose of radiation. Several studies have demonstrated that the cervix and uterus move considerably due to tumour regression and variations in bladder and rectal filling3,4,5 and that uterine motion is greater than cervical motion. Uterine motion is predominantly influenced by bladder filling, with uterine fundus displacement of up to 48 mm6, and by cervical motion via rectal filling7.
RT with a full bladder is usually preferred because of the greater sparing of the small bowel8. However, it has been demonstrated that even with detailed instruction, when drinking a fixed volume of water at a fixed time (30–60 min) prior to each fraction, patients were unable to maintain consistent bladder filling9, 10. The use of cone-beam computed tomography (CBCT) scans enabled more accurate target definition and more precise tumour localization during radiation11, but the improvement was not sufficient to handle all the intra- and inter-fraction variabilities. An inherent drawback is the delayed bladder volume (BV) value. Following CBCT, the radiation oncologist must contour the entire outer surface of the bladder quickly and calculate the BV value to test its consistency. As a result, patient waiting time is inevitably prolonged. In addition, the time constraint is an important factor. More time is required for the execution of CBCT for each fraction12, 13. Time efficiency is crucial for RT centres where demand is high. The cost of CBCT is a further drawback.
Recently, Luo reported that a consistent and reproducible BV could be acquired using a portable bladder scanner. It was concluded that the CTV-to-PTV (PTV: planning target volume) margin was reduced from 11.1 to 6.4 mm in the superior–inferior direction14, 15. However, multiple measurements might be required until the BV is consistent with that of the planning computed tomography (CT). This possibility raised concerns regarding patient comfort, additional labour for the technician, and increases in waiting time and cost.
The aim of this study was to propose a model that predicted the likely BV and to reduce the number of pre-treatment ultrasound (US) measurements. The model could be incorporated into the current protocol of image-guided radiotherapy (IGRT). By applying the enhanced protocol, we could achieve more consistent BVs and reduce patient pain and discomfort (due to holding the urine for a long time). In addition, several validation tests assessed the model accuracy based on US measurements of variable bladder filling. The possibility of a reduction in workload and patient burden were also analysed by examining an appropriate number of US measurements.
Materials and Methods
Patients
Forty-nine patients with stage IIb cervical cancer but without angiocardiopathy or diabetes were included. Ethics approval for this study was obtained from Chongqing Cancer Hospital’s ethics committee, and screening was performed. All patients gave written informed consent. All methods were performed in accordance with the relevant guidelines and regulations. The patients’ mean age was 55.3 yr (median, 55 yr; range, 40–75 yr), and their mean BMI was 23.0 kg/m2 (median, 23.2 kg/m2; range, 17.6–28.2 kg/m2). The mean daily water consumption was 319 ml (median, 300 ml; range, 60–600 ml). Definitive RT was performed consecutively on all patients. The patients were randomly divided into two groups: a model group (25 patients) and a test group (24 patients). The datasets in the model group were collected to establish a parameterized model, and those in the test group were used to validate this model. Patients in both groups were asked to empty their bladders and then underwent controlled water intake prior to the planning CT and each subsequent RT fraction. Water intake prior to each fraction depended on the patient’s initial BV at the time of the planning CT.
Bladder scan protocol
The BV measurements were made with a portable three-dimensional ultrasound bladder scanner (BVI9400, Verathon Medical B.V., Europe) for all patients. Then, on the CT day, a CT scan was performed. US measurements were taken throughout the treatment period, followed by pre-RT CBCT and post-RT bladder voiding. The post-void residual urine was also evaluated. The times at which the planning CT scan, US measurement, CBCT, and urination occurred were recorded.
For each patient in the model group, the first BV measurement occurred one hour after the patient drank the required amount of water but before each fraction was taken. Thereafter, multiple measurements might be required at regular 10-min intervals until the BV was consistent with that of the planning CT. Based on a previous report by our group, the default tolerance of BV is recommended to be within ±15% to maintain a constant BV14,15,16. This value might be updated after dosimetry data supporting the clinical effects are analysed.
In the test group, the first US measurement was taken at the predicted time based on the parameterized model. If the BV was not consistent with the expected value, which depended on the parameterized model with a correction factor, the patient was instructed to void his or her bladder or to wait for another measurement.
A parameterized model for mean urinary inflow rate
A parameterized model was developed based on several assumptions:
-
(1)
The BV increases linearly over time, and the mean urinary inflow rate (v tot ) is constant for each patient;
-
(2)
V tot is different for different patients;
-
(3)
V tot is affected mainly by three independent variables, namely, age (P age ), water consumption (P wat ), and body mass index (P bmi );
-
(4)
The correlation between v tot and P age , P wat or P bmi is linear, and v tot is a linear superposition of these functions. Thus,
where v 0 is a constant, called the net urinary inflow rate; v age , v wat , and v bmi are the corresponding partial inflow rates affected by age, water intake, and body mass index, respectively; and k age , k wat , and k bmi are the coefficients.
Data analysis
The entire outer surface of the bladder was contoured on the axial slices using CT or CBCT images by experienced radiologists and radiation oncologists. Statistical analyses were performed using the Statistical Package of Social Sciences (IBM SPSS statistics version 22, SPSS22) program, where the threshold for statistical significance was p < 0.05. Due to the Gaussian distribution of the BV variation, the linearity of the time trend of BV and the correlation between v tot and P age , P wat or P bmi were tested using Pearson’s coefficient. The relationships among these variables were tested by performing multivariable linear regression with SPSS22. In addition, an iterative fitting method was proposed to double-check these correlations. A detailed description of this analysis is presented in the next section. The difference in the mean urinary inflow rate between the two methods was analysed using paired t-tests.
An iterative fitting method
In an investigation of the parameters that influence the urinary inflow rate, Heidi et al. showed that the impact of body mass index (P bmi ) on the urinary inflow rate was negligible17. Hence, k bmi = 0 and Equation 1 were simplified to only two arguments, P wat and P age , and one constant, v 0 . In the model group, 4 patients had the same P wat value. Using the datasets of these patients, k wat was assigned a specific value, and Equation 1 was simplified again to only one argument, P age . The mean value of k age was derived from the above four patients as an initial value of k age (k age, ini ) to calculate the initial v age , while the rest of the equation, (v tot − k age, ini * P age ), was set as the initial value of (v 0 + v wat ). The correlations of initial v age vs. P age and initial (v 0 + v wat ) vs. P wat were derived simultaneously from linear fitting. The fitted parameters were considered the new values of k age and k wat , and they were used to calculate the new v age and (v 0 + v wat ). The new v age vs. P age and the initial (v 0 + v wat ) vs. P wat were fit. These loops were repeated until the difference between the fitted parameter and the two consecutive loops was less than 0.01. In addition, the correlation between v bmi vs. P bmi was tested using linear fitting.
Results
Time trend of bladder volume
The relative BV was defined as
and the relative deviation of the BV was defined as ΔV(t)/V 0 , where t is the time of the measurement. V 0 was the BV from the first US scan (t = 0 min), and V(t) was the measured BV at regular 10-min intervals until BV consistency was reached. ΔV(t) was the corresponding difference.
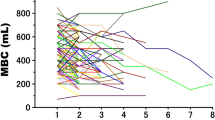
The relative BV as a function of time showed a linear behaviour for all 25 patients (p < 0.01). Figure 1 shows the BV as a function of time for one individual (R = 0.996, p = 0.00), and the error bars indicate the standard deviation. Due to the pre-RT variable waiting times and the repeated US measurements, the positions of the data points in Fig. 1 are around the predefined time measurements (i.e.,10, 20, 30, 40, 50 and 60 min). Two types of linear fitting were performed: 1) no uncertainty in scanning time, v tot = 3.356 ± 0.060 ml/min with χ2 (the goodness of the fit)/degrees of freedom (ndf) = 5.647/11 (probability, prob = 0.896); 2) maximum uncertainty in time due to multi-scanning, v tot = 3.534 ± 0.157 ml/min (χ 2 /ndf = 4.145/10, prob = 0.940). The width of the brown box in Fig. 1 represents the maximum uncertainty of 2.18 min. The sub-panel in Fig. 1 shows the corresponding relative deviation of the BV, and Gaussian fitting was performed with χ 2 /ndf = 8.873/16 (prob = 0.918).
A parameterized model
The v tot was correlated with P age (R = −0.53, p = 0.01) and P wat (R = 0.84, p = 0.00), and no relation between v tot and P bmi was found (p > 0.05).
A multivariable linear regression method
The relations between v tot and P age , P wat or P bmi could be expressed as follows:
The impact of P bmi on v tot was negligible and was verified once again in Equation 9. Thus, only two variables, P age and P wat , were used to represent v tot .
An iterative fitting method
The optimal solutions of Equation 1 (following several loops) are shown on the left-side of Fig. 2. The top panel of Fig. 2 shows (v 0 + v wat ) as a function of water intake in millilitres, and the middle panel shows v age as a function of age in years. V bmi was plotted as a function of P bmi , as shown in the bottom panel of Fig. 2. Linear fitting was performed. The values of k wat , k age or k bmi are the slope of the corresponding linear fitting lines, and v 0 is the ordinate at the origin; thus,
The left panel: v bm i as a function of BMI in kg/m2 together with linear fitting (bottom); v age as a function of age in years together with linear fitting (middle); (v 0 + v wat ) as a function of water intake in millilitres together with linear fitting (top). The right panel: The mean urinary inflow rates of all patients, including the measured value and the calculated value using two methods (top); the difference between the measured value and the calculated value (bottom).
All linear fits had high probabilities ((v 0 + v wat ): prob = 0.9997; v age : prob = 0.9997; v bmi : prob = 1).
The urinary inflow rates for the 25 patients are shown in Fig. 2 (right, top). The red points are the mean inflow rates for the individuals, and the error bars indicate standard deviations. Despite the provided drinking instructions, there was large inter-patient variation in the inflow rate (0.19 to 5.13 ml/min). No significant difference was observed between the calculated inflow rates using the two methods (blue line: the fitting method; pink line: the regression method). The difference between the measured and the calculated v tot is shown in the lower right panel of Fig. 2. Two linear fits were used to obtain the mean relative deviation of v tot (the fitting method: 0.07 ± 0.34; the regression method: −0.02 ± 0.40). Therefore, the maximum relative deviation of 0.42 was used as the tolerance in the validation test of this parameterized model.
The validation test of this model
Through US measurements taken in the test group 0 and 10 min after emptying the bladder before drinking water, two BV values were obtained, and the net urinary inflow rate, v 0 , was calculated using the expression (BV(10 min) −BV(0 min))/10. The mean of all 24 measured values was 2.51 ± 1.76 ml/min (black star in the left panel of Fig. 3). The calculated v 0 was 2.59 ± 0.63 ml/min (dash-dot line plus yellow band, iterative fitting) or 3.12 ± 1.13 ml/min (dotted line plus green band, linear regression) using the parameterized model. These values were consistent with v 0 within one standard deviation.
The comparison of net urinary inflow rates in the test group between measured and calculated values (left); the mean urinary inflow rates of all patients in ml/min in the test group, including the measured value and the calculated value using two methods (right, top) and the difference between two methods (right, bottom); SD: standard deviation.
After plugging the values of P age and P wat into Equations 14 and 19 for all cases in the test group, the mean urinary inflow rate v tot was obtained, as shown in the upper right panel of Fig. 3 (purple band plus yellow-green band). The bands represent the range of the calculated v tot using two methods, and the red stars are the measured v tot using the US scanner. Only 5 cases showed substantial discrepancies, and the relative deviation between the calculated v tot and the measured v tot was larger than 0.42 (purple band), as seen in the lower right panel of Fig. 3. Nineteen patients (79.2% of all patients) showed a high degree of consistency (≤0.42).
US scanning number and waiting time before RT
The waiting time and US scanning number before RT are shown in Fig. 4. In this work, the waiting time was defined as the amount of time that elapsed between one hour after drinking water (the model group) and the true RT or the predicted RT based on the parameterized model (the test group). In the model group, 6.5% of the patients had zero wait time for one US, while this percentage was 41.2% in the test group. In 71.2% of the RT fractions, the waiting time ranged from 12 min to 60 min in the model group, while this was the case for 54.2% of the cases in the test group. For the US scanning number, the peak value of the distribution was 1 for the test group (41.2%) and 3 for the model group (34.1%). All of the RT fractions achieved BV consistency within 5 US scans in the test group, but 3.5% of cases in the model group could not achieve the desired consistency.
Discussion
In the previous literature, a portable ultrasonic instrument was adopted to evaluate bladder filling with a short measurement time. This instrument showed a high precision volume readout for standard bladder phantom and actual urine15. In this study, we used this US scanner to investigate the BV change and mean urinary inflow rate (v tot ) in relation to age (P age), water consumption (P wat ), and body mass index (P bmi ). We found that v tot was only correlated with P wat and P age (R = −0.53), which was consistent with Lotz’s report in which v tot was negatively correlated with age (R = −0.50, p = 0.038)17. No relation between P bmi and v tot was found. Therefore, we proposed a model to predict v tot and tried to provide a comfortable RT strategy for new patients with cervical cancer.
A patient’s respiration and level of stress, as well as technician proficiency, have been found to affect the accuracy of BV measurements. Multiple US scans (>5) were adopted to reduce this deviation in the current study. A US scan usually takes approximately 12 seconds. Therefore, a complete BV measurement for a patient might take approximately 1 min. When we calculated v tot , the mean measurement time value was used, and the maximum time uncertainty was approximately 30 seconds. In view of this uncertainty, we used two linear fitting methods, and the results from these two methods were consistent within one standard deviation. In fact, the true time uncertainty was far less than 2.18 min. The discrepancy between them was very small. Therefore, we obtained v tot using the first method without uncertainty in the scanning time.
Figures 2 and 3 indicate a large intra-patient variation in the inflow rate (0.19 to 5.13 ml/min), but the net urinary inflow rate v 0 was 2.59–3.12 ml/min. In Sabrina’s report, the bladder inflow rate was 3.0 ± 2.7 ml/min over all treatment fractions, agreeing with our results within one standard deviation. In addition, we found a significant correlation between the bladder inflow rate and the average length of the systematic cervix-uterus intra-fraction displacement18. The inflow rate might be used to predict the cervix-uterus position during treatment. In the future, we will focus on modelling the correlation between the inflow rate and the cervix-uterus position, which could help to design an automatic plan for cervical cancer.
In this study, the median pre-treatment water consumption was 300 ml and ranged from 60 to 600 ml. These values covered most of the water-drinking amounts. Figure 4 shows that the patients without model assistance achieved BV consistency in 6.5% of RT fractions one hour after water intake, which was higher than those at other time points. This finding might be one of reasons for the success of most commonly used instructions involving drinking 300, 500 or 600 ml of water 1 hour before RT9, 18,19,20. At the same time, we found that BV consistency was achieved in 77.6% of the fractions within 2 hours after water intake. This finding indirectly verified Sabrina’s instruction to drink 300 ml of water 2 hours pre-RT18. In the clinical practice, 79.2% of patients’ v tot could be predicted accurately, and 41.2% of the treatment fractions could be performed successfully after one US scan with the parameterized model using the US scanner. However, in more than 50% treatments, the parameterized model should be modified due to changes in weather, treatment reactions, and the timing of each appointment during RT fraction.
Considering the above findings, we suggested that patients drink a specified amount of water until they had a comfortably full bladder after emptying their bladder. Then, the BV was measured at a specific time for each RT fraction, which depended on the v tot (v tot, old ) calculated by Equation 14 or 19. If the first measured BV was consistent with the value on the planning-CT day, RT could be delivered. If not, a new v tot (v tot, new ) was calculated using the BV at the specific time divided by the time interval. The difference between v tot, old and v tot, new was regarded as a correction factor to guide the next US scan until BV consistency was achieved. In our practice, 95.5% of fractional RT was performed within 3 US scans, as shown in Fig. 4.
Over the course of RT, a systematic reduction in mean BV was found21. A decrease in mean BVs from 156 to 88 cm3 between the first and last weeks of treatment was reported3. Some adaptive strategies with weekly magnetic resonance imaging (MRI) and daily CBCT were discussed in previous studies16, 22. James reported that all patients achieved clinically acceptable target coverage while maintaining organ-at-risk dose sparing after weekly re-planning19. Recently, Luo found that the mean BV during the course of RT was approximately equal to the initial BV with US-scanner assistance15.
In view of the results from this study and the above-noted previous reports and certain impact factors including the price of a US scan (approximately 1/13 CBCT cost; approximately 1/17 MRI cost) and technician labour, we propose a new IGRT protocol for cervical cancer involving daily US measurements and weekly CBCT and MRI scans based on this model. We only need one specialized technician to calculate the US scanning and RT time and measure BV in a clinical preparation room, while treatment technicians deliver RT and conduct the patient’s weekly imaging in order to perform an adaptive RT. This strategy could reduce the patient discomfort caused by holding onto the urine, as well as reduce technician labour and cost.
References
Kidd, E. A. et al. Clinical outcomes of definitive intensity-modulated radiation therapy with fluorodeoxyglucose-positron emission tomography simulation in patients with locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 77, 1085–1091 (2010).
Hasselle, M. D. et al. Clinical outcomes of intensity-modulated pelvic radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 80, 1436–1445 (2011).
Beadle, B. M. et al. Cervix regression and motion during the course of external beam chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys 73, 235–241 (2009).
Haripotepornkul, N. H., Nath, S. K., Scanderbeg, D., Saenz, C. & Yashar, C. M. Evaluation of intra- and inter-fraction movement of the cervix during intensity modulated radiation therapy. Radiother Oncol. 98, 347–351 (2011).
Latifi, K., Zhang, G. G., Moros, E. G. & Harris, E. E. Assessment of intact cervix motion using implanted fiducials in patients treated with helical tomotherapy with daily MVCT positioning. J Radiat Oncol 2, 323–329 (2013).
Taylor, A. & Powell, M. E. An assessment of interfractional uterine and cervical motion: Implications for radiotherapy target volume definition in gynaecological cancer. Radiother Oncol. 88, 250–257 (2008).
Jadon, R. et al. A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin Oncol. 26, 185–196 (2014).
Georg, P., Georg, D., Hillbrand, M., Kirisits, C. & Pötter, R. Factors influencing bowel sparing in intensity modulated whole pelvic radiotherapy for gynaecological malignancies. Radiother Oncol. 80, 19–26 (2006).
Knight, K. A. Patient positioning and treatment instructions used during radiation therapy of the prostate: Results of an Australian and New Zealand survey. J. Med. Radiat. Sci 52, 8–13 (2005).
Jhingran, A., Salehpour, M., Sam, M., Levy, L. & Eifel, P. J. Vaginal motion and bladder and rectal volumes during pelvic intensity-modulated radiation therapy after hysterectomy. Int J Radiat Oncol Biol Phys 82, 256–262 (2012).
Tirado, J. L. et al. Evaluation of image guided radiotherapy (IGRT) in lung cancer. Is weekly cone beam CT (CBCT) enough? Radiother Oncol. 115, s1 (2015).
Ottosson, W., Baker, M., Hedman, M., Behrens, C. F. & Sjöström, D. Evaluation of setup accuracy for NSCLC patients; studying the impact of different types of cone-beam CT matches based on whole thorax, columna vertebralis, and GTV. Acta Oncol. 49, 1184–1191 (2010).
Matthias, G. Image-guided radiotherapy based on kilovoltage cone-beam computed tomography-A review of technology and clinical outcome. Eur Oncol Haemato 7, 121–124 (2011).
Luo, H. L. et al. Comparative study on evaluating the bladder volume between BladderScan BVI9400 and ultrasound system iU22. Chin. J. of Med. Instrum 39, 295–298 (2015).
Luo, H. L. et al. Interfractional variation in bladder volume and its impact on cervical cancer radiotherapy: Clinical significance of portable bladder scanner. Med. Phys. 43, 4412–4419 (2016).
Chan, P. et al. Inter- and intrafractional tumor and organ movement in patients with cervical cancer undergoing radiotherapy: a cinematic-MRI point-of-interest study. Int J Radiat Oncol Biol Phys 70, 1507–1515 (2008).
Lotz, H. T. et al. Reproducibility of the bladder shape and bladder shape changes during filling. Med. Phys. 32, 2590–2597 (2005).
Heijkoop, S. T. et al. Quantification of intra-fraction changes during radiotherapy of cervical cancer assessed with pre- and post-fraction Cone Beam CT scans. Radiother Oncol. 117, 536–541 (2015).
Stewart, J. et al. Automated weekly replanning for intensity-modulated radiotherapy of cervix cancer. Int J Radiat Oncol Biol Phys 78, 350–358 (2010).
Bondar, L. et al. Toward an individualized target motion management for IMRT of cervical cancer based on model-predicted cervix-uterus shape and position. Radiother Oncol. 99, 240–245 (2011).
Collen, C. et al. Volumetric imaging by megavoltage computed tomography for assessment of internal organ motion during radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 77, 1590–1595 (2010).
Tyagi, N. et al. Daily online cone beam computed tomography to assess interfractional motion in patients with intact cervical cancer. Int J Radiat Oncol Biol Phys 80, 273–280 (2011).
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant No. 11575038, the Scientific and Technological Research Program of Chongqing Municipal Education Commission (No. KJ1400102) and the Medicine Scientific Research Project of Chongqing Health Bureau (No. 2015MSXM229).
Author information
Authors and Affiliations
Contributions
Conception and design: F.J., H.-L.L., J.Z. Development of methodology: F.J., H.-L.L. Acquisition of data: H.-L.L., D.-Y.Y., L.Y., X.-Q.Y., Y.-N.H., H.Y., C.L., M.-S.Z., D.Q., X.-F.L., Q.-C.L., G.-L.H. Analysis and interpretation of data: F.J., H.-L.L., X.-Q.Y. Writing, review and/or revision of the manuscript: F.J., H.-L.L., J.Z., Y.W. Administrative, technical, or material support: F.J., H.-L.L., Y.W. Study supervision: F.J., H.-L.-L., J.Z., D.-Y.Y., L.Y., X.-Q.Y., Y.-N.H., H.Y., C.L., M.-S.Z., D.Q., X.-F.L., Q.-C.L., G.-L.H.,Y.W.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jin, F., Luo, HL., Zhou, J. et al. A parameterized model for mean urinary inflow rate and its preliminary application in radiotherapy for cervical cancer. Sci Rep 7, 280 (2017). https://doi.org/10.1038/s41598-017-00356-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00356-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.