Abstract
Hepatocytes are a major parenchymal cell type in the liver and play an essential role in liver function. Hepatocyte-like cells can be differentiated in vitro from induced pluripotent stem cells (iPSCs) via definitive endoderm (DE)-like cells and hepatoblast-like cells. Here, we explored the in vitro differentiation time-course of hepatocyte-like cells. We performed methylome and transcriptome analyses for hepatocyte-like cell differentiation. We also analyzed DE-like cell differentiation by methylome, transcriptome, chromatin accessibility, and GATA6 binding profiles, using finer time-course samples. In this manuscript, we provide a detailed description of the dataset and the technical validations. Our data may be valuable for the analysis of the molecular mechanisms underlying hepatocyte and DE differentiations.
Similar content being viewed by others
Background & Summary
Hepatocytes compose approximately 70–85% of the liver mass and are crucial for the normal functioning of the liver. They are involved in activities such as detoxification, glycolytic and urea metabolism, control of blood cholesterol levels, and production of bile and hormones. Hepatocyte deficiencies such as hepatitis, non-alcoholic steatohepatitis (NASH), cirrhosis, and liver cancer result in severe health problems.
There is a great need for hepatocytes in medical and pharmaceutical applications. Because the liver is a central organ for foreign compound metabolism, hepatocytes are sensitive to drug toxicity. Therefore, hepatocytes isolated from the liver are also used to analyze pharmacokinetics and hepatotoxicity ex vivo. In addition, liver transplantation is an effective approach to the treatment of hepatic disorders, particularly end-stage liver disease. Ex vivo generation of the hepatocyte/liver is a promising alternative to the use of in vivo liver for such purposes.
During embryogenesis, hepatocytes are sequentially differentiated from pluripotent stem cells (PSCs) in the inner cell mass, definitive endoderm (DE) cells, and hepatoblasts. Many protocols for in vitro hepatic differentiation from PSCs, such as embryonic stem cells and induced pluripotent stem cells (iPSCs), have been described with different efficiencies and functionalities1,2,3,4,5,6,7,8. One of the earliest protocols, which is a standardized serum- and feeder-free differentiation protocol, showed highly efficient near-homogenous hepatocytic differentiation from a large panel of hPSC lines8. This protocol essentially mimics in vivo differentiation in three steps. First, PSCs differentiate into DE-like cells. Second, DE-like cells are committed to hepatoblast-like cells via the ventral foregut-like cells. Finally, the hepatoblast-like cells differentiated into fetal-like hepatocyte-like and hepatocyte-like cells. Because in vitro differentiated hepatocyte-like cells express key enzymes for detoxification, they are expected to be used in drug metabolism models.
During this process, precise successive alteration of gene expression profiles is essential for hepatic differentiation, which is governed by several key transcription factors. For example, GATA6 is a pivotal transcription factor for DE commitment and is, therefore, essential for liver development9.
In addition to transcription factors, gene expression is regulated by epigenome and chromatin levels. Importantly, the epigenome and chromatin structure are also controlled by proteins, indicating a complex regulatory mechanism among these factors.
In the present study, we explored changes in gene expression and DNA methylation using cap analysis gene expression (CAGE) and Infinium MethylationEPIC human methylation beadchips with a robust, standardized serum- and feeder-free hepatocyte differentiation protocol8. Furthermore, we analyzed gene expression, DNA methylation, chromatin accessibility, and GATA6 binding profile in the time range of DE-like cell commitment. Using this dataset, we previously investigated the regulatory relationship between transcription factors, epigenome, and chromatin structure10. In addition, our comprehensive time-course omics dataset can be reused for a detailed analysis of the molecular mechanisms underlying hepatocyte-like cell differentiation. Notably, taking advantage of CAGE, which provides highly quantitative transcriptional start sites and their activity, analysis of enhancers, lncRNAs, and alternative promoters is also available.
Methods
Study design
Figure 1A illustrates the dataset acquired in this study. We obtained two in vitro differentiation time-course datasets: iPSCs to hepatocyte-like cells and iPSCs to DE-like cells. The hepatic differentiation was performed using the Cellartis® Hepatocyte Differentiation Kit (Takara Bio Inc., Shiga, Japan). We collected the samples every seven days until day 28 (day 0, day 7, day 14, day 21, and day 28 of the differentiation). Each time point represents a different stage of hepatic differentiation. Days 0 and 7 represent the stages of undifferentiated iPSCs and DE-like cells, respectively. DE marker expression is shown in Fig. 1B. Day 14 is the endpoint of the cultivation with the Progenitor medium, an intermediate stage between DE-like cells and hepatocyte-like cells. Day 21 and Day 28 correspond to the stage of the hepatocyte-like cell. The characteristics of the hepatocyte-like cells were shown in our previous report10. Methylome data were acquired using Infinium MethylationEPIC beadchips, and transcriptome data were acquired by CAGE. The DE-like cell differentiation was performed using the Cellartis® Definitive Endoderm Differentiation Kit (Takara Bio Inc.), equivalent to the DE-like cell differentiation step of the Cellartis® Hepatocyte Differentiation Kit (Takara Bio Inc.). The DE-like cell differentiation time-course samples were obtained starting iPS cells and every 6 hours from 48 hours to 72 hours (0, 48, 54, 60, 66, and 72 hours) to cover the timing of GATA6 upregulation. Methylome data acquired by Infinium MethylationEPIC beadchips, transcriptome data acquired by CAGE, GATA6 binding profile acquired by chromatin immunoprecipitation, and chromatin accessibility acquired by OmniATAC-seq were obtained.
Data generation. (A) Time-course samples of in vitro hepatocyte differentiation were obtained at 0, 7, 14, 21, and 28 days after initiation of the differentiation. The obtained samples were subjected to Infinium MethylationEPIC beadschips and CAGE. Time-course samples of in vitro DE differentiation were obtained 0, 48, 54, 60, 66, and 72 hours (h) after initiation of the differentiation. The obtained samples were subjected to Infinium MethylationEPIC beadschips, CAGE, GATA6 ChIPmentation, and OmniATAC-seq. The time points when samples were collected are shown as bold-red characters. The culture condition of hepatocyte and DE differentiation are shown at the upper and lower of each schematic, respectively. (B) Immunocytochemistry for FOXA2 and SOX17 in Day 7 DE-like cells. The scale bar is 100 μm.
iPS cell culture condition
The 201B7 human iPS cell line, which was derived from the skin of a 36-year-old female with retroviral vectors of the four Yamanaka factors (Oct3/4, Sox2, Klf4, c-Myc), was acquired from the RIKEN BioResource Center (BRC). The iPS cells cultured with mouse STO feeder cells were converted to feeder-free culture conditions using the Cellartis® DEF-CS™ Culture System (Takara Bio Inc.).
Definitive endoderm-like cell and Hepatocyte-like cell differentiations
iPS cells were sub-cultured at a density of 4.2 × 104 cells/cm2 three days before the in vitro differentiation. iPSCs were differentiated into DE-like cells using the Cellartis® Definitive Endoderm Differentiation Kit (Takara Bio Inc.) for seven days according to the manufacturer’s instructions. The obtained DE cells were detached and dissociated using TrypLE Select (Thermo Fisher Scientific Inc., Waltham, MA). The DE cells were seeded at 1.3 × 105 cells/cm2 and were differentiated into hepatocyte-like cells using Cellartis® Hepatocyte Differentiation Kit (Takara Bio Inc.).
Immunocytochemistry
The Day 7 DE-like cells cultured on a cover glass were fixed in 4% formaldehyde for 10 min, followed by blocking with 1% skim milk. The cells were incubated for 24 hours at 4 °C with anti-human FOXA2(Abcam, Cambridge, UK; cat no. ab60721, lot no. GR3426450-2) and anti-human SOX17(Abcam; cat no. ab224637, lot no. GR3449941-5) antibodies diluted to 1:00 by the antibody reaction buffer (1% BSA 0.2% Triton-X100 containing D-PBS(+/+)) for 24 hours at 4 °C. After washing in D-PBS(+/+) twice, the cells were incubated for 1 hour at RT with Alexa Fluor 488-conjugated anti-mouse IgG (Thermo Fisher Scientific Inc., Waltham, MA, USA) and Alexa Fluor 594-conjugated anti-rabbit IgG(Thermo Fisher Scientific Inc.) secondary antibodies diluted to 1:0000 by the antibody reaction buffer. The cells were mounted in slow-fade (Thermo Fisher Scientific Inc.) and analyzed by a BZ-X810 fluorescent microscope (Keyence Corporation, Osaka, Japan).
DNA extraction
Cells were detached using TrypLE Select (Thermo Fisher Scientific Inc.) and pelletized. The cells were stored at −80 °C until use. DNA extraction was performed using NucleoSpin Tissue (Takara Bio Inc.) according to the manufacturer’s instructions.
Methylation array
Bisulfite conversion was performed using the EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA, USA) with 500 μg of genomic DNA. Bisulfite-converted DNA was hybridized to Infinium MethylationEPIC BeadChips according to the manufacturer’s instructions.
RNA extraction
Cells were detached using TrypLE Select (Thermo Fisher Scientific Inc.) and lysed in 350 μL lysis buffer (LBP) of NecleoSpin RNA Plus (Takara Bio Inc.). The lysed cells were stored at −80 °C until use. RNA extraction was performed using NucleoSpin RNA plus (Takara Bio Inc.) according to the manufacturer’s instructions.
Cap analysis gene expression
For CAGE library construction, 3 μg of extracted total RNA was reverse-transcribed using SuperScript III reverse transcriptase (Thermo Fisher Scientific Inc.), and a diol residue of the cap structure was biotinylated, followed by RNase I treatment using RNase ONE ribonuclease (Promega Corporation, Madison, WI, USA). The RNA-cDNA hybrids were captured using streptavidin-coated magnetic beads (Thermo Fisher Scientific Inc.), and only single-stranded cDNAs were released from the beads. Barcoded 5′- and 3′- linkers were ligated to the single-stranded cDNA, followed by 2nd strand synthesis using Deep Vent (exo-) DNA polymerase (New England BioLabs, Ipswich, MA, USA). CAGE library construction was performed using three biological replicates. Eight hepatocyte differentiation time-course or seven DE differentiation libraries were equally multiplexed. A multiplexed library was sequenced in a 50 bp single-end on one lane of the Hiseq. 2500 (Illumina Inc., San Diego, CA, USA).
OmniATAC-sequencing
Cells were detached using TrypLE Select (Thermo Fisher Scientific Inc.) and stored at −80 °C in STEM-CELLBANKER GMP grade (Takara Bio Inc.) until use. The cells were quickly defrozen in a 37 °C water bath. The 5 × 104 cells were washed in PBS twice, and nuclei were extracted in l cold ATAC-Resuspension Buffer (RSB) containing 0.1% NP40, 0.1% Tween20, and 0.01% Digitonin. The nuclei were resuspended in a transposition mixture (25 ul 2x TD buffer, 2.5 ul transposase (100 nM final), 16.5 ul PBS, 0.5 ul 1% digitonin, 0.5 ul 10% Tween-20, 5 ul H2O) and incubate at 37 °C for 30 min in a thermomixer with 1,000 RPM mixing. DNA was extracted from the reaction using the Zymo DNA Clean and Concentrator Kit (Zymo Research, Irvine, CA, USA). The sequencing library was generated using NEBNext Ultra DNA Library Prep Kit for Illumina (New England BioLabs) with five cycles followed by three to seven cycles of pre- and PCR-amplification, respectively. The amplified library was purified with Zymo DNA Clean and Concentrator kit (Zymo Research), followed by size-selection with SPRIselect (1:0.6 and 1:0.2 sample vol. to beads vol.; Beckman Coulter, CA, USA). The fragment size of the OmniATA-seq libraries was checked by Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA). The fragment size of each library was mainly between 200 bp and 600 bp. OmniATAC was performed in two biological replicates. The concentration of the OmniATAC libraries was measured using KAPALibraryQuantificationKits (F. Hoffmann-La Roche, Ltd., Basel, Swiss Confederation). All the OmniATAC libraries were equally multiplexed and sequenced in a 50 bp single end on one lane of the Hiseq. 2500 (Illumina Inc.).
GATA6 ChIPmentation sequencing
Cells were detached using TrypLE Select (Thermo Fisher Scientific Inc.) and fixed in 1% formaldehyde for 8 min at RT. The fixation was quenched by adding glycine solution at a final concentration of 200 mM and incubated for 10 min at RT. The fixed cells were snap-frozen in liquid nitrogen and stored at −80 °C until use. ChIP was performed using auto ChIPmentation for TF kit (Diagenode SA., Seraing, Belgium) according to the manufacturer’s instructions. Briefly, two million fixed cells were lysed and sonicated using Picoruptor® (Diagenode SA.) in 1.5 mL Bioruptor Microtubes for ten cycles (1 cycle: 30 s sonication and 30 s “off”) at 4 °C. Magnetic immunoprecipitation and tagmentation were performed on the SX-8G IP-STAR® Compact Automated System (Diagenode SA.) with an anti-GATA6 antibody (D61E4, Cell Signaling Technology, Inc.). Chromatin was extracted from the magnetic beads in the stripping reagent for 30 min at 50 °C, followed by end-repair and reverse cross-linking. Illumina sequencer convertible libraries were generated and amplified by nine cycles of PCR. The optimal size of the sequencing libraries (200 bp) was purified using AMPure XP beads (1:1.8 sample vol. to beads vol.; Beckman Coulter), and the purification result was confirmed by Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA). ChIPmentation was performed in two biological replicates. The concentration of the ChIPmentation libraries was measured using KAPALibraryQuantificationKits (F. Hoffmann-La Roche, Ltd). All the ChIPmentation libraries were equally mixed and sequenced using 150 bp paired-end reads on one lane of the HiSeq X (Illumina Inc.).
Computational methods
Methylation array data processing
Raw intensity data (IDAT) of Infinium MethylationEPIC BeadChips were read into MethyLumiSet objects using the readEPIC function implemented in the watermelon package (version 1.34.0) of R. Color balance adjustment and normalization by quantile normalization were performed using lumiMethyC and lumiMethyN functions of the lumi package (version 2.42) of R.
CAGE data processing
Raw sequencing data quality was checked by fastQC, and the results were summarized using MultiQC (Fig. 2). Raw CAGE sequence data were processed using MOIRAI, a web-based CAGE data processing workflow11. Briefly, the sequences were trimmed using a fastx-trimmer and fastx_clipper FASTX-toolkit (version 0.0.13). Ribosomal RNA reads were removed using rRNAdust (version 1.02) (fantom.gsc.riken.jp/5/suppl/rRNAdust/). Then, the sequences were mapped to the hg19 genome using STAR (version 2.5.3a). Mapped CAGE tags were counted for each FANTOM5 promoter (https://fantom.gsc.riken.jp/5/datafiles/latest/extra/CAGE_peaks/hg19.cage_peak_phase1and2combined_ann.txt.gz) using bedtools (version 2.26.0) and normalized as tags per million reads (TPM). The number of reads and mapping rate of each sample are shown in Table 1.
OmniATAC-seq data processing
Raw sequencing data quality was checked by fastQC, and the results were summarized using MultiQC (Fig. 2). Sequence reads were mapped to the hg19 genome using bowtie2 (version 2.3.0). The mapped reads were frozen, and reads mapped to the mitochondrial genome were removed using the removeChrom.py script of the Harvard ATAC-seq module. Peak calling was performed using MACS2 (version 2.1.1.20160309) with the following parameters:--shift −37,–extsize 73, -B –SPMR, and -p 10e-6. Peaks that overlapped with the ENCODE blacklist regions were removed. Bigwig coverage files were generated using the bam2wig.py script (version 2.6.4) of RSeQC with wigToBigWig (version 2.8). The number of reads and mapping rate of each sample are shown in Table 1.
ChIPmentation sequence data processing
Raw sequencing data quality was checked by fastQC, and the results were summarized using MultiQC (Fig. 2). Sequence reads were mapped to the hg19 genome using bowtie2(version 2.3.0) and reads mapped to the mitochondrial genome, and PCR duplicates were removed using the removeChrom.py script of the Harvard ATAC-seq module and samtools (version 1.9). Peak calling was performed using MACS2 (version 2.1.1.20160309) with a cut-off p-value < 10−6, and peaks that overlapped with the ENCODE blacklist regions were removed. Bigwig coverage files were generated, as described above. The number of reads and mapping rate of each sample are shown in Table 1.
Data Records
The raw fastq files, CTSS bed files and expression tables of CAGE, bigwig coverage files of omniATAC-seq, bigwig coverage files, and peak summit bed files were deposited at the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) under the accession ID of SuperSeries GSE16333112. The SuperSeries comprised the methylation array data SubSeries for hepatocyte-like cell differentiation (GSE163324) and DE-like cell differentiation (GSE163322), CAGE data SubSeries for hepatocyte-like cell differentiation (GSE163329), DE-like cell differentiation (GSE163328), omniATAC-seq data SubSeries for DE-like cell differentiation (GSE163327), and GATA6 chromatin immunoprecipitation data for DE-like cell differentiation (GSE163330).
Technical Validation
Validation of CAGE reproducibility
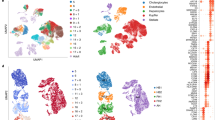
To evaluate the reproducibility of CAGE, we performed a principal component analysis based on the expression table (Fig. 3A,B). The replicate data for each time point were closely plotted in the 2D space, indicating high reproducibility.
De novo motif analysis of GATA6 ChIPmentation
To examine the enriched sequences by GATA6 ChIPmentation, de novo motif analysis was performed. Because there were few peaks at 0 h and 48 h (Fig. 4A), we analyzed time points of 56, 60, 66, and 72 h. At all analyzed time points, we detected GATA sequences containing motifs that were significantly similar to the known GATA motifs (Fig. 4B).
Technical validation of GATA6 ChIPmentation data. (A) A bar plot represents the peak count of each time point. The peaks were identified using MACS2 with a p-value < 10−6. (B) Motif overrepresented at ChIPpeaks. The top tiers with a gray background are the overrepresented de novo motifs. The lower tiers are known transcription factor-binding motifs similar to the overrepresented de novo motifs.
Read coverage distribution of OmniATAC-seq
Highly accessible regions are enriched in gene regulatory regions, such as promoters13. Therefore, we analyzed the distribution of OmniATAC-seq reads around known gene models. The OmniATAC-seq reads were highly enriched at transcription start sites (TSSs) (Fig. 5).
Methylation-level distribution of methylation array
The M-value of methylation assay data typically shows a bimodal distribution, representing hypomethylated and hypermethylated CpGs14. Our methylation array M-values also showed bimodal distribution (Fig. 6).
Code availability
The code for the data pre-processing and technical validations are available on GitHub (https://github.com/RIKEN-CFCT/hep_methyl_data).
References
Hay, D. C. et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proceedings of the National Academy of Sciences 105, 12301–12306 (2008).
Agarwal, S., Holton, K. L. & Lanza, R. Efficient Differentiation of Functional Hepatocytes from Human Embryonic Stem Cells. Stem Cells 26, 1117–1127 (2008).
Touboul, T. et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 51, 1754–1765 (2010).
Nakamura, N. et al. Feeder-free and serum-free production of hepatocytes, cholangiocytes, and their proliferating progenitors from human pluripotent stem cells: application to liver-specific functional and cytotoxic assays. Cell Reprogram 14, 171–185 (2012).
Liu, H., Kim, Y., Sharkis, S., Marchionni, L. & Jang, Y. Y. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med 3 (2011).
Si-Tayeb, K. et al. Highly efficient generation of human hepatocyte–like cells from induced pluripotent stem cells. Hepatology 51, 297–305 (2010).
Hannan, N. R. F., Segeritz, C. P., Touboul, T. & Vallier, L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc 8, 430–437 (2013).
Asplund, A. et al. One Standardized Differentiation Procedure Robustly Generates Homogenous Hepatocyte Cultures Displaying Metabolic Diversity from a Large Panel of Human Pluripotent Stem Cells. Stem Cell Rev Rep 12, 90–104 (2016).
Tiyaboonchai, A. et al. GATA6 Plays an Important Role in the Induction of Human Definitive Endoderm, Development of the Pancreas, and Functionality of Pancreatic β Cells. Stem Cell Reports 8, 589–604 (2017).
Suzuki, T. et al. GATA6 is predicted to regulate DNA methylation in an in vitro model of human hepatocyte differentiation. Communications Biology 2022 5:1 5, 1–12 (2022).
Hasegawa, A., Daub, C., Carninci, P., Hayashizaki, Y. & Lassmann, T. MOIRAI: A compact workflow system for CAGE analysis. BMC Bioinformatics 15, 1–7 (2014).
Suzuki, T. et al. GATA6 is predicted to regulate DNA methylation in an in vitro model of human hepatocyte differentiation. Gene Expression Omnibus https://identifiers.org/geo:GSE163331 (2022).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for multimodal regulatory analysis and personal epigenomics. Nat Methods 10, 1213 (2013).
Du, P. et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics 11, 1–9 (2010).
Acknowledgements
We thank Chung-Chau Hon for his useful advice in the data analysis. We are grateful to RIKEN IMS, Laboratory for Comprehensive Genomic Analysis, for Hiseq 2500 sequencing.
Author information
Authors and Affiliations
Contributions
Y.T. carried out the molecular biology studies and drafted the manuscript. S.M., E.F. and M.K. performed the molecular biology experiments. H.S. helped draft the manuscript and acquired funding. T.S. participated in the study’s design, devised the methodology, performed the computational analyses, acquired the funding, drafted the manuscript, and supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanaka, Y., Furuhata, E., Maeda, S. et al. A dataset of definitive endoderm and hepatocyte differentiations from human induced pluripotent stem cells. Sci Data 10, 93 (2023). https://doi.org/10.1038/s41597-023-02001-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-023-02001-9