Abstract
Asparagus bean (Vigna. unguiculata ssp. sesquipedialis), known for its very long and tender green pods, is an important vegetable crop broadly grown in the developing Asian countries. In this study, we reported a 632.8 Mb assembly (549.81 Mb non-N size) of asparagus bean based on the whole genome shotgun sequencing strategy. We also generated a linkage map for asparagus bean, which helped anchor 94.42% of the scaffolds into 11 pseudo-chromosomes. A total of 42,609 protein-coding genes and 3,579 non-protein-coding genes were predicted from the assembly. Taken together, these genomic resources of asparagus bean will help develop a pan-genome of V. unguiculata and facilitate the investigation of economically valuable traits in this species, so that the cultivation of this plant would help combat the protein and energy malnutrition in the developing world.
Design Type(s) | sequence assembly objective • sequence annotation objective |
Measurement Type(s) | genome assembly |
Technology Type(s) | DNA sequencing |
Factor Type(s) | |
Sample Characteristic(s) | Vigna unguiculata subsp. sesquipedalis • leaf |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Similar content being viewed by others
Background & Summary
Asparagus bean (Vigna unguiculata ssp. sesquipedialis, 2n = 2× = 22) is a warm-season and drought-tolerant subspecies of cowpea (Vigna unguiculata) with a wide cultivation area in East and Southeast Asia1. This plant is also known as yardlong bean because of its characteristic pod that grows up to 50–100 cm in length2. The long pod trait is believed to be the result of intensive local domestication after it was brought to Asia from sub-Saharan Africa3. Unlike the grain-type subspecies common cowpea (Vigna. unguiculata ssp. unguiculata, or black-eyed pea), asparagus bean is harvested while its pod is still tender, thereby providing a very good source of protein, minerals, vitamins, and dietary fiber4. Due to the low requirement for cultivation management and its high nutritional value, asparagus bean is one of the top crops that help combat malnutrition and food insecurity in most developing countries5.
As the DNA sequencing technologies became more advanced and affordable for the past decade, previous research had mainly focused on delineating the genome of common cowpea (estimated genome size of 620 Mb6). The first study of cowpea genomics was reported in 2008, in which the gene-rich space of cowpea was sequenced and assembled into 52,149 assemblies (41,260 assemblies were annotated) and 70,679 singletons7. Then the common cowpea (variety IT97K-499-35) genomic resources including a partial 323 Mb whole-genome shotgun assembly8, a 497 Mb bacterial artificial chromosome physical map8, and consensus genetic maps based on either 10 K9 or 50 K single nucleotide polymorphisms (SNPs) were available8. A more recent research reported two survey genomes of common cowpea (varieties IT97K-499-35 and IT86D-1010) with substantially improved assembly sizes (568 Mb and 609 Mb, respectively)10. In addition, a draft IT97K-499-35 variety reference genome was assembled by incorporating the single molecule real-time technology, yielding an assembly size of 519.4 Mb into 722 scaffolds and 11 pseudo-chromosomes11. Three genetic maps were derived from either simple sequence repeat markers12,13 or restriction-site associated DNA sequencing for asparagus bean14. Most of these genetic resources are focus on the grain-type cowpea, but there are many differences between the two types of cowpea, such as morphology, growing environments and parts for use12.
In this study, we aimed to fill the knowledge gap with regard to the asparagus bean genome and provide new genetic resources for breeding cowpea and related legume species. A schematic workflow of the research is shown in Fig. 1. In brief, a series of short-insert and large-insert Illumina libraries were sequenced on an Illumina HiSeq 4000 platform, yielding a total of 222.9 Gb clean data (Table 1). Since the genome size of asparagus bean was estimated to be about 590 Mb using the K-mer distribution analysis (Table 2) (Fig. 2), the clean data used for genome assembly represented about 340× coverage. The software SOAPdenovo15 was used to generate a draft contig assembly of 549.8 Mb with a contig N50 size of 15.2 kb (Table 3). After scaffolding and gap closing, the final asparagus genome was 632.8 Mb (549.81 Mb non-N size) in size with scaffold N50 size of 2.7 Mb (Table 3). We also obtained 536,824 high-confident SNPs from the whole-genome sequencing data of 97 asparagus bean F2 individuals and two parents from a well-controlled selfing population. These SNPs were used to construct a high-density genetic map for asparagus bean, in which 1,556 scaffolds were successfully anchored onto 11 pseudo-chromosomes (Table 4). Furthermore, the asparagus bean genome contained 294.95 Mb of transposable elements, accounting for 46.47% of the assembly (Tables 5 and 6). The gene prediction was performed on a combination of de novo, homologous, and RNA-Seq-based approaches. It resulted in 42,609 protein-coding genes and 3,579 non-protein-coding genes, respectively (Table 7).
Methods
Materials
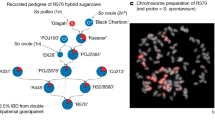
All plant accessions were provided by Hubei Natural Science Resource Center for Edible Legumes in Wuhan of China. A single plant of the widely cultivated asparagus bean variety ‘Xiabao II’ (Vigna unguiculata ssp. sesquipedialis var. ‘Xiabao II’) was used for de novo sequencing and genome assembly. A F2 sequencing population was obtained for making the genetic map according to the following procedure. First, the F1 population were obtained by crossing ‘Xiabao II’ (male, same plant used for de novo sequencing) with a cultivar from the other subspecies, ‘Duanjiangdou’ (Vigna unguiculata ssp. unguiculata var. ‘Duanjiangdou’; female). This step yielded 17 seeds, from which only 12 seeds survived till flowering. These F1 individuals were bagged to promote selfing, which produced 561 seeds in total (the F2 generation). Only 367 of the F2 individuals were able to germinate and mature into full plants. We selected 97 of the 367 F2 individuals for genome sequencing and genetic map construction.
Whole-genome shotgun sequencing
Young leaves were collected from a single ‘Xiabao II’ plant and used for genomic DNA extraction by the CTAB method16. About 10 µg of genomic DNA were used for library construction. Four short-insert libraries (350 bp, 445 bp, 758 bp, and 912 bp) and five large-insert libraries (2 kb, 3 kb, 5 kb, 9 kb, and 15 kb) were constructed with NEBNext Ultra II DNA Kit (NEB, America) and Nextera Mate Pair Sample Preparation Kit (Illumina, America), respectively. These libraries were sequenced on an Illumina HiSeq 4000 platform. To ensure high-quality reads for the subsequent de novo assembly step, we filtered out the low-quality data by the following criteria: (a) reads with >2% unidentified nucleotides (N) or with poly-A structure; (b) reads with ≥40% bases having low quality for short insert-size libraries and ≥60% for large insert-size libraries; (c) reads with adapters or PCR duplication; (d) reads with 20 bp in 5′ terminal and 5 bp in 3′ terminal. Subsequently, about 222.9 Gb clean data were retrieved17,18, covering 341.66-fold of the estimated genome (Table 1).
The genomic DNA was extracted with the same procedure for the parents and all 97 F2 individuals in the resequencing population. Each DNA was used to construct 500 bp insert size libraries, which were then sequenced on an Illumina HiSeq 4000 platform. Each individual was sequenced to at least 4× coverage. NGSQCToolkit_v2.3.319 was used to filter low-quality reads (parameters: −l 70 −s 25) and trim the poor-quality terminal bases (parameters: −l 5 −r 5). A total of 882.67 Gb clean bases were kept, which represented 99% of the raw sequencing data17,18.
Estimation of the genome size
The genome size of asparagus bean was estimated by the k-mer analysis approach using 69.42 Gb filtered short-insert sequencing data. The number of effective k-mers and the peak depth of a series of k values (17, 19, 21, 23, 25, 27 29 and 31) were generated by Jeffyfish (v2.2.6)20 with the C-setting and the genome size was estimated to be about 590 Mb, according to the formula Genome_Size = (Total k-mers - Erroneous k-mers)/Peak_depth (Table 2). It is worth noting that this number could be an underestimate, in that the GC rich regions and repetitive sequences could not be properly resolved by k-mer analysis. Nonetheless, our estimated genome size was within the range of previously reported sizes (560.3 Mb11~620 Mb6). The rate of genome heterozygosity was calculated with the k-mer frequency distribution by the GenomeScope (v1.0.0)21 and the result was around 0.77% (Fig. 2).
De novo genome assembly
Clean data from short insert-size libraries were corrected with the Error Correction program in SOAPdenovo package15. Genome assembly was performed based on the de Bruijn graph algorithm using SOAPdenovo package22 by the following steps: (1) the paired-end reads of all libraries were used to construct the contig sequences while the K-mer values were set as 95 and 85 at the pregraph step and map step, respectively; (2) mapped paired reads were used to construct scaffolds; (3) The GapCloser package was used to map reads to the flanking sequences of gaps and to close gaps between the scaffolds; (4) genome sequence was randomly broken to re-scaffold with SSPACE package. Gaps were then filled again by GapCloser to obtain the final assembly. In the end, there were 54,864 out of 80,696 contigs with sizes longer than 1 kb. The total length of the contig assembly was 549.81 Mb (Table 3). The longest scaffold was 14,145,393 bp, and a total of 5,621 scaffolds were longer than 1,000 bp17,23,24,25. The total length of the scaffold assembly was 632.8 Mb (Table 3).
High-density genetic map construction and genome assembly anchoring
All clean data obtained from the two parents and the 97 F2 individuals were mapped to the asparagus bean scaffold assembly using the Burrows-Wheeler-Alignment tool (BWA)26 mem algorithm. The SAM files were converted to BAM files using SAMtools27. Then the bam files were used to call SNP by the GATK software package19 with parameters “-T HaplotypeCaller -stand_call_conf 30.0 -stand_emit_conf 10.0” and “-T SelectVariants -selectType SNP”. The SNPs were filtered using GATK with parameters as the following:–filterExpression “QD <2.0 || ReadPosRankSum <−8.0 || FS >60.0 || MQ <40.0 || SOR >3.0 || MQRankSum <−10.0 || QUAL <30” –logging_level ERROR–missingValuesInExpressionsShouldEvaluateAsFailing. After genotyping, the raw SNPs were filtered with the following criteria: missing rate <0.3 and heterozygous genotypes <0.5, resulting in a total of 836,933 high-confidence SNPs23.
For the genetic map construction, 50 SNPs were selected to generate bin markers from the two termini and middle part of each scaffold. These bin markers were grouped into 11 linkage groups by JoinMap v4.128 with the regression mapping algorithm. The grouped bins were then sorted and genetic distance was calculated by MSTmap with the Kosambi model29. According to this linkage map, scaffolds were anchored onto 11 pseudo-chromosomes. The SNPs were then assigned chromosome positions and a sliding window method (window size of 50 SNPs; step size of one SNP) was adopted to identify recombination events for each individual. All the recombination sites were merged and sorted with 20 kb intervals30. In the end, the filtered 536,824 SNPs23 were combined into 2,013 bins23. According to the distribution of bins, low-recombination regions were indicated (Fig. 3). These were used to construct 11 linkage maps, resulting in 2180.14 cM spanning the whole genome23, which was within the reported genetic map size ranged from 643 cM31 to 2,670 cM32. It is worth noting that the map lengths could be inflated by genotyping errors, as well as some biological phenomena causing double recombinations33. The sliding window method and bin markers were used to reduce genotype errors. Since the parents were not 100% homozygous, the F1 plants were not identical, which might result inflated genetic size. In addition, 1,556 scaffolds with 597.52 Mb were anchored23, accounting for 94.42% of the assembled genome (Table 4).
Transposable elements annotation
Transposable elements (TEs) annotation were performed by a combination of homology-based and de novo prediction approaches. Homology-based approach involved searching commonly used databases for known TEs at both DNA and protein level. With default parameters, RepeatMasker 3.3.034 was used to identify TEs against the Repbase TE library 18.0735 and RepeatProteinMask34 was used to identify TEs at the protein level in the genome assembly. For de novo prediction, RepeatModeler software (http://www.repeatmasker.org/) was used in constructing the de novo repeat library. Tandem repeats were then predicted by TRF36 with parameters set to “Match = 2, Mismatch = 7, Delta = 7, PM = 80, PI = 10, Minscore = 50 and MaxPeriod = 2000”. In total, we identified 294.95 Mb of the transposable elements, accounting for 46.47% of the asparagus bean genome (Tables 5 and 6). Among all TEs, long terminal repeat (LTR), which are important determinants of angiosperm genome size variation, constituted 19.24% of the assembled genome. DNA TEs accounted for 7.2% of the total sequence.
Gene annotation
We used de novo, homology and RNA-Seq-based prediction methods to annotate protein-coding genes in the asparagus bean genome. Three de novo prediction programs, Augustus37, Genscan38 and GlimmerHMM38 were used to annotate protein-coding genes while gene model parameters were trained from Arabidopsis thaliana. For homology-based prediction, protein sequences of all the protein-coding genes of eleven species including common bean (Phaseolus vulgaris), soybean (Glycine max), pigeonpea (Cajanus cajan), chickpea (Cicer arietinum), mungbean (Vigna radiata), adzuki bean (Vigna angularis), lotus (Lotus japonicus), medick (Medicago truncatula), Arabidopsis (Arabidopsis thaliana), grape (Vitis vinifera), and rice (Orzya sativa), were first mapped to the asparagus bean genome using TblastN with the parameter E-value = 10−5. GeneWise39 was then used to predict gene structure within each protein-coding region. RNA-Seq data of root and stem tissues40 were aligned to the asparagus bean genome using TopHat on default settings. Finally, the predicted genes were merged by EvidenceModeler (EVM)41 to generate a consensus and non-redundant gene set. This process produced 42,609 protein-coding genes with an average length of 3,156 bp (Table 7).
With BLASTP (E-value ≤ 10−5), gene functions were assigned according to the best hit of alignment to SwissProt42, TrEMBL43, and KEGG44 database. Functional domains and motifs of asparagus bean genes were determined by InterProScan45, which analyzed peptide sequences against protein databases including SMART, ProDom, Pfam, PRINTS, PROSITE and PANTHER. Gene Ontology (GO) terms for each gene were extracted from the corresponding InterPro entries. The result showed that 75.40% (32,126) of the total genes were supported by TrEMBL, 56.22% (23,953) by Swiss-Prot, and 59.27% (25,254) by InterPro. In addition, 10,096 (23.69%) genes could not be functionally annotated with current databases (Table 8).
The tRNA genes were identified by tRNAscan-SE software46 with default parameters. The rRNA genes were identified based on homology search to previously published plant rRNA sequences using BLASTN with parameters of “E-value = 10−5”. The snRNA and miRNA genes were identified by INFERNAL v1.047 software against the Rfam database with default parameters. In all, 3,579 non-protein-coding genes were identified in the asparagus bean genome, including 1593 tRNAs, 1,076 rRNAs, 350 snRNAs, and 210 microRNAs (Table 9).
Data Records
The authors declare that all data reported here are fully and freely available from the date of publication. The data resulting from each experimental and analytic step are indicated in a table (Table 10). The assembly genome and annotation are available at CNSA17, figshare23, GenBank and have accessions CP03934524 to CP03935525. Raw read files of genome sequencing are available at NCBI Sequence Read Archive18 and CNSA17. The SNP sets of each pseudo chromosome, the anchored scaffolds information, the filtered SNPs set identified by GATK, the information of bin markers and the linkage map constructed by bin markers are deposited in figshare23. The RNA-seq data was deposited in figshare40.
Technical Validation
DNA sample quality
DNA was quantified using 0.8% agarose gel electrophoresis and Qubit Fluorometer (Invitrogen, US). DNA concentrations were normalized to 100 ng/µl for subsequent library construction.
Assessment of the genome assembly and annotation
Completeness of the genome assembly was assessed with default settings using the Benchmarking Universal Single-Copy Orthologs (BUSCO)48 approach with a total of 1440 orthologue groups from the Embryophyta Dataset. The results showed that 93.2% of the core orthologs could be found in the asparagus bean genome, indicating a high-integrity assembly superior to the other four legume genomes. We aligned the raw reads from short insert-size sequencing back to the assembly and showed that approximately 94.88% of short reads could be successfully mapped. Furthermore, a previously reported high-density linkage map (“ZZ” linkage map v.2)5 was used to assess the quality of anchored scaffolds. The sequences of 7,964 SNPs markers were aligned onto the 11 pseudo chromosomes using BLAT with parameters of “-fine”49. High accordance was shown between the assembled genome and the linkage map (Fig. 4). Whole genome comparative analysis was also conducted between this assembly and the cowpea genome available from Phytozome11 by the MUMmer with nucmer50, presenting high collinearities with four inconsistent areas, which are located in the low-recombination regions (Fig. 5).
Comparison of asparagus bean genome with published common cowpea genomes
A comparison was performed (Table 11) between the asparagus bean genome and previously published common cowpea assemblies8,10,11. The asparagus bean genome assembly (549.81 Mb, non-N) was significantly larger than the first published IT97K-499-35b genome8. Its size was close to the other three common cowpea survey assemblies (IT97K-499-35a11,IT97K-499-35c and IT86D-1010)10. The scaffold N50 size of our asparagus bean genome was 2.7 Mb, longer than the other three genomes assembled by the next-generation sequencing technology. Moreover, the asparagus bean assembly had about 94% of the scaffolds anchored into 11 pseudo-chromosomes according to the high-density genetic map. In addition, a set of 42,287 common cowpea coding sequences (CDS) derived from the single molecule real-time technology11 could be blasted back to our asparagus bean genome with 90% similarity. All these results showed that the asparagus bean genome was of high quality.
Code Availability
All tools used in this study were properly cited in the sections above. Settings and parameters were also clearly described.
References
Ehlers, J. D. & Hall, A. E. Cowpea (Vigna unguiculata L. Walp.). Field Crops Research 53, 187–204 (1997).
Xu, P. et al. Development and polymorphism of Vigna unguiculata ssp. unguiculata microsatellite markers used for phylogenetic analysis in asparagus bean (Vigna unguiculata ssp. sesquipedialis (L.) Verdc.). Molecular Breeding 25, 675–684 (2010).
Fang, J., Chao, C.-C. T., Roberts, P. A. & Ehlers, J. D. Genetic diversity of cowpea [Vigna unguiculata (L.) Walp.] in four West African and USA breeding programs as determined by AFLP analysis. Genetic Resources and Crop Evolution 54, 1197–1209 (2006).
Jayathilake, C. et al. Cowpea: an overview on its nutritional facts and health benefits. J Sci Food Agric 98, 4793–4806 (2018).
Xu, P. et al. Genomic regions, cellular components and gene regulatory basis underlying pod length variations in cowpea (V. unguiculata L. Walp). Plant Biotechnology Journal 15, 547–557 (2017).
Chen, X., Laudeman, T. W., Rushton, P. J., Spraggins, T. A. & Timko, M. P. CGKB: an annotation knowledge base for cowpea (Vigna unguiculata L.) methylation filtered genomic genespace sequences. BMC Bioinformatics 8, 1–9 (2007).
Timko, M. P. et al. Sequencing and analysis of the gene-rich space of cowpea. BMC Genomics 9, 1–23 (2008).
Munoz-Amatriain, M. et al. Genome resources for climate-resilient cowpea, an essential crop for food security. Plant J 89, 1042–1054 (2017).
Muchero, W. et al. A consensus genetic map of cowpea [Vigna unguiculata (L) Walp.] and synteny based on EST-derived SNPs. Proc. Natl. Acad. Sci. USA 106, 18159–18164 (2009).
Spriggs, A. et al. Assembled genomic and tissue-specific transcriptomic data resources for two genetically distinct lines of Cowpea (Vigna unguiculata (L.) Walp). Gates Open Research 2, 1–13 (2018).
Lonardi, S. et al. The genome of cowpea (Vigna unguiculata [L.] Walp.). Plant J 98, 767–782 (2019).
Xu, P. et al. A SNP and SSR based genetic map of asparagus bean (Vigna. unguiculata ssp. sesquipedialis) and comparison with the broader species. PLoS One 6, e15952 (2011).
Kongjaimun, A. et al. An SSR-based linkage map of yardlong bean (Vigna unguiculata (L.) Walp. subsp. unguiculata Sesquipedalis Group) and QTL analysis of pod length. Genome 55, 81–92 (2012).
Pan, L. et al. A High Density Genetic Map Derived from RAD Sequencing and Its Application in QTL Analysis of Yield-Related Traits in Vigna unguiculata. Front Plant Sci 8, 1–13 (2017).
Luo, R. et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience 1, 1–6 (2012).
Porebski, S., Bailey, L. G. & Baum, B. R. Modification of a CTAB DNA Extraction Protocol for Plants Containing High Polysaccharide and Polyphenol Components. Plant Molecular Biology Reporter, 15, 8–15 (1997).
China National GeneBank, https://db.cngb.org/search/?q=CNP0000264&from=CNSA (2019).
NCBI Sequence Read Archive, https://identifiers.org/ncbi/insdc.sra:SRP144706 (2019).
Patel, R. K. & Jain, M. NGS QC Toolkit: A Toolkit for Quality Control of Next Generation Sequencing Data. PLoS One 7(2), e30619 (2012).
Marcais, G. & Kingsford, C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 27, 764–770 (2011).
Vurture, G. W. et al. GenomeScope: fast reference-free genome profiling from short reads. Bioinformatics 33, 2202–2204 (2017).
Boetzer, M. & Pirovano, W. SSPACE-LongRead: scaffolding bacterial draft genomes using long read sequence information. BMC Bioinformatics 15, 211 (2014).
Xia, Q. J. An improved genome assembly and genetic linkage map for asparagus bean, Vigna unguiculata ssp. sesquipedialis. Figshare, https://doi.org/10.6084/m9.figshare.8131823 (2019).
GenBank, https://identifiers.org/ncbi/insdc:CP039345 (2019).
GenBank, https://identifiers.org/ncbi/insdc:CP039355 (2019).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Behrend, A., Borchert, T., Spiller, M. & Hohe, A. AFLP-based genetic mapping of the “bud-flowering” trait in heather (Calluna vulgaris). BMC Genetics 14, 64 (2013).
Wu, Y., Bhat, P. R., Close, T. J. & Lonardi, S. Efficient and accurate construction of genetic linkage maps from the minimum spanning tree of a graph. PLoS Genet 4, e1000212 (2008).
Ling, H. Q. et al. Genome sequence of the progenitor of wheat A subgenome Triticum urartu. Nature 557, 424–428 (2018).
Muchero, W., Ehlers, J. D., Close, T. J. & Roberts, P. A. Mapping QTL for drought stress-induced premature senescence and maturity in cowpea [Vigna unguiculata (L.) Walp.]. Theor Appl Genet 118, 849–863 (2009).
Ouédraogo, J. T. et al. An improved genetic linkage map for cowpea (Vigna unguiculata L.) Combining AFLP, RFLP, RAPD, biochemical markers, and biological resistance traits. Genome 45, 175–188 (2002).
Cartwright, D. A., Troggio, M., Velasco, R. & Gutin, A. Genetic mapping in the presence of genotyping errors. Genetics 176, 2521–2527 (2007).
Tarailo-Graovac, M. & Chen, N. Using RepeatMasker to Identify Repetitive Elements in Current Protocols in Bioinformatics Ch.4 (Wiley Interscience, 2009).
Bao, W., Kojima, K. K. & Kohany, O. Repbase Update, a database of repetitive elements in eukaryotic genomes. Mob DNA 6, 11 (2015).
Benson, G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Research 2, 573–580 (1999).
Stanke, M., Steinkamp, R., Waack, S. & Morgenstern, B. AUGUSTUS: a web server for gene finding in eukaryotes. Nucleic Acids Research 32, W309–W312 (2004).
Burge, C. & Karlin, S. Prediction of Complete Gene Structures in Human Genomic DNA. J. Mol. Biol 268, 78–94 (1997).
Birney, E. & Durbin, R. Using GeneWise in the Drosophila Annotation Experiment. Genome Research 10, 547–548 (2000).
Xia, Q. J. RNA-Seq data of root and stem tissues of asparagus bean. Figshare, https://doi.org/10.6084/m9.figshare.8131535 (2019).
Haas, B. J. et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol 9, R7 (2008).
Bairoch, A. & Apweiler, R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Research 1, 45–48 (2000).
Boeckmann, B. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Research 31, 365–370 (2003).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research 1, 27–30 (2000).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240 (2014).
Schattner, P., Brooks, A. N. & Lowe, T. M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res 33, W686–W689 (2005).
Nawrocki, E. P., Kolbe, D. L. & Eddy, S. R. Infernal 1.0: inference of RNA alignments. Bioinformatics 25, 1335–1337 (2009).
Simão, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V. & Zdobnov, E. M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015).
Kent, W. J. BLAT–the BLAST-like alignment tool. Genome Res 12, 656–664 (2002).
Kurtz, S. et al. Versatile and open software for comparing large genomes. Genome Biology 5, R12.11–R12.19 (2004).
Acknowledgements
This study was funded by State Key Laboratory of Agricultural Genomics, BGI-Shenzhen, Shenzhen, China (No. 2011DQ782025). Financial support for de novo assembly was from the Key Lab of Agricultural Genomics, Chinese Ministry of Agriculture, BGI-Shenzhen, Shenzhen. China, Guangdong Provincial Key Laboratory of core collection of crop genetic resources research and application (No. 2011A091000047). Genetic map construction was supported by the fund from Shenzhen Municipal Government of China: Shenzhen Engineering laboratory of Crop Molecular design breeding. RNA-seq was supported by the fund from National Natural Science Foundation of China (No. 31501369).
Author information
Authors and Affiliations
Contributions
Y.D., W.C., J.H.M., H.M.Y. and W.W. conceived and led the project. Y.D., X.M.N. and Y.L. contributed to secure funding. L.P. and C.Y.C. provided the sequencing samples and RNA-seq data. R.Z., Y.Z.W. and L.K. performed the sequencing. Q.J.X., R.Z. and Y.G. performed genome assembly and annotation. Q.J.X., X.D. and Z.Z. constructed the genetic map and anchored. Q.J.X., R.Z. and W.C. wrote the article.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ISA-Tab metadata file
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata files associated with this article.
About this article
Cite this article
Xia, Q., Pan, L., Zhang, R. et al. The genome assembly of asparagus bean, Vigna unguiculata ssp. sesquipedialis. Sci Data 6, 124 (2019). https://doi.org/10.1038/s41597-019-0130-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-019-0130-6
This article is cited by
-
Translocations and inversions: major chromosomal rearrangements during Vigna (Leguminosae) evolution
Theoretical and Applied Genetics (2024)
-
Heat stress and cowpea: genetics, breeding and modern tools for improving genetic gains
Plant Physiology Reports (2020)
-
Chromosome-level genome assembly of golden pompano (Trachinotus ovatus) in the family Carangidae
Scientific Data (2019)