Abstract
A comprehensive database of chemical properties on a vast set of transition metal surfaces has the potential to accelerate the discovery of novel catalytic materials for energy and industrial applications. In this data descriptor, we present such an extensive study of chemisorption properties of important adsorbates - e.g., C, O, N, H, S, CHx, OH, NH, and SH - on 2,035 bimetallic alloy surfaces in 5 different stoichiometric ratios, i.e., 0%, 25%, 50%, 75%, and 100%. To our knowledge, it is the first systematic study to compile the adsorption properties of such a well-defined, large chemical space of catalytic interest. We propose that a collection of catalytic properties of this magnitude can assist with the development of machine learning enabled surrogate models in theoretical catalysis research to design robust catalysts with high activity for challenging chemical transformations. This database is made publicly available through the platform www.Catalysis-hub.org for easy retrieval of the data for further scientific analysis.
Design Type(s) | chemical reaction data analysis objective • modeling and simulation objective |
Measurement Type(s) | Chemical Properties |
Technology Type(s) | digital curation |
Factor Type(s) | purity • ratio • Material |
Sample Characteristic(s) |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Similar content being viewed by others
Background & Summary
Electronic structure calculations from Density functional theory (DFT)1,2 is a well established approach for predicting a large range of material properties3. In the field of heterogeneous catalysis and electrocatalysis, DFT has provided a deeper understanding of catalytic activity and reaction mechanisms4, and has guided the exploration of new catalytic materials. Importantly, the adsorption energy of chemical species to the surface obtained from DFT, has been found to be a strong descriptor for catalytic activity of surfaces5,6.
Chemical reactions of interest for sustainable energy applications, including the conversion of CO2 and syngas to carbon-based fuels7,8, fuel-cell operation9,10, and electrochemical water splitting11, noble metals such as Pt, Ru, Ag, Ir and Cu are the most active materials. However, a key challenge for large-scale sustainable energy technologies is to identify catalytic materials that are also of high abundance and low cost. In this search, it is instructive to investigate metal alloys, which span a vast set of materials, with the potential to mimic the catalytic properties of the highly active pure metals. Several bimetallic alloys with high catalytic activity have already been identified, including CoMo12, BiPt13 and Pt-lanthanide alloys such as Pt3Y14.
Here, we present a large-scale DFT study of chemical adsorption and hydrogenation on 1,998 bimetallic alloy and 37 pure metal surfaces. Consisting of more than 90,000 systematic DFT calculations, this dataset is intended for machine learning model generation. The alloys were chosen by combining 37 selected metals and transition metals (outlined in the periodic table in Fig. 1) to form alloys in the L12 and L10 Strukturbericht designation, which corresponds to face-centered cubic crystal structures with A3B and AB stoichiometries, respectively. The 37 pure metals in the A1 (FCC) structure were included in addition to the 1,998 bimetallic alloys resulting from all possible combinations, such that stoichiometric A:B ratios of 0%, 25%, 50%, 75% and 100% are sampled. The metal surfaces were modeled by cleaving three-layer slabs with a (111) termination for A1 and L12 and a (101) termination for L10, although this termination is also referred to as the (111) miller index15,16 when cleaved from the cubic bulk unit cell which is not the standard conventional form.
The periodic table outlining five adsorbate elements and the 37 metals included in the dataset. This includes six metals from group 13–15, 17 transition metals, and Lanthanum. Surface geometry and enumerated adsorption sites for the three structures are provided in the lower panel, where top, bridge, and hollow sites are shown in red, white, and green, respectively.
Atomic adsorption of H, C, N, O, and S was studied for all 2,035 surfaces. In order to systematically sample the adsorption energies, all unique adsorption sites were considered. The unique sites for each of the surface structures are shown in Fig. 1 where the number of sites are 4, 9, and 10 for the A1, L12, and L10 surfaces respectively. This gives a total of 96,015 unique surfaces, adsorbate and site combinations (including the empty slabs), where roughly 65,000 calculations are completed. Also, the adsorption of the hydrogenated species CH, NH, CH2, CH3, SH, OH and H2O has been studied for a smaller subset of alloy surfaces, where alloys formed from 16 metals of particular interest for catalysis have been chosen, with approximately 25,000 calculations completed. We note that due to reorientation of adsorbates during structure relaxation, the number of unique surface structures are lower than the number of initially sampled configurations. More than 90,000 adsorption and reaction energies have been parsed from the dataset, where approximately 30,000 adsorption energies stems from the monoatomic adsorbates (H, C, N, O and S), and 10,000 adsorption and reaction energies stems from the hydrogenated adsorbates. The remaining reaction energies are generated by decomposing a set of gas phase molecules of interest for catalysic applications, such as CH4(g), NH3(g), CO2(g), CH2CH2(g), CH3OH(g), H2O2(g), CH3COOH(g), into their atomic constituents on the surfaces.
The dataset is made available from the open repository Catalysis-Hub.org17, where reaction energies and barriers from more than 50 publications and datasets can be accessed.
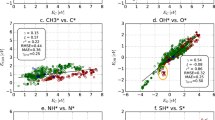
Examples of calculated adsorption energies are given in Fig. 2, showing the results for the most stable adsorption sites for atomic carbon (C), oxygen (O), and nitrogen (N). In Fig. 2(a,b) the adsorption energies are plotted as a function of metal A and B, that are arranged on an improved Pettifor scale18,19, with small adjustments for magnetic elements, which ensures a smooth variation of the adsorption energies with composition. Grey areas in the figure can be seen for structures where converged adsorption energies could not be obtained due to surface reconstruction, mismatch in the magnetic structure of the slab and the adsorbate-slab structure or convergence problems for the electronic structure calculation.
Adsorption energies of selected atomic species. In (a,b) the C adsorption energy is shown for the 666 L10 (AB) and 1332 L12 (A3B) alloy surfaces respectively. Results for the 37 pure metals are shown along the diagonal. Adsorption energies of atomic (c) O and (d) N are plotted with respect to the C adsorption energy for all materials. References are taken with respect to the reactions: CH4(g) − 2 H2(g) + * → C*, 0.5 N2(g) * → N* and H2O(g) − H2(g) + * → O* with all species adsorbed to their lowest energy site.
Another approach for visualizing adsorption energy trends is to plot the adsorption energy of two adsorbates against each other, which often gives rise to linear scaling relationships for similar surface geometries. Utilizing scaling relationships is a well established approach in theoretical catalysis to model and understand catalytic activity and selectivity6,20. In the lower panel of Fig. 2 the correlation between the adsorption of carbon with (c) oxygen and (d) nitrogen is shown. Metals containing a partially filled d-band versus a filled or empty d-band is labeled as d- and non-d metals respectively. All alloys containing a non-d metal are labeled as non-d alloys. While a close to linear relationship between the adsorption of C and O can be seen for the d and non-d pure metals separately, the correlation between the atomic adsorption energies on the alloys are more complicated, emphasizing the need for more sophisticated methods for modelling these systems, such as data-driven approaches. A link to the script used to plot Fig. 2 by fetching the data directly with the Catalysis-Hub Python API is provided in the Methods section.
Methods
Adsorption energies were calculated with DFT and obtained from the equation:
where the gas phase species are chosen among CH4(g), H2(g), N2(g), H2O(g) and H2S(g). A full list of studied adsorbates and references used are given in Table 1.
The Catalysis Kit (CatKit)21 software was used to generate the slab structures from optimized bulk systems and to systematically enumerate all high-symmetry adsorption sites. The generated structures were stored and handled with the ASE database22.
DFT calculations were performed in the Quantum Espresso (QE) electronic structure code23, using the BEEF-vdw exchange correlation functional24, a 500 eV plane-wave cutoff, and a 5,000 eV density cutoff. Monkhorst-Pack k-point grids of (12, 12, 12) for bulk and (6, 6, 1) for slab calculations were used, with a 0.15 eV Fermi smearing. Spin-polarized calculations were performed only for alloys containing Fe, Ni, Co, and Mn, while allowing the magnetic moments to converge during the electronic structure optimization. Initial magnetic moments of 3, 3, 2, 1 μB was chosen for Fe, Mn, Co and Ni respectively, and set to zero for all other elements. For the A1 and L12 structures, lattice constants were obtained from bulk alloy calculations with an equation of state combined with an energy minimization in QE. For L10 structures we used a variable cell optimization in QE with a high plane wave cutoff (800 eV) and then used the resulting lattice constants as initial guess for the final energy minimization with respect to lattice constant parameters - i.e., ‘a’ and ‘c’ - using the Scipy fmin optimizer25. Slab geometries were optimized by fixing the two bottom layers and allowing the top layer and adsorbates to relax. Due to the large number of calculations, job submissions were handled with FireWorks26 and the CatFlow submodule of CatKit, that provides a FireWorks interface to QE and other electronic structures calculators supported by ASE.
Upon relaxation we found that reconstructions of the metal surfaces, e.g. horizontal sliding or dissociation of the top layer from the slab, are quite common. Also, we found that the adsorbates often reorient into other sites. The relaxed geometries were therefore post-processed with a tailored classification method to label reconstructed surfaces and reclassify the adsorption sites. Only non-reconstructed surfaces have been used to generate adsorption energies, although, as the reconstructed structures could be of interest for model generation, the atomic structures are still made available via the web and python APIs discussed in the Usage Notes section.
Data Records
All the DFT calculations stems from one dataset, generated by O. Mamun, K. Winther, and J. Boes, in the group of Thomas Bliggard at the SUNCAT-Center for Interface Science and Catalysis. The data are made available from two platforms, where the open electronic structure database Catalysis-Hub.org17, is the main resource for easy access to parsed adsorption energies. The dataset has been assigned its own permanent link at https://www.catalysis-hub.org/publications/MamunHighT2019. The Catalysis-Hub web interface enables in-browser search for reactions and chemical compositions, with a visualization of atomic geometries, that can be downloaded in several formats including CIF, JSON, VASP POSCAR and Quantum ESPRESSO input. A description on how to download reaction energies and atomic structures with the Catalysis-Hub (CatHub) Python API, available from the Zenodo repository27, is provided in the Usage Notes section.
In addition, all the raw text output files from the Quantum Espresso calculations have been uploaded to the Materials Cloud archive28. The output files can be downloaded and inspected with any text editor, or opened with ASE22 to create Atoms objects containing the atomic structures and the results of the calculations.
Technical Validation
To ensure the quality of the adsorption properties reported herein, the convergence with respect to all calculation parameters have been carefully checked. Adsorption and reaction energies have only been included for surface structures that do not undergo reconstruction upon relaxation. In the case of magnetic surface structures, we have only parsed adsorption and reaction energies if the discrepancy in total magnetization between the empty surface and the surface with the adsorbate is less than 4 in atomic units.
To illustrate the validity of the data, we compare the lattice constant reported in reputed journal articles to the computed lattice constant. We found excellent agreement between our results and previously computed lattice constants which are presented in Tables 2, 3 and 4. In Table 5, we also show a comparison between previously computed adsorption energies to those reported in this article. We also see good agreement between the reported and the computed adsorption energies with slight deviation which may be an artifact of different calculation setup and/or system size, i.e., pseudopotential, smearing scheme, number of layers and lateral size of the slab. For example, the differences in adsorption energies between this work and ref.8, which is also available at https://www.catalysis-hub.org/publications/SchumannSelectivity2018, can be attributed to the use of a 4 layer (3 × 3) repeated surface slab model, compared to the 3 layer (2 × 2) slab used in this study.
Usage Notes
The CatHub software module, which is available from the Zenodo repository27, provides a Python API which is better suited for fetching a large amount of data from the Catalysis-Hub repository. A small script for obtaining pre-parsed adsorption energies in Python is provided below:
from cathub.query import get_reactions
get_reactions(pubId = ‘MamunHighT2019’,
n_results = 2,
surfaceComposition = ‘Mo + Ru’,
reactants = “CH4gas + H2”,
sites = “~hollow”,
products = ‘C’)
which returns a JSON dictionary on the form:
{‘reactions’:
{‘edges’: [x‘
{‘node’:
{‘Equation’: ‘CH4(g) - 2.0H2(g) + * - > C*‘,
‘activationEnergy’: None,
‘chemicalComposition’: ‘Mo3Ru9’,
‘coverages’: {‘C’: 0.25},
‘dftCode’: ‘Quantum ESPRESSO 5.1’,
‘dftFunctional’: ‘BEEF-vdW’,
‘facet’: ‘111’,
‘products’: {‘Cstar’: 1},
‘pubId’: ‘MamunHighT2019’,
‘reactants’: {‘star’: 1, ‘H2gas’: -2.0,
‘CH4gas’: 1.0}’,
‘reactionEnergy’: 1.2068607934897955,
‘sites’: {‘C’: ‘hollow|A_A_A|HCP’},
‘surfaceComposition’: ‘Ru3Mo’}
},
{‘node’:…}
],
‘totalCount’: 10}
}
}.
Note that each data entry is given as a ‘node’ in a list of ‘edges’, utilizing the graph-theory based query language GraphQL (https://graphql.org/). Since the Catalysis-Hub repository contains several datasets from different publications, the “pubId = ‘MamunHighT2019’” argument must be assigned in the script above in order to query only this dataset. The script above queries for entries with hollow-site adsorption of C (product) with respect to the relevant gas phase species (reactants), on surfaces containing Mo as well as Ru. The reaction and product entries must be chosen (and matched) among the adsorbates and gas phase references in Table 1. A more specific query for adsorption site can be made by using the site names specified in Table 6.
Furthermore, easy access to all the atomic structures, calculation results and parameters in the study, can be obtained with the ASE database interface22, where the CatHub module features a convenient wrapper around the ASE db command line interface (CLI), used directly from a terminal. For example, the query:
cathub ase ‘pub_id = MamunHighT2019, relaxed = 1’
will return a list with the first 20 results (out of approximately 90,000) for the relaxed configurations in the study. The initial geometries can be queried by setting ‘relaxed = 0’. The atomic structures are labeled with an several key-value-pair metadata, that can be used to query the dataset. For example:
cathub ase ‘Pt,pub_id = MamunHighT2019,relaxed = 1,
reconstructed = 0,SB_symbol = L10
-c formula,energy,adsorbate,site,site_type -L 100’
--gui
will return the 100 first relaxed and non-reconstructed structures containing Pt in the L10 structure, as a table containing the chemical formula, the DFT total energy, the name of the adsorbate, and site information as well as opening all the matching structures in the ASE gui visualizer. A description of ASE native columns as well as special key value pairs assigned in this study is given in Table 7. We refer to ref.17 for a detailed description of the Catalysis-Hub database structure.
Code Availability
The CatHub python API and the CatKit software packages are available open-source from the GitHub repository at https://github.com/SUNCAT-Center/. In addition, the latest stable version of the CatHub module is available from the Zenodo repository27. The Python scripts used for plotting the data shown in Fig. 2 is made available as a tutorials at https://github.com/SUNCAT-Center/CatHub/tree/master/tutorials/1_bimetallic_alloys/. The code used to classify the adsorption sites is made available at https://github.com/SUNCAT-Center/CatHub/tree/master/cathub/classification.py.
References
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Physical Review 136, B864 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Physical Review 140, A1133 (1965).
Jones, R. O. Density functional theory: Its origins, rise to prominence, and future. Reviews of Modern Physics 87, 897 (2015).
Nørskov, J. K., Abild-Pedersen, F., Studt, F. & Bligaard, T. Density functional theory in surface chemistry and catalysis. Proceedings of the National Academy of Sciences 108, 937–943 (2011).
Nørskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nature Chemistry 1, 37 (2009).
Medford, A. J. et al. From the sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. Journal of Catalysis 328, 36–42 (2015).
Subramani, V. & Gangwal, S. K. A review of recent literature to search for an efficient catalytic process for the conversion of syngas to ethanol. Energy & Fuels 22, 814–839 (2008).
Schumann, J. et al. Selectivity of synthesis gas conversion to C2+ oxygenates on fcc(111) transition-metal surfaces. ACS Catalysis 8, 3447–3453 (2018).
Shao, M., Chang, Q., Dodelet, J.-P. & Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chemical Reviews 116, 3594–3657 (2016).
Debe, M. K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486, 43 (2012).
Wang, J. et al. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Advanced Materials 28, 215–230 (2016).
Jacobsen, C. J. et al. Catalyst design by interpolation in the periodic table: Bimetallic ammonia synthesis catalysts. Journal of the American Chemical Society 123, 8404–8405 (2001).
Greeley, J., Jaramillo, T. F., Bonde, J., Chorkendorff, I. & Nørskov, J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nature Materials 5, 909–913 (2006).
Escudero-Escribano, M. et al. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 352, 73–76 (2016).
Chen, Z.-X., Neyman, K. M., Lim, K. H. & Rösch, N. CH3O decomposition on PdZn (111), Pd (111), and Cu (111). A theoretical study. Langmuir 20, 8068–8077 (2004).
Hensley, A. J. R., Schneider, S., Wang, Y. & McEwen, J.-S. Adsorption of aromatics on the (111) surface of PtM and PtM3 (M = Fe, Ni) alloys. RSC Advances 5, 85705–85719 (2015).
Winther, K. T., Hoffmann, M. J., Mamun, O., Boes, J. R. & Bligaard, T. Catalysis-Hub.org, an open electronic structure database for surface reactions. Scientific Data 6, 81 (2019).
Pettifor, D. G. A chemical scale for crystal-structure maps. Solid State Communications 51, 31–34 (1984).
Glawe, H., Sanna, A., Gross, E. & Marques, M. A. The optimal one dimensional periodic table: A modified pettifor chemical scale from data mining. New Journal of Physics 18, 093011 (2016).
Abild-Pedersen, F. et al. Scaling properties of adsorption energies for hydrogen-containing molecules on transition-metal surfaces. Physical Review Letters 99, 016105 (2007).
Boes, J. R., Mamun, O., Winther, K. & Bligaard, T. Graph theory approach to high-throughput surface adsorption structure generation. The Journal of Physical Chemistry A 123, 2281–2285 (2019).
Larsen, A. H. et al. The atomic simulation environment—a Python library for working with atoms. Journal of Physics: Condensed Matter 29, 273002 (2017).
Giannozzi, P. et al. Advanced capabilities for materials modelling with QUANTUM ESPRESSO. Journal of Physics: Condensed Matter 29, 465901 (2017).
Wellendorff, J. et al. Density functionals for surface science: Exchange-correlation model development with bayesian error estimation. Physical Review B 85, 235149 (2012).
Nelder, J. A. & Mead, R. A simplex method for function minimization. The Computer Journal 7, 308–313 (1965).
Jain, A. et al. Fireworks: a dynamic workflow system designed for high-throughput applications. Concurrency and Computation: Practice and Experience 27, 5037–5059 (2015).
Winther, K. T. et al. CatHub: A Python API for the Surface Reactions Database on Catalysis-Hub.org. Zenodo. https://doi.org/10.5281/zenodo.2600391 (2019).
Mamun, O., Winther, K. T., Boes, J. R. & Bligaard, T. High-throughput calculations of catalytic properties of bimetallic alloy surfaces. Materials Cloud Archive. https://doi.org/10.24435/materialscloud:2019.0015/v1 (2019).
Haas, P., Tran, F. & Blaha, P. Calculation of the lattice constant of solids with semilocal functionals. Physical Review B 79, 085104 (2009).
Donohue, J. Structures of the Elements (John Wiley and Sons, 1974).
Li, B., Greeley, J. & Prakash, J. Understanding the oxygen reduction reaction on Pd-based alloys (Pd-M, M = Ni, Co) surfaces using density functional theory calculations. ECS Transactions 19, 109–116 (2009).
Arıkan, N. The first-principles study on Zr3Al and Sc3Al in L12 structure. Journal of Physics and Chemistry of Solids 74, 794–798 (2013).
Liu, Z., Lei, Y. & Wang, G. First-principles computation of surface segregation in L1 CoPt magnetic nanoparticles. Journal of Physics: Condensed Matter 28, 266002 (2016).
Montoya, J. H., Tsai, C., Vojvodic, A. & Nørskov, J. K. The challenge of electrochemical ammonia synthesis: A new perspective on the role of nitrogen scaling relations. ChemSusChem 8, 2180–2186 (2015).
Wellendorff, J. et al. A benchmark database for adsorption bond energies to transition metal surfaces and comparison to selected DFT functionals. Surface Science 640, 36–44 (2015).
Hansen, M. H., Nørskov, J. K. & Bligaard, T. First principles micro-kinetic model of catalytic non-oxidative dehydrogenation of ethane over close-packed metallic facets. Journal of Catalysis 374, 161–170 (2019).
Acknowledgements
This work was supported by the U.S. Department of Energy, Chemical Sciences, Geosciences, and Biosciences (CSGB) Division of the Office of Basic Energy Sciences, via Grant DE-AC02-76SF00515 to the SUNCAT Center for Interface Science and Catalysis.
Author information
Authors and Affiliations
Contributions
The dataset was generated by O. Mamun in collaboration with J. Boes and K. Winther. K. winther was responsible for parsing and uploading data to the database platforms, and surface geometries were generated by J. Boes. T. Bligaard formed the vision and scope of the work. The manuscript was written by K. Winther and O. Mamun, and has been revised and approved by all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ISA-Tab metadata file
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata files associated with this article.
About this article
Cite this article
Mamun, O., Winther, K.T., Boes, J.R. et al. High-throughput calculations of catalytic properties of bimetallic alloy surfaces. Sci Data 6, 76 (2019). https://doi.org/10.1038/s41597-019-0080-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-019-0080-z
This article is cited by
-
Automatic feature engineering for catalyst design using small data without prior knowledge of target catalysis
Communications Chemistry (2024)
-
Accurate energy barriers for catalytic reaction pathways: an automatic training protocol for machine learning force fields
npj Computational Materials (2023)
-
The importance of a charge transfer descriptor for screening potential CO2 reduction electrocatalysts
Nature Communications (2023)
-
Enlightening the bimetallic effect of Au@Pd nanoparticles on Ni oxide nanostructures with enhanced catalytic activity
Scientific Reports (2023)
-
Electronic structure factors and the importance of adsorbate effects in chemisorption on surface alloys
npj Computational Materials (2022)