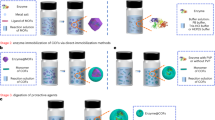
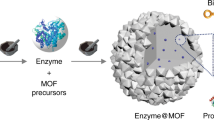
Abstract
Enzymes are outstanding natural catalysts with exquisite 3D structures, initiating countless life-sustaining biotransformations in living systems. The flexible structure of an enzyme, however, is highly susceptible to non-physiological environments, which greatly limits its large-scale industrial applications. Seeking suitable supports to immobilize fragile enzymes is one of the most efficient routes to ameliorate the stability problem. This protocol imparts a new bottom-up strategy for enzyme encapsulation using a hydrogen-bonded organic framework (HOF-101). In short, the surface residues of the enzyme can trigger the nucleation of HOF-101 around its surface through the hydrogen-bonded biointerface. As a result, a series of enzymes with different surface chemistries are able to be encapsulated within a highly crystalline HOF-101 scaffold, which has long-range ordered mesochannels. The details of experimental procedures are described in this protocol, which involve the encapsulating method, characterizations of materials and biocatalytic performance tests. Compared with other immobilization methods, this enzyme-triggering HOF-101 encapsulation is easy to operate and affords higher loading efficiency. The formed HOF-101 scaffold has an unambiguous structure and well-arranged mesochannels, favoring mass transfer and understanding of the biocatalytic process. It takes ~13.5 h for successful synthesis of enzyme-encapsulated HOF-101, 3–4 d for characterizations of materials and ~4 h for the biocatalytic performance tests. In addition, no specific expertise is necessary for the preparation of this biocomposite, although the high-resolution imaging requires a low-electron-dose microscope technology. This protocol can provide a useful methodology to efficiently encapsulate enzymes and design biocatalytic HOF materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Data availability
The main data discussed in this protocol are available within our primary research papers (refs. 18,21) and Supplementary Information. Source data are provided with this paper.
References
Thornberry, N. A. & Lazebink, Y. Caspases: enemies within. Science 281, 1312–1316 (1998).
Henzler-Wildman, K. & Kern, D. Dynamic personalities of proteins. Nature 450, 964–972 (2007).
Sheldon, R. A. & Woodley, J. M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 118, 801–838 (2018).
Intasian, P. et al. Enzymes, in vivo biocatalysis, and metabolic engineering for enabling a circular economy and sustainability. Chem. Rev. 121, 10367–10451 (2021).
Devine, P. N. et al. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Rev. Chem. 2, 409–421 (2018).
Schmid, A. et al. Industrial biocatalysis today and tomorrow. Nature 409, 258–268 (2001).
Wong, L. S., Khan, F. & Micklefield, J. Selective covalent protein immobilization: strategies and applications. Chem. Rev. 109, 4025–4053 (2009).
Sheldon, R. A., Basso, A. & Brady, D. New frontiers in enzyme immobilisation: robust biocatalysts for a circular bio-based economy. Chem. Soc. Rev. 50, 5850–5862 (2021).
Huang, S., Chen, G. & Ouyang, G. Confining enzymes in porous organic frameworks: from synthetic strategy and characterization to healthcare applications. Chem. Soc. Rev. 51, 6824–6863 (2022).
Liang, W. et al. Metal–organic framework-based enzyme biocomposites. Chem. Rev. 121, 1077–1129 (2021).
Lian, X. et al. Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 46, 3386–3401 (2017).
Wang, B., Lin, R.-B., Zhang, Z., Xiang, S. & Chen, B. Hydrogen-bonded organic frameworks as a tunable platform for functional materials. J. Am. Chem. Soc. 142, 14399–14416 (2020).
Li, P., Ryder, M. R. & Stoddart, J. F. Hydrogen-bonded organic frameworks: a rising class of porous molecular materials. Acc. Mater. Res. 1, 77–87 (2020).
Song, X. et al. Design rules of hydrogen-bonded organic frameworks with high chemical and thermal stabilities. J. Am. Chem. Soc. 144, 10663–10687 (2022).
Lin, R.-B. & Chen, B. Hydrogen-bonded organic frameworks: chemistry and functions. Chem 8, 2114–2135 (2022).
Liang, W. et al. Enzyme encapsulation in a porous hydrogen-bonded organic framework. J. Am. Chem. Soc. 141, 14298–14305 (2019).
Wied, P. et al. Combining a genetically engineered oxidase with hydrogen-bonded organic frameworks (HOFs) for highly efficient biocomposites. Angew. Chem. Int. Ed. 61, e202117345 (2022).
Chen, G. et al. Protein-directed, hydrogen-bonded biohybrid framework. Chem 7, 2722–2742 (2021).
Tang, Z. et al. A biocatalytic cascade in an ultrastable mesoporous hydrogen-bonded organic framework for point-of-care biosensing. Angew. Chem. Int. Ed. 60, 23608–23613 (2021).
Tang, J. et al. In-situ encapsulation of protein into nanoscale hydrogen-bonded organic frameworks for intracellular biocatalysis. Angew. Chem. Int. Ed. 60, 22315–22321 (2021).
Chen, G. et al. Hydrogen-bonded organic framework biomimetic entrapment allowing non-native biocatalytic activity in enzyme. Nat. Commun. 13, 4816 (2022).
Nelson, J. & Griffin, E. G. Adsorption of invertase. J. Am. Chem. Soc. 38, 1109–1115 (1916).
Hartmann, M. & Kostrov, X. Immobilization of enzymes on porous silicas—benefits and challenges. Chem. Soc. Rev. 42, 6277–6289 (2013).
Pierre, A. C. The sol-gel encapsulation of enzymes. Biocatal. Biotransformation 22, 145–170 (2004).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013).
Cote, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Lykourinou, V. et al. Immobilization of MP-11 into a mesoporous metal–organic framework, MP-11@mesoMOF: a new platform for enzymatic catalysis. J. Am. Chem. Soc. 133, 10382–10385 (2011).
Li, P. et al. Nanosizing a metal–organic framework enzyme carrier for accelerating nerve agent hydrolysis. ACS Nano 10, 9174–9182 (2016).
Li, P. et al. Hierarchically engineered mesoporous metal-organic frameworks toward cell-free immobilized enzyme systems. Chem 4, 1022–1034 (2018).
Sun, Q. et al. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks. J. Am. Chem. Soc. 140, 984–992 (2018).
Lyu, F., Zhang, Y., Zare, R. N., Ge, J. & Liu, Z. One-pot synthesis of protein-embedded metal−organic frameworks with enhanced biological activities. Nano Lett. 14, 5761–5765 (2014).
Liang, K. et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 6, 7240 (2015).
Li, M. et al. Fabricating covalent organic framework capsules with commodious microenvironment for enzymes. J. Am. Chem. Soc. 142, 6675–6681 (2020).
Zheng, Y. et al. Green and scalable fabrication of high-performance biocatalysts using covalent organic frameworks as enzyme carriers. Angew. Chem. Int. Ed. 61, e202208744 (2022).
Gao, R. et al. Mechanochemistry-guided reticular assembly for stabilizing enzymes with covalent organic frameworks. Cell Rep. Phys. Sci. 3, 101153 (2022).
Hu, C. et al. Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis. Sci. Adv. 6, eaax5785 (2020).
Liang, W. et al. Enhanced activity of enzymes encapsulated in hydrophilic metal–organic frameworks. J. Am. Chem. Soc. 141, 2348–2355 (2019).
Tong, L. et al. Atomically unveiling the structure-activity relationship of biomacromolecule-metal-organic frameworks symbiotic crystal. Nat. Commun. 13, 951 (2022).
Wu, X. et al. Packaging and delivering enzymes by amorphous metal-organic frameworks. Nat. Commun. 10, 5165 (2019).
Feng, Y. et al. Defect engineering of enzyme-embedded metal-organic frameworks for smart cargo release. Chem. Eng. J. 439, 135736 (2022).
Wu, X. et al. A versatile competitive coordination strategy for tailoring bioactive zeolitic imidazolate framework composites. Small 17, e2007586 (2021).
Huang, W. et al. Photodynamic hydrogen-bonded biohybrid framework: a photobiocatalytic cascade nanoreactor for accelerating diabetic wound therapy. JACS Au 2, 2048–2058 (2022).
Ye, N. et al. Hydrogen-bonded biohybrid framework-derived highly specific nanozymes for biomarker sensing. Anal. Chem. 93, 13981–13989 (2021).
Wijesundara, Y. H. et al. Carrier gas triggered controlled biolistic delivery of DNA and protein therapeutics from metal–organic frameworks. Chem. Sci. 13, 13803–13814 (2022).
Chen, T.-T., Yi, J.-T., Zhao, Y.-Y. & Chu, X. Biomineralized metal-organic framework nanoparticles enable intracellular delivery and endo-lysosomal release of native active proteins. J. Am. Chem. Soc. 140, 9912–9920 (2018).
Maddigan, N. K. et al. Protein surface functionalisation as a general strategy for facilitating biomimetic mineralisation of ZIF-8. Chem. Sci. 9, 4217–4223 (2018).
Shen, B. et al. A single-molecule van der Waals compass. Nature 592, 541–544 (2021).
Lazić, I. et al. Single-particle cryo-EM structures from iDPC–STEM at near-atomic resolution. Nat. Methods 19, 1126–1136 (2022).
Gao, L. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2, 577–583 (2007).
Wei, H. & Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 42, 6060–6093 (2013).
Acknowledgements
We acknowledge financial support from projects of the National Natural Science Foundation of China (22174164, to G.C.; 22104159, to S.H.; 21904146, to G.C.).
Author information
Authors and Affiliations
Contributions
G.C. designed the research. G.C. and G.O. directed the project. G.C. organized the in situ encapsulation and material characterizations. G.C. and S.H. organized the tables and the figures. X.M. organized the text about the cryo-EM characterization. R.H. participated in the discussion and helped with the data analysis. G.C. and G.O. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Kang Liang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Chen, G. et al. Chem 7, 2722–2742 (2021): https://doi.org/10.1016/j.chempr.2021.07.003
Chen, G. et al. Nat. Commun. 13, 4816 (2022): https://doi.org/10.1038/s41467-022-32454-2
Ye, N. et al. Anal. Chem. 93, 13981−13989 (2021): https://doi.org/10.1021/acs.analchem.1c03381
Extended data
Extended Data Fig. 1 In situ encapsulation of HRP within HOF-101.
Snapshots of HRP@HOF-101 sediment after assembly (a) and the dried product (b). c, Cryo-EM image of HRP@HOF-101. d, The nitrogen isotherms of standard HOF-101 and HRP@HOF-101, respectively. e, The catalytic kinetics of HRP, HRP@HOF-101 and HRP@ZIF-8. The HRP dosages in each group are the same (0.4 μg/ml). The H2O2 concentration is 0.1 mM. ΔAbs, change in absorbance. a–c and e adapted with permission from ref. 18, Elsevier.
Supplementary information
Supplementary Information
Supplementary Method 1 and Tables 1 and 2
Source data
Source Data Fig. 7
Unprocessed PXRD and FT-IR data
Source Data Fig. 8
Unprocessed N2 isotherm data for Cyt c@HOF-101
Source Data Extended Data Fig. 1d
Unprocessed N2 isotherm and bioactivity data for HRP@HOF-101
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, G., Huang, S., Ma, X. et al. Encapsulating and stabilizing enzymes using hydrogen-bonded organic frameworks. Nat Protoc 18, 2032–2050 (2023). https://doi.org/10.1038/s41596-023-00828-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00828-5
This article is cited by
-
Supramolecular polynuclear clusters sustained cubic hydrogen bonded frameworks with octahedral cages for reversible photochromism
Nature Communications (2024)
-
Pros and Cons in Various Immobilization Techniques and Carriers for Enzymes
Applied Biochemistry and Biotechnology (2024)
-
Green synthesis of stable hybrid biocatalyst using a hydrogen-bonded, π-π-stacking supramolecular assembly for electrochemical immunosensor
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.