Abstract
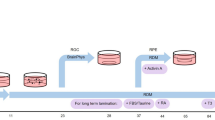
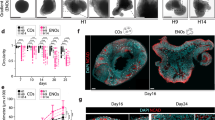
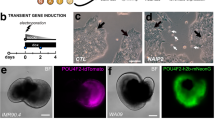
Induced pluripotent stem cell-derived brain organoids enable the developmental complexities of the human brain to be deconstructed. During embryogenesis, optic vesicles (OVs), the eye primordium attached to the forebrain, develop from diencephalon. However, most 3D culturing methods generate either brain or retinal organoids individually. Here we describe a protocol to generate organoids with both forebrain entities, which we call OV-containing brain organoids (OVB organoids). In this protocol, we first induce neural differentiation (days 0–5) and collect neurospheres, which we culture in a neurosphere medium to initiate their patterning and further self-assembly (days 5–10). Then, upon transfer to spinner flasks containing OVB medium (days 10–30), neurospheres develop into forebrain organoids with one or two pigmented dots restricted to one pole, displaying forebrain entities of ventral and dorsal cortical progenitors and preoptic areas. Further long-term culture results in photosensitive OVB organoids constituting complementary cell types of OVs, including primitive corneal epithelial and lens-like cells, retinal pigment epithelia, retinal progenitor cells, axon-like projections and electrically active neuronal networks. OVB organoids provide a system to help dissect interorgan interactions between the OVs as sensory organs and the brain as a processing unit, and can help model early eye patterning defects, including congenital retinal dystrophy. To conduct the protocol, experience in sterile cell culture and maintenance of human induced pluripotent stem cells is essential; theoretical knowledge of brain development is advantageous. Furthermore, specialized expertise in 3D organoid culture and imaging for the analysis is needed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are provided with the original paper5. Any other data are available from the corresponding author. The data presented here have not been published previously.
References
Mariani, J. & Vaccarino, F. M. Breakthrough moments: Yoshiki Sasai’s discoveries in the third dimension. Cell Stem Cell 24, 837–838 (2019).
Giandomenico, S. L., Sutcliffe, M. & Lancaster, M. A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 16, 579–602 (2021).
Bergmann, S. et al. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 13, 2827–2843 (2018).
Gabriel, E. et al. CPAP promotes timely cilium disassembly to maintain neural progenitor pool. EMBO J. 35, 803–819 (2016).
Gabriel, E. et al. Human brain organoids assemble functionally integrated bilateral optic vesicles. Cell Stem Cell 28, 1740–1757.e8 (2021).
Gopalakrishnan, J. The emergence of stem cell-based brain organoids: trends and challenges. BioEssays 41, e1900011 (2019).
Ramani, A. et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 39, e106230 (2020).
Sloan, S. A., Andersen, J., Pașca, A. M., Birey, F. & Pașca, S. P. Generation and assembly of human brain region-specific three-dimensional cultures. Nat. Protoc. 13, 2062–2085 (2018).
Quadrato, G. et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 (2017).
Goranci-Buzhala, G. et al. Rapid and efficient invasion assay of glioblastoma in human brain organoids. Cell Rep. 31, 107738 (2020).
Birey, F. et al. Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59 (2017).
Huschke, E. Uber einige streitpunkte aus der anatomie des auges [German]. Z. Opthalmol. 4, 273–295 (1835).
Pander, H. Beiträge zur Entwickelungsgeschichte des Hühnchens im Eye [German] (1817).
Adelmann, H. Marcello Malpighi and the Evolution of Embryology Vol. 3 (Cornell Univ. Press, 1966).
Zhong, X. et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 5, 4047 (2014).
Capowski, E. E. et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 146, dev171686 (2019).
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012).
Cowan, C. S. et al. Cell types of the human retina and its organoids at single-cell resolution. Cell 182, 1623–1640.e34 (2020).
Vergara, M. N. et al. Three-dimensional automated reporter quantification (3D-ARQ) technology enables quantitative screening in retinal organoids. Development 144, 3698–3705 (2017).
Meyer, J. S. et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 29, 1206–1218 (2011).
Eldred, K. C. et al. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science 362, eaau6348 (2018).
Eldred, K. C. & Reh, T. A. Human retinal model systems: strengths, weaknesses, and future directions. Dev. Biol. 480, 114–122 (2021).
Fligor, C. M. et al. Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids. Stem Cell Rep. 16, 2228–2241 (2021).
Graw, J. Eye development. Curr. Top. Dev. Biol. 90, 343–386 (2010).
Rao, R. C., Stern, J. H. & Temple, S. The eyeball’s connected to the brain ball. Cell Stem Cell 28, 1675–1677 (2021).
Fuhrmann, S. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 93, 61–84 (2010).
Adler, R. & Canto-Soler, M. V. Molecular mechanisms of optic vesicle development: complexities, ambiguities and controversies. Dev. Biol. 305, 1–13 (2007).
Dupacova, N., Antosova, B., Paces, J. & Kozmik, Z. Meis homeobox genes control progenitor competence in the retina. Proc. Natl Acad. Sci. USA 118, e2013136118 (2021).
Mann, I. C. The Development of the Human Eye 1st edn (Univ. Press, 1928).
O’Rahilly, R. The early development of the eye in staged human embryos. Contrib. Embryol. 38, 1–42 (1966).
O’Rahilly, R. The prenatal development of the human eye. Exp. Eye Res. 21, 93–112 (1975).
Gabriel, E. & Gopalakrishnan, J. Generation of iPSC-derived human brain organoids to model early neurodevelopmental disorders. J. Vis. Exp. 14, 55372 (2017).
Gabriel, E. et al. Recent zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell. Stem Cell. 20, 397–406.e5 (2017).
Zhang, W. et al. Modeling microcephaly with cerebral organoids reveals a WDR62-CEP170-KIF2A pathway promoting cilium disassembly in neural progenitors. Nat. Commun. 10, 2612 (2019).
Rosen, D. & Mahabadi, N. Embryology, Optic Cup (StatPearls Publishing LLC, updated 8 May 2022); https://www.ncbi.nlm.nih.gov/books/NBK545150/
Cvekl, A. & Wang, W. L. Retinoic acid signaling in mammalian eye development. Exp. Eye Res. 89, 280–291 (2009).
Janesick, A., Wu, S. C. & Blumberg, B. Retinoic acid signaling and neuronal differentiation. Cell. Mol. Life Sci. 72, 1559–1576 (2015).
Morizane, A., Doi, D., Kikuchi, T., Nishimura, K. & Takahashi, J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J. Neurosci. Res. 89, 117–126 (2011).
Jin, M., Yuan, Q., Li, S. & Travis, G. H. Role of LRAT on the retinoid isomerase activity and membrane association of Rpe65. J. Biol. Chem. 282, 20915–20924 (2007).
Hu, J. & Bok, D. The use of cultured human fetal retinal pigment epithelium in studies of the classical retinoid visual cycle and retinoid-based disease processes. Exp. Eye Res. 126, 46–50 (2014).
Francis, P. J. Genetics of inherited retinal disease. J. R. Soc. Med. 99, 189–191 (2006).
Takagi, S. et al. Evaluation of transplanted autologous induced pluripotent stem cell-derived retinal pigment epithelium in exudative age-related macular degeneration. Ophthalmol. Retin. 3, 850–859 (2019).
Dahl-Jensen, S. & Grapin-Botton, A. The physics of organoids: a biophysical approach to understanding organogenesis. Development 144, 946–951 (2017).
He, Z. et al. Lineage recording in human cerebral organoids. Nat. Methods 19, 90–99 (2022).
Hu, S. et al. Effects of cellular origin on differentiation of human induced pluripotent stem cell-derived endothelial cells. JCI Insight 1, e85558 (2016).
Phetfong, J. et al. Cell type of origin influences iPSC generation and differentiation to cells of the hematoendothelial lineage. Cell Tissue Res. 365, 101–112 (2016).
Bardy, C. et al. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc. Natl Acad. Sci. USA 112, E2725–E2734 (2015).
Slembrouck-Brec, A. et al. Reprogramming of adult retinal Müller glial cells into human-induced pluripotent stem cells as an efficient source of retinal cells. Stem Cells Int. 2019, 7858796 (2019).
Karch, C. M. et al. A comprehensive resource for induced pluripotent stem cells from patients with primary tauopathies. Stem Cell Rep. 13, 939–955 (2019).
Baharvand, H., Salekdeh, G. H., Taei, A. & Mollamohammadi, S. An efficient and easy-to-use cryopreservation protocol for human ES and iPS cells. Nat. Protoc. 5, 588–594 (2010).
Rivera, T., Zhao, Y., Ni, Y. & Wang, J. Human-induced pluripotent stem cell culture methods under cGMP conditions. Curr. Protoc. Stem Cell Biol. 54, e117 (2020).
Klingberg, A. et al. Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy. J. Am. Soc. Nephrol. 28, 452–459 (2017).
Maiorano, N. A. & Hindges, R. Restricted perinatal retinal degeneration induces retina reshaping and correlated structural rearrangement of the retinotopic map. Nat. Commun. 4, 1938 (2013).
Crish, S. D., Sappington, R. M., Inman, D. M., Horner, P. J. & Calkins, D. J. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc. Natl Acad. Sci. USA 107, 5196–5201 (2010).
Foxton, R., Osborne, A., Martin, K. R., Ng, Y. S. & Shima, D. T. Distal retinal ganglion cell axon transport loss and activation of p38 MAPK stress pathway following VEGF-A antagonism. Cell Death Dis. 7, e2212 (2016).
Hou, M. et al. Age-related visual impairments and retinal ganglion cells axonal degeneration in a mouse model harboring OPTN (E50K) mutation. Cell Death Dis. 13, 362 (2022).
Stoeckel, K., Schwab, M. & Thoenen, H. Role of gangliosides in the uptake and retrograde axonal transport of cholera and tetanus toxin as compared to nerve growth factor and wheat germ agglutinin. Brain Res. 132, 273–285 (1977).
Conte, W. L., Kamishina, H. & Reep, R. L. Multiple neuroanatomical tract-tracing using fluorescent alexa fluor conjugates of cholera toxin subunit B in rats. Nat. Protoc. 4, 1157–1166 (2009).
Huberman, A. D., Dehay, C., Berland, M., Chalupa, L. M. & Kennedy, H. Early and rapid targeting of eye-specific axonal projections to the dorsal lateral geniculate nucleus in the fetal macaque. J. Neurosci. 25, 4014–4023 (2005).
Mikkelsen, J. D. Visualization of efferent retinal projections by immunohistochemical identification of cholera toxin subunit B. Brain Res. Bull. 28, 619–623 (1992).
Yao, F. et al. Did you choose appropriate tracer for retrograde tracing of retinal ganglion cells? The differences between cholera toxin subunit B and fluorogold. PLoS ONE 13, e0205133 (2018).
Chen, Y. et al. Cited2 is required for the proper formation of the hyaloid vasculature and for lens morphogenesis. Development 135, 2939–2948 (2008).
Eiraku, M. & Sasai, Y. Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr. Opin. Neurobiol. 22, 768–777 (2012).
Parfitt, D. A. et al. Identification and correction of mechanisms underlying inherited blindness in human iPSC-derived optic cups. Cell Stem Cell 18, 769–781 (2016).
Susaimanickam, P. J. et al. Generating minicorneal organoids from human induced pluripotent stem cells. Development 144, 2338–2351 (2017).
Foster, J. W. et al. Cornea organoids from human induced pluripotent stem cells. Sci. Rep. 7, 41286 (2017).
Hallam, D. et al. Human-induced pluripotent stem cells generate light responsive retinal organoids with variable and nutrient-dependent efficiency. Stem Cells 36, 1535–1551 (2018).
Kim, S. et al. Generation, transcriptome profiling, and functional validation of cone-rich human retinal organoids. Proc. Natl Acad. Sci. USA 116, 10824–10833 (2019).
Zerti, D. et al. IGFBPs mediate IGF-1’s functions in retinal lamination and photoreceptor development during pluripotent stem cell differentiation to retinal organoids. Stem Cells 39, 458–466 (2021).
Kuwahara, A. et al. Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat. Commun. 6, 6286 (2015).
Reichman, S. et al. Generation of storable retinal organoids and retinal pigmented epithelium from adherent human ips cells in xeno-free and feeder-free conditions. Stem Cells 35, 1176–1188 (2017).
Gagliardi, G. et al. Characterization and transplantation of CD73-positive photoreceptors isolated from human iPSC-derived retinal organoids. Stem Cell Rep. 11, 665–680 (2018).
Sullivan-Brown, J., Bisher, M. E. & Burdine, R. D. Embedding, serial sectioning and staining of zebrafish embryos using JB-4 resin. Nat. Protoc. 6, 46–55 (2011).
Gu, L., Cong, J., Zhang, J., Tian, Y. Y. & Zhai, X. Y. A microwave antigen retrieval method using two heating steps for enhanced immunostaining on aldehyde-fixed paraffin-embedded tissue sections. Histochem. Cell Biol. 145, 675–680 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Risso, D., Ngai, J., Speed, T. P. & Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 32, 896–902 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Bodenhofer, U., Kothmeier, A. & Hochreiter, S. APCluster: an R package for affinity propagation clustering. Bioinformatics 27, 2463–2464 (2011).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Albanna, W. et al. Electroretinographic assessment of inner retinal signaling in the isolated and superfused murine retina. Curr. Eye Res. 42, 1518–1526 (2017).
Acknowledgements
This study was supported by the SPP2127-GO 2301/5-2 ‘Gene and Cell-Based Therapies to Counteract Neuroretinal Degeneration’ (J.G. and E.G.) and by the Fritz Thyssen Stiftung (E.G). V.B. acknowledges support by the Volkswagen Foundation (Freigeist—A110720) and the Deutsche Forschungsgemeinschaft SPP2127-BU 2974/4-1, EXC-2151-390873048-Cluster of Excellence—ImmunoSensation2 at the University of Bonn.
Author information
Authors and Affiliations
Contributions
J.G. and E.G.: conception and protocol design. The rest of the authors have contributed their expertise in the respective methods of the protocol. E.G.: organoid generation and characterization; G.P. and V.B.: scRNA-seq analysis; W.A. and T.S.: ERGs; A.P. and N.J.: RNA-seq analyses; A.R. and A.M.: 3D imaging; M.G.R. and G.C.: TEM and analysis; O.G.: iPSCs, organoids and data analysis; C.M.K.: iPSC lines and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using the protocol
Gabriel, E. et al. EMBO J. 35, 803–819 (2016): https://doi.org/10.15252/embj.201593679
Gabriel, E. et al. Cell Stem Cell 20, 397–406.e5 (2017): https://doi.org/10.1016/j.stem.2016.12.005
Gabriel, E. et al. Cell Stem Cell 28, 1740–1757.e8 (2021): https://doi.org/10.1016/j.stem.2021.07.010
Supplementary information
Supplementary Video 1
Microinjection of Cholera toxin B (CTB-488 and CTB-647) to label axon-like projections. Representative movie of volume-rendered optic vesicles from specimen shown in Fig. 5c microinjected with CTB-488 and CTB-647 at two distinct sites. The injection sites are marked with arrows (00:03 min). Individual channels (GFP and Cy5) have been shown to highlight the distribution of injected toxins. The converging nature of optic tracts is demonstrated by the strong co-localization of axons labeled by both CTB-488 and CTB-647. Scale bar, 200 μm. The representative movie, cell line IMR-90.
Supplementary Table 1
Visual pathway genes
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gabriel, E., Albanna, W., Pasquini, G. et al. Generation of iPSC-derived human forebrain organoids assembling bilateral eye primordia. Nat Protoc 18, 1893–1929 (2023). https://doi.org/10.1038/s41596-023-00814-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00814-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.