Abstract
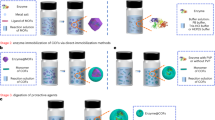
Strategies for the covalent immobilization of enzymes depend on the type of functional group selected to perform the coupling reaction, and on the relative importance of selectivity, loading capacity, immobilization time and activity/stability of the resulting immobilized preparation. However, no single strategy is applicable for all covalent immobilization methods or can meet all these criteria, exemplifying the challenge of introducing a versatile process broadly compatible with the residues on the surface of proteins and the functional groups of common linkers. Here, we describe the use of isocyanide-based multi-component reactions for the carrier-bound and carrier-free covalent immobilization of enzymes. Isocyanide-based multi-component reactions can accept a wide variety of functional groups such as epoxy, acid, amine and aldehyde, as well as many commercially available bi-functional linkers, and are therefore suitable for either covalent coupling of enzymes on a solid support or intermolecular cross-linking of enzymes. In this strategy, an isocyanide is directly added to the reaction medium, the enzyme supplies either the exposed amine or carboxylic acid groups, and the support (in carrier-bound immobilization) or the bi-functional cross-linking agent (in carrier-free immobilization) provides another reactive functional group. The protocol offers operational simplicity, high efficiency and a notable reduction in time over alternative strategies, and can be performed by users with expertise in chemistry or biology. The immobilization reactions typically require 1–24 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the findings of this study are present in the paper. All other data supporting the findings of this study are available from the corresponding author upon request. No datasets or custom code was generated in this study.
References
Bolivar, J. M., Woodley, J. M. & Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 51, 6251–6290 (2022).
Souza, P. M. P. et al. Enzyme-support interactions and inactivation conditions determine Thermomyces lanuginosus lipase inactivation pathways: functional and florescence studies. Int. J. Biol. Macromol. 191, 79–91 (2021).
Sheldon, R. A. & van Pelt, S. Enzyme immobilisation in biocatalysis: why, what and how. Chem. Soc. Rev. 42, 6223–6235 (2013).
Rodrigues, R. C. et al. Immobilization of lipases on hydrophobic supports: immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 37, 746–770 (2019).
Guzik, U., Hupert-Kocurek, K. & Wojcieszyńska, D. Immobilization as a strategy for improving enzyme properties—application to oxidoreductases. Molecules 19, 8995–9018 (2014).
da Fonseca, A. M. et al. Synthesis, biological activity, and in silico study of bioesters derived from bixin by the CALB enzyme. Biointerface Res. Appl. Chem. 12, 5901–5917 (2022).
Sheldon, R. A., Basso, A. & Brady, D. New frontiers in enzyme immobilisation: robust biocatalysts for a circular bio-based economy. Chem. Soc. Rev. 50, 5850–5862 (2021).
Cao, L. Carrier-Bound Immobilized Enzymes: Principles, Application and Design (John Wiley & Sons, 2006).
Monteiro, R. R. et al. Improvement of enzymatic activity and stability of lipase A from Candida antartica onto halloysite nanotubes with Taguchi method for optimized immobilization. Appl. Clay Sci. 228, 106634 (2022).
Pierre, S. J. et al. Covalent enzyme immobilization onto photopolymerized highly porous monoliths. Adv. Mater. 18, 1822–1826 (2006).
Silva, A. R. et al. The chemistry and applications of metal–organic frameworks (MOFs) as industrial enzyme immobilization systems. Molecules 27, 4529 (2022).
Nunes, Y. L. et al. Chemical and physical chitosan modification for designing enzymatic industrial biocatalysts: how to choose the best strategy? Int. J. Biol. Macromol. 181, 1124–1170 (2021).
Mohammadi, M. et al. Rapid and high-density covalent immobilization of Rhizomucor miehei lipase using a multi component reaction: application in biodiesel production. RSC Adv. 5, 32698–32705 (2015).
Mohammadi, M., Gandomkar, S., Habibi, Z. & Yousefi, M. One pot three-component reaction for covalent immobilization of enzymes: application of immobilized lipases for kinetic resolution of rac-ibuprofen. RSC Adv. 6, 52838–52849 (2016).
Mohammadi, M., Ashjari, M., Garmroodi, M., Yousefi, M. & Karkhane, A. A. The use of isocyanide-based multicomponent reaction for covalent immobilization of Rhizomucor miehei lipase on multiwall carbon nanotubes and graphene nanosheets. RSC Adv. 6, 72275–72285 (2016).
Zhu, J. & Bienaymé, H. Multicomponent Reactions (John Wiley & Sons, 2006).
Ashjari, M., Garmroodi, M., Ahrari, F., Yousefi, M. & Mohammadi, M. Soluble enzyme cross-linking via multi-component reactions: a new generation of cross-linked enzymes. Chem. Commun. 56, 9683–9686 (2020).
Ahrari, F., Yousefi, M., Habibi, Z. & Mohammadi, M. Application of undecanedicarboxylic acid to prepare cross-linked enzymes (CLEs) of Rhizomucor miehei lipase (RML); selective enrichment of polyunsaturated fatty acids. Mol. Catal. 520, 112172 (2022).
Chen, N. et al. Cross-linked enzyme aggregates immobilization: preparation, characterization, and applications. Crit. Rev. Biotechnol. 1–15 (2022).
Jegan Roy, J. & Emilia Abraham, T. Strategies in making cross-linked enzyme crystals. Chem. Rev. 104, 3705–3722 (2004).
Noori, R., Perwez, M. & Sardar, M. Cross-linked enzyme aggregates: current developments and applications. Biocatalysis 83–112 (2019).
Jiaojiao, X., Yan, Y., Bin, Z. & Feng, L. Improved catalytic performance of carrier-free immobilized lipase by advanced cross-linked enzyme aggregates technology. Bioprocess Biosyst. Eng. 45, 147–158 (2022).
Mateo, C. et al. Immobilization of enzymes on heterofunctional epoxy supports. Nat. Protoc. 2, 1022–1033 (2007).
Moosavi, F., Ahrari, F., Ahmadian, G. & Mohammadi, M. Sortase-mediated immobilization of Candida antarctica lipase B (CalB) on graphene oxide; comparison with chemical approach. Biotechnol. Rep. 34, e00733 (2022).
Fernandez-Lorente, G. et al. Solid-phase chemical amination of a lipase from Bacillus thermocatenulatus to improve its stabilization via covalent immobilization on highly activated glyoxyl-agarose. Biomacromolecules 9, 2553–2561 (2008).
Zimmermann, J. L., Nicolaus, T., Neuert, G. & Blank, K. Thiol-based, site-specific and covalent immobilization of biomolecules for single-molecule experiments. Nat. Protoc. 5, 975–985 (2010).
Amini, Y. et al. A multi-component reaction for covalent immobilization of lipases on amine-functionalized magnetic nanoparticles: production of biodiesel from waste cooking oil. Bioresour. Bioprocess. 9, 1–15 (2022).
Gao, Z. et al. Co-immobilization of laccase and TEMPO onto amino-functionalized magnetic Fe3O4 nanoparticles and its application in acid fuchsin decolorization. Bioresour. Bioprocess. 5, 1–8 (2018).
Vashist, S. K., Lam, E., Hrapovic, S., Male, K. B. & Luong, J. H. Immobilization of antibodies and enzymes on 3-aminopropyltriethoxysilane-functionalized bioanalytical platforms for biosensors and diagnostics. Chem. Rev. 114, 11083–11130 (2014).
Moreira, Kd. S. et al. Taguchi design-assisted co-immobilization of lipase A and B from Candida antarctica onto chitosan: characterization, kinetic resolution application, and docking studies. Chem. Eng. Res. Des. 177, 223–244 (2022).
Kannan, K. & Jasra, R. V. Improved catalytic hydrolysis of carboxy methyl cellulose using cellulase immobilized on functionalized meso cellular foam. J. Porous Mater. 18, 409–416 (2011).
Habibi, Z., Mohammadi, M. & Yousefi, M. Enzymatic hydrolysis of racemic ibuprofen esters using Rhizomucor miehei lipase immobilized on different supports. Process Biochem. 48, 669–676 (2013).
Mohammadi, M., Habibi, Z., Gandomkar, S. & Yousefi, M. A novel approach for bioconjugation of Rhizomucor miehei lipase (RML) onto amine-functionalized supports; application for enantioselective resolution of rac-ibuprofen. Int. J. Biol. Macromol. 117, 523–531 (2018).
Barbosa, O. et al. Heterofunctional supports in enzyme immobilization: from traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 14, 2433–2462 (2013).
Shahedi, M., Yousefi, M., Habibi, Z., Mohammadi, M. & As’ habi, M. A. Co-immobilization of Rhizomucor miehei lipase and Candida antarctica lipase B and optimization of biocatalytic biodiesel production from palm oil using response surface methodology. Renew. Energy 141, 847–857 (2019).
Babaki, M., Yousefi, M., Habibi, Z., Brask, J. & Mohammadi, M. Preparation of highly reusable biocatalysts by immobilization of lipases on epoxy-functionalized silica for production of biodiesel from canola oil. Biochem. Eng. J. 101, 23–31 (2015).
Ramon-Marquez, T., Medina-Castillo, A. L., Fernandez-Sanchez, J. F. & Fernández-Gutiérrez, A. Evaluation of different functional groups for covalent immobilization of enzymes in the development of biosensors with oxygen optical transduction. Anal. Methods 7, 2943–2949 (2015).
Kutorglo, E. M. et al. Carboxyethyl-functionalized 3D porous polypyrrole synthesized using a porogen-free method for covalent immobilization of urease. Micropor. Mesopor. Mat. 311, 110690 (2021).
Je, H. H. et al. Cellulose nanofibers for magnetically-separable and highly loaded enzyme immobilization. Chem. Eng. J. 323, 425–433 (2017).
Orrego, A. H. et al. Stabilization of enzymes by multipoint covalent attachment on aldehyde-supports: 2-picoline borane as an alternative reducing agent. Catalysts 8, 333 (2018).
Bolivar, J. M. et al. Stabilization of a formate dehydrogenase by covalent immobilization on highly activated glyoxyl-agarose supports. Biomacromolecules 7, 669–673 (2006).
Ashjari, M. et al. Application of multi-component reaction for covalent immobilization of two lipases on aldehyde-functionalized magnetic nanoparticles; production of biodiesel from waste cooking oil. Process Biochem. 90, 156–167 (2020).
Barbosa, O. et al. Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 4, 1583–1600 (2014).
Salami, F., Habibi, Z., Yousefi, M. & Mohammadi, M. Covalent immobilization of laccase by one pot three component reaction and its application in the decolorization of textile dyes. Int. J. Biol. Macromol. 120, 144–151 (2018).
Weltz, J. S., Kienle, D. F., Schwartz, D. K. & Kaar, J. L. Reduced enzyme dynamics upon multipoint covalent immobilization leads to stability-activity trade-off. J. Am. Chem. Soc. 142, 3463–3471 (2020).
Acknowledgements
This work was financially supported by National Institute of Genetic Engineering and Biotechnology of Iran (grants 672 and 587), by a grant from PhosAgro/UNESCO/IUPAC partnership program (4500283719-A1) and by a grant from the Iran National Science Foundation (INSF) (95830819).
Author information
Authors and Affiliations
Contributions
M. Mohammadi and M.Y. conceived the project. M. Mohammadi and M.S. wrote the manuscript with support from M.Y., Z.H., M. Mostafavi and F.A. F.A. performed the experiments. M. Mohammadi, Z.H., M.Y. and M.S. analyzed the data. M. Mohammadi and M.Y. supervised the project. M. Mostafavi provided the figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Rénato Froidevaux and J. C. S. dos Santos for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Mohammadi, M. et al. RSC Adv. 5, 32698–32705 (2015): https://doi.org/10.1039/C5RA03299G
Mohammadi, M. et al. RSC Adv. 6, 52838–52849 (2016): https://doi.org/10.1039/C6RA11284F
Ashjari, M. et al. Chem. Commun. 56, 9683–9686 (2020): https://doi.org/10.1039/D0CC03429K
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammadi, M., Shahedi, M., Ahrari, F. et al. Isocyanide-based multi-component reactions for carrier-free and carrier-bound covalent immobilization of enzymes. Nat Protoc 18, 1641–1657 (2023). https://doi.org/10.1038/s41596-023-00812-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00812-z
This article is cited by
-
Pros and Cons in Various Immobilization Techniques and Carriers for Enzymes
Applied Biochemistry and Biotechnology (2024)
-
CATase-immobilized hydrogel platform molded by photo-enzyme coupling polymerization for effectively preventing postoperative abdominal adhesion
Science China Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.